Abstract
Tendons connect muscles to bones, ensuring joint movement. With advanced age, tendons become more prone to degeneration followed by injuries. Tendon repair often requires lengthy periods of rehabilitation, especially in elderly patients. Existing medical and surgical treatments often fail to regain full tendon function.
The development of novel treatment methods has been hampered due to limited understanding of basic tendon biology. Recently, it was discovered that tendons, similar to other mesenchymal tissues, contain tendon stem/progenitor cells (TSPCs) which possess the common stem cell properties.
The current strategies for enhancing tendon repair consist mainly of applying stem cells, growth factors, natural and artificial biomaterials alone or in combination. In this review, we summarise the basic biology of tendon tissues and provide an update on the latest repair proposals for tendon tears.
Cite this article: EFORT Open Rev 2017;2:332-342. DOI: 10.1302/2058-5241.2.160075
Keywords: tendon repair, cell-based therapy, tendon stem/progenitor cells, mesenchymal stem cells, growth factors, biomaterials
Tendons basic biology
Anatomy
Tendons are anatomical structures connecting muscles to bones which generate transmission of forces, thereby ensuring joint movements.1 Due to overuse or age-related degeneration, tendon injuries have become a common clinical problem. Damaged tendons heal slowly and rarely retain the structural integrity and mechanical strength of a healthy tendon, which often results in clinical challenges as well as patient burden.
According to clinical observations and statistical data, certain tendons are prone to a higher possibility of injury. These are the rotator cuff, forearm extensors, Achilles tendon, tibialis posterior and patellar tendons.2,3 The attachment of tendon to bone is termed an osteotendinous junction or enthesis.4,5 The attachment between muscles and the tendon is called a myotendinous junction; a highly specialised region where tendinous collagen fibrils are inserted into deep recesses formed by myocytes. This arrangement allows first, the transmission of tension generated by intracellular, muscular contractile proteins to the tendinous collagen fibres,6 and second, reduces the exerted tensile stress that is applied to the tendon.1 The enthesis reduces and dissipates stress concentration at the hard-soft tissue junction, prevents collagen fibre bending, sharing and failure.7-10 There are two types of entheses: fibrous entheses and fibrocartilaginous entheses. In a fibrous enthesis, the collagenous tendon or ligament directly attaches to the bone, whereas the fibrocartilaginous interface encompasses different transitional zones, namely, uncalcified fibrocartilage, calcified fibrocartilage and bone.
Architecture and molecular composition
Microscopically, healthy tendons are known to be brilliant white in colour with a glistening appearance. They belong to the group of dense connective tissues and are predominantly composed of parallel, closely packed collagen fibres and cells within a well-ordered extracellular matrix (ECM). The basic structure of tendons is of collagen type I, which is arranged in hierarchical levels of complexity and constitutes 65% to 80% of the tendon dry mass. There are also 2% to 3% of other collagen types, such as collagen type II in the cartilaginous zones, collagen type III in reticular fibres of blood vessels, collagen type V in vascular membranes, collagen type IV in capillary membranes, collagen type X in the mineralised fibrocartilage within the osteotendinous junction and collagen type XII, XIV as well as XV as fibril-associated collagens.3,11,12
The smallest collagen unit is tropocollagen which is synthesised inside the tendon cell, aggregates into a triple-helix polypeptide consisting of two α1 chains and one α2 chain, and is secreted into the ECM. There, the triple-helix self-assembles via intermolecular crosslinks into parallel organised collagen fibrils, which are 100 nm to 500 nm in diameter and rotate about 90°, in the Achilles tendon, descending to the calcaneus. This structure is responsible for the crimp and wave-like appearance of the tendon. Fibrils in turn are bundled mainly longitudinally into collagen fibres, sub-fascicles (primary bundle), fascicles (secondary bundle), tertiary bundles and the tendon itself. Each tendon fibre, the smallest unit visible under light microscopy, is surrounded by a thin reticular network of connective tissue: the endotenon. Tertiary bundles, as well as the whole tendon, are covered by a fine, loose connective tissue sheath (the epitenon) ensuring vascular, lymphatic and nerve supply.1,5,13
Tendons which bend sharply around joints, e.g. in the hand and foot, are enclosed by a tendon sheath with synovial fluid to reduce sliding friction. Instead of a true synovial sheath, the Achilles tendon has a ‘false sheath’ called the paratenon: a loose areolar connective tissue which is composed of collagen fibrils, some elastic fibrils, an inner lining of synovial cells, blood vessels and nerves. Taken together, the paratenon provides the vascularisation of the epitenon and endotenon, reduces friction and permits free tendon movement against surrounding tissues.2,3,5
The ground substance of the ECM in tendons, surrounding the collagen and tendon cells, is composed of 1% to 5% proteoglycans and glycoproteins, 2% elastin and 0.2% inorganic molecules, including copper, manganese and calcium.14 Via their glycosaminoglycan (GAG) side chains, proteoglycans bind to the collagen fibrils in order to interconnect the fibrils in a parallel alignment and to ensure gliding of collagen fibrils during locomotion. They also enable rapid diffusion of water-soluble molecules and the migration of cells. Major GAG components of the tendon are dermatan and chondroitin sulphates.1,15 The tendon ECM contains several proteoglycans and glycoproteins such as tenascin C, cartilage oligo-matrix protein (COMP), decorin, fibromodulin, biglycan, lumican and tenomodulin. The glycoprotein tenascin C is a member of the tenascin gene family. It is abundantly found in the ECM of developing vertebrate embryos, interacts with fibronectin and binds to integrins and ECM components such as collagens.16,17 The pentameric, non-collagenous ECM-protein COMP belongs to the thrombospondin family of extracellular calcium-binding proteins. It consists of a five-stranded coiled coil including five identical glycoprotein subunits and its 3D structure is stabilised by disulfide bonds. COMP plays a catalytic role in the assembly of the tendon ECM by binding to collagen.18,19 Decorin, fibromodulin, biglycan and lumican belong to the small leucine-rich proteoglycan family and consist of a protein core containing leucine repeats with varying GAG chains. The GAG chains of decorin consist of either dermatan or chondroitin sulphates. Fibromodulin has four keratin sulphates. Biglycan has GAG chains consisting of either chondroitin or dermatan sulphates20-22 and the GAG chains of lumican contain keratin sulphates. Decorin is known to bind directly to collagen type I fibrils and has been implicated in the lateral collagen fibrillogenesis.23 Similar to decorin, fibromodulin, biglycan and lumican bind to collagen I fibrils participating in the lateral tendon collagen fibrillogenesis.21,22 The importance of fibromodulin for the tendon collagen network was demonstrated in a study in which fibromodulin-null mice exhibited irregular collagen fibrils accompanied by an abnormal tissue organisation in tail tendons.20 The function of decorin in collagen fibrillogenesis was reported by Reed and Iozzo.24 They showed that decorin ‘knockout’ mice also had abnormal tendon phenotypes which revealed themselves by altered tendon collagen fibrils and reduced tensile strength.24 Tenomodulin (Tnmd) is a member of the type II transmembrane glycoproteins family with C-terminal anti-angiogenic domain, which is highly expressed in tendon tissue and is known to be one of the key factors for tendon maturation.25,26 Elastin plays an important role in various tissues and organs. As an essential component in ECM, elastin has been reported to cooperate with collagen mainly functioning in elastic stretch and recoil, especially for tensile resistance, and regulates the interactions between cells and ECM.
Taken together, the tendon architecture and molecular composition are well described; however, the exact protein signature, distinguishing it from other musculoskeletal tissues as well as from ligament or among sub-variants of tendons, remain to be identified.
Cellular content
In the very beginning, since no cells could be found in the tissue staining, for a long time tendon had been considered an inert tissue. Later, it was found that the tendon tissue contains one major cell type and the rest of the components are a scarce amount of fat, neuronal and endothelial cells. Nowadays various cell types have been discovered, such as uncommitted cells and more mature cells. Even in the uncommitted fraction there are several stem cell sub-types. Only in the last few years has there been clarification of the discrete steps of the tenogenic cell cascade.27 However, there is still great need of tools; for example, specific markers (single or combinational) or genetic lineage tracing to well separate each discrete cell subset. We have summarised the cellular content of tendon in Table 1.28-33
Table 1.
Tendon cellular content
| Cell type | Alternative name | Location/Incidence | Function | Reference |
|---|---|---|---|---|
| Tenocyte | Tendon-derived cell (TDC) Tendon fibroblast Tenoblast |
Tendon/enthesis tissue | Building up and maintaining tendon structure; interacting with collagen | Fu et al28
Mienaltowski et al29 |
| Tendon stem/progenitor cell | Tendon stem cell (TSC) Tendon-derived stem cell (TDSC) Tendon-derived progenitor cell (TDPC) |
Debatable | Multipotent and clonogenic, tissue homeostasis and repair; exact involvement is to be elucidated | Kohler at al30
Bi et al31 |
| Perivascular cell | Pericyte Tendon mesenchymal stem cell (TMSC) |
Vessel, paratenon, epitenon, tendon sheets | Regulation of stem cells; regeneration of various tissues; immunomodulation | Tempfer et al32 |
| Intra-fascicular matrix cell | Endotenon Sheet stem cell |
Around tendon fascicles Endotenon sheets |
Maintenance; exact identity and function are to be elucidated | Mienaltowski et al29
Thorpe et al33 |
Blood supply and innervation
The blood supply of tendons is assured by vessels originating from three different sites: the myotendinous junction; the osteotendinous junction; and the tendon sheaths. At the myotendinous junction, the muscle provides the tendon with blood vessels running down to the proximal third of the tendon.26 The sparse blood supply from the osteotendinous junction is restricted to the insertion site.34 The main vascularisation of the tendon is provided by the paratenon, where a vascular network penetrates the epitenon deep into the tendon and reaches the endotenon sheets.35,36 Studies have shown that the Achilles tendon has a hypovascular zone, 2 cm to 6 cm proximal to the osteotendinous junction.7,34,36-39 However, Aström40 has demonstrated, by using laser Doppler flowmetry, an even distribution of the blood flow within the Achilles tendon. This suggests that, despite the regional vascularity of the tissue, the blood-derived nutrients can penetrate the tissue evenly. Further research is necessary to investigate precisely the tendon fluid dynamics. Because tendon tissues are predominantly extracellular and have a low metabolic rate, the vascularity and healing capability of tendons is much inferior compared with many other tissues.2,3,41
Tendon has been considered as hyponeural, which is in accordance with the relative hypovascularity. Sensory nerves are mostly located on the surface of the Achilles tendon. Most of the nerves originate from cutaneous, muscular and peritendinous nerve trunks and terminate as nerve endings on the paratenon. Only few sensory nerve fibres enter the main body of the tendon as they follow the vascular network of the endotenon.3,42 The nerve endings act as specialised sensory receptors in order to sense changes in pressure, tension or pain. Tension receptors are also known as Golgi tendon organs and are mainly located at the myotendinous junction. Each Golgi tendon organ consists of collagen fibres enclosed in a capsule with nerve fibres which branch as spiral endings between tendon collagen bundles.1,3 In recent years, the production of nerve signal substances has been reported in tendons. Studies have identified neurotransmitters such as glutamate, acetylcholine and substance P in human Achilles tendons.43-45
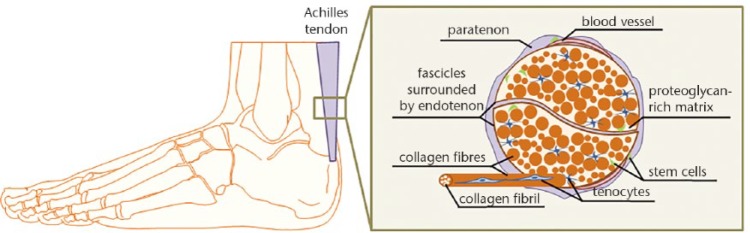
Figure 146 represents a simplified cartoon model of the tendon anatomical structure.
Fig. 1.
Simplified cartoon model of Achilles tendon anatomical structure. Paratenon sheet surrounds the whole tendon, while endotenon sheets surround individual tendon fascicles. Scarce blood vessels and nerve fibres (not shown) are located within the sheets. Each fascicle is composed of collagen fibres with variable diameter size situated in proteoglycan-rich matrix, while each collagen fibre is composed of multiple collagen type I fibrils. In between fibres, tenocytes (terminally differentiated cells) are found. Tendon stem cells are suggested to be located in the tendon sheets, near the blood vessels or within the intra-fascicular matrix. Based on Docheva et al.46
Tendon diseases
Tendon disorders are medical conditions including ruptures and overuse injuries accompanied by inflammatory and degenerative changes such as tendinopathies. In the United States, 33 million musculoskeletal injuries have been reported per year, 50% involving tendon and ligament injuries.5 The Achilles tendon is one of the most injured tendons in the body often due to long-term overuse and repetitive activities.3,47-50 Tendon injuries, however, do not occur only in physically active adults and adolescents (especially men are affected), but they also appear among the sedentary population with a moderate physical activity. Next to sport, several intrinsic factors, including body weight, nutrition and age, may be responsible for tendon injuries and other conditions. Genetics might also affect tendon disorders. Genetic diseases are not specifically associated with tendon injuries, but there are common genetic connective tissue disorders affecting bone, skin, cornea, muscle, vessel or tendon/ligament. Tendinopathies such as tendonitis, peri-tendonitis and retrocalcaneobursitis are tendon disorders accompanied by inflammation and pain, whereas tendinosis and ruptures are caused by intertendinous degeneration without the evidence of inflammatory processes.51,52
Achilles ruptures (80% to 90% of cases) typically occur in the low vascularised region, 2 cm to 6 cm to the osteotendinous junction, and often follow tendinopathies.53 The latter belongs to the most common running-associated disorders before knee and shin-splint injuries.54 In recent decades, the prevalence of Achilles tendinopathies and ruptures has risen, due to both an increase in the elderly population and a higher participation in excessive physical activities.55 In the general population, the incidence of Achilles tendon ruptures, a typical injury among 30- and 50-year-old men, is up to 1%.56 Leppilahti and Orava57 reported that 80% of these ruptures occurred during sporting activities, while 10% of these patients suffered from pre-existing Achilles tendon problems. Achilles tendinopathies appear more frequently with a lifetime risk of 52% in former elite male runners52 with 5.9% among sedentary people, 24% among competitive athletes and 18% among athletes aged younger than 45 years.55
Tendon pathophysiology
Histological features of tendinopathies and ruptures are characterised by a loss of the glistening-white to an amorphous, grey-brown appearance of the tendon. Furthermore, collagen degeneration, fibre disorientation and thinning, hypercellular, rounded tenocytes nuclei, increased vascular in-growth and a change towards fibrocartilaginous composition can be observed microscopically.8,58,59 Occasionally, tendon degeneration also includes fibrin deposits, calcifications and lipid accumulations.41
The tendon degeneration process can be considered as a failure of matrix adaptation and remodelling due to an imbalance between matrix decomposition and synthesis caused by a variety of stresses and mechanical loads.1 The imbalance of metalloproteinases and their endogenous inhibitors, the so-called tissue inhibitors of metalloproteinases (TIMPs), is considered to play a crucial role in the degeneration process. In recent decades, biochemical and molecular studies of tendon disorders have advanced our understanding of the underlying degenerative process.16 The major structural and molecular changes include: upregulation of collagen type I and collagen type III mRNA; a shift to a higher collagen type III abundance in relation to collagen type I in the ECM; elevated levels of fibronectin, tenascin C, GAGs, aggrecan and biglycan.58 There are also changes in the activity of various matrix metalloproteinases (MMPs), disintegrin and metalloproteinases (ADAMs); disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs); and TIMPs which contribute to the weakening of the tendon ECM.1,51 Downregulation of MMP-3 and upregulation of MMP-2 and vascular endothelial growth factor (VEGF) has been reported in Achilles tendinopathy.60 In tendon ruptures it has been reported that levels of MMP-1, -9, -19, -25 and TIMP1 were increased, while the expression of MMP-3, -7, TIMP2, were decreased.16,58 Tendinopathy also involves an increase in inflammatory mediators such as prostaglandin E2 and interleukin-1, an enhanced expression of cyclo-oxygenase-2, growth factors including TGF-β and platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1) and neurotransmitters such as glutamate and substance B.1,58
The changes occurring during tendinopathy are considered a functional adaptation to compressional loading. Currently, there are three main hypotheses explaining the reasons for tendon degeneration: (1) mechanical overuse; (2) vascularisation; and (3) aging. The human tendon has the ability to adapt to loading through an increased collagen synthesis and metalloproteinase activity. This adaptation modifies the mechanical strength and the visco-elastic properties, and furthermore, decreases the stress-susceptibility which, in turn, leads to a higher load resistance.61,62 Nevertheless, the tendon has to withstand tremendous forces during repetitive activities, making it prone to overuse injuries. Over time, repetitive tensile loading under the tendon injury threshold can lead to an accumulation of microdamage which elevates the risk for tendinopathy or rupture.8,63 As a result of microdamage, scattered vascular in-growth, including necrotic capillaries, contributes to vascular compromise.64 This leads to local tissue hypoxia, which is considered to increase the risk for degeneration and tendinopathy. Aging, in turn, alters the tendon mechanical properties and the metabolism, thereby increasing the susceptibility for microdamage and the prevalence of degenerative changes.8,65
To date, the underlying mechanisms of tendon aging are largely unknown. However, in recent decades further knowledge about the aging process in general has been gained. The process of cellular and organismal aging is complex and not only influenced by genetic factors, but also by external factors such as obesity, diabetes, mutagens like alcohol and tobacco smoke, or intrinsic stresses including reactive oxygen species and telomere erosion.66 Tuite et al67 reported that tendon aging is underlined by a degenerative process characterised by cellular senescence, an overall decline in tissue tensile strength, mechanical properties, blood flow and an enhanced lipid formation. Other studies have indicated that the reduced regenerative potential of adult tissues is linked to a functional decline of the stem cell pool which has been associated with human aging and age-associated diseases such as osteoporosis, tendon disorders, sarcopenia, anaemia, dementia, cancer or impaired wound healing.68-74 Thus, it is assumed that aging is partly driven by an age-associated decline in the number and repair capacity of tissue-specific adult stem cells. Recently, Zhou et al73 have successfully isolated TSPC from tendon tissue of young and aged rats and revealed a decline in clonogenicity, proliferation rate and chondrogenic potential. However osteogenic and adipogenic differentiation capacities were unaffected. Kohler et al30 propose that during human tendon aging and degeneration, the TSPC pool is becoming exhausted in terms of size and functional fitness, which could provide an explanation for the often-observed delayed healing in elderly patients. Further research is needed to elucidate the precise mechanisms involved in tendon aging and degeneration; the gained knowledge can be then transferred to development of highly specific preventing therapies.
Tendon healing
Tendons possess the capability to heal by a repair process controlled by tendon cells and their surrounding ECM. Immediately after tendon injury, scar formation and tissue repair is initiated. This process contains three overlapping stages: (1) tissue inflammation; (2) cell proliferation; and (3) ECM remodelling.
The initial inflammatory stage begins with the formation of a haematoma shortly after injury. Blood cells, such as platelets, neutrophils, monocytes and erythrocytes, are attracted to the injury site by pro-inflammatory cytokines. Components of the ECM, predominantly collagen type III, are synthesised by recruited fibroblasts. Then, secreted angiogenic factors initiate the formation of a vascular network which is responsible for the preliminary stabilisation of the injury site.5,75,76 After a few days, the proliferation stage takes place accompanied by the synthesis of abundant ECM components, such as proteoglycans and collagens (mostly collagen type III), which are arranged in a random manner. Further features of this stage are increased cellularity and absorption of large amounts of water.5,77 The remodelling stage includes two sub-stages; it begins after six to eight weeks after injury and takes around one to two years, depending on the age and condition of the patient. The first sub-stage, consolidation, is characterised by a decrease in cellularity and matrix production, as the tissue becomes more fibrous through the replacement of collagen type III to collagen type I. Collagen fibres then start to organise along the longitudinal axis of the tendon, thereby restoring tendon stiffness and tensile strength.78,79 After approximately ten weeks, the maturation stage occurs, which includes an increase of collagen fibril crosslinking and the formation of a tendon-like tissue.80
In general, tendon healing is composed of two overlapping mechanisms: extrinsic and intrinsic healing. Extrinsic healing starts first and implies the invasion of inflammatory cells from the periphery to the injury site, which in turn synthesise the initial collagen matrix and promote the repair process. Intrinsic healing contributes to the repair process by the recruitment and local stem/progenitor cells.81 The healed tendon does not usually regain the mechanical properties of the uninjured tissue.5,81 The reduced strength of the scar tissue compared with the native tendon results from a reduced integration of collagen fibres with a higher ratio of collagen type III (smaller diameter) to collagen type I. As a consequence, the tendon thickens and stiffens to overcome the lower mechanical strength and, hence, the tendon quality and its functional activity are reduced.82
The healing process is supported by the secretion of certain inflammatory cytokines, such as interleukin-6 and interleukin-1β.78 Tendon cells synthesise ECM components as well as enzymes such as metalloproteinases which are capable of degrading the majority of the tendon matrix and have been implicated in the tendon remodelling process.83 Matrix remodelling is a slow, continuous process consisting of degradation and deposition of ECM proteins. These primarily include proteoglycans and collagens.
Various MMPs, ADAMs and ADAMTSs participate in the tendon repair process. For example, collagenases, such as MMP-1, -2, -8, -13 and -14, are capable of cleaving native collagen fibrils resulting in denatured collagen, which in turn is further degraded by gelatinases such as MMP-2 and -9.54,84 The maintenance of tissue homoeostasis is assured by the balance of MMPs, ADAMs, ADAMTSs and their endogenous antagonists TIMPs.85
During tendon repair, additional support is ensured by a variety of growth factors which are released by cells located at the injury site. When these growth factors bind to their specific cell surface receptors, a signalling cascade is initiated, leading to the transcription of regulatory genes and the regulation of cellular responses. TGF-β, IGF-1, PDGF, bFGF, VEGF and bone morphogenetic proteins (BMP) are known to be involved in different phases of the healing process with diverse molecular effects.5
The TGF-β family consists of three isoforms, TGF-β1, TGF-β2 and TGF-β3, which are expressed in almost every tissue in the human body and play roles in diverse events such as regulation of the cell cycle, embryogenesis, stimulation of cell migration and proliferation86 as well as wound healing.87 IGF-1 induces the production of collagens and proteoglycans as well as fibroblast proliferation as shown in flexor tendon injury models.8 PDGF promotes the expression of other growth factors such as IGF-1 along with the stimulation of cell proliferation and collagen synthesis as shown in animal models in which PDGF was delivered during tendon repair.89,90 The treatment of MSC with low doses of bFGF, increased cell proliferation91 and wound healing models attested a faster wound closure with bFGF treatment.5,92 VEGF is known to promote angiogenesis and, post-operatively, VEGF has been shown to be expressed by a majority of cells within the injury site.93 BMPs, a sub-group of the TGF-β superfamily, mostly induce bone and cartilage formation, but the delivery of BMP-12, -13 and -14 (also known as GDF-7, -6 and -5) in ectopic sites of rat models led to neo-tendon formation.94-97
Despite the immense progress in deciphering regulatory molecular factors and cells involved in tendon healing, no major breakthrough has been achieved to enhance significantly tendon repair. Therefore, the experimental research has aimed at novel strategies to augment and shorten this lengthy process which we summarise in the following section.
New repair proposals for tendon injuries
Tendon injuries are currently treated by conservative therapies or surgery. However, scientific evidence has stated that, in general, non-surgical treatment is less successful – only 60% of the restored tendons are functional.8 Up to 29% of patients need to be treated by surgery after failure of conservative therapies.43,52 Thus, surgery remains the main option followed by allogenic transplantation. Tendon treatment often requires lengthy periods of rehabilitation, while the original biological properties and mechanical strengths are rarely restored, and results in chronic pain.8 Current conservative, unsatisfactory strategies for the treatment of tendon disorders include a combination of extracorporeal shock wave therapy, eccentric exercise therapy, ultrasound treatment and low-intensity laser treatment. The use of non-steroidal anti-inflammatory drugs for pain relief is controversial based on the high risk of spontaneous post-injection tendon ruptures.8,98 Current treatments and newly developed therapies of tendinopathies are summarised in Table 2.99-121
Table 2.
New repair proposals for tendon injuries
| New proposals | Type of material | Study model | Outcome | Reference |
|---|---|---|---|---|
| Stem cells | Tendon-derived stem cells (TDSC) | Rat; patellar tendon; surgical window defect, 1 mm in width; TDSC-fibrin constructs transplantation; analysis at 2, 4 and 8 wks. | The treated TDSCs accelerated and enhanced the quality of tendon repair compared with untreated TDSCs up to week 8, which was better than that in the controls up to week 16 as shown by histology, ultrasound imaging and biomechanical testing. | Lui et al99 Lui et al99 |
| Periodontal ligament cells (PDL), Achilles tendon-derived cells | Rat; Achilles tendon; surgical transection 3 mm; 3D cell pellet transplantation; analysis at 16 weeks. | PDL group showed advanced tissue maturation, less ectopic fibrocartilage formation, more organised collagen fibres, tendon matrix expression corresponding to the final healing stage, and better cell morphometry parameters when compared with the control group. | Hsieh et al100 | |
| Human mesenchymal stem cells (hMSC) and scleraxis (hMSC-Scx) -programmed tendon progenitors | Rat; Achilles tendon; surgical transection 3 mm; 3D cell pellet transplantation; analysis at 16 weeks. | Implantation of hMSC-Scx, in contrast to hMSC and empty defect, results in smaller diameters, negligible ectopic calcification and advanced cellular organisation and matrix maturation in the injured tendons. | Hsieh et al101 | |
| Rat bone marrow mesenchymal (BMSC) and tendon-derived stem cells | Rat; Achilles tendon; surgical transection 5 mm; TDSCs or BMSCs were injected; analysis at 1, 2 and 4 wks. | TDSCs showed better biomechanical properties and higher tendency in Col-I/III gene expression level during wks 1 and 2. Immunofluorescent assay revealed higher expression of Tenascin-C in TDSCs at week 1. |
Al-ani et al102 | |
| Human embryonic stem cell-derived mesenchymal stem cells (MSC) | Rat; Achilles tendon; surgical transection 1.5-7.5 mm; Knitted Silk-Collagen Scaffold loaded with human embryonic stem cell-derived MSC; analysis at 2 and 4 weeks. | Enhanced regeneration process as shown by histological scores; superior mechanical performance but still much lower than normal tissues. | Chen et al103 | |
| Rat BMSC. | Rat; Achilles tendon; surgical transection 2 mm; MSC injection; analysis at 2 and 4 wks. | Partially positive effects on tendon remodelling in the initial stages by biomechanical test; negative or negligible effects on biomechanical results of tendon remodelling. | Kraus et al104 | |
| Rat MSC | Rat; Achilles tendon; surgical transection 3 mm proximally; MSC-loaded mesh; analysis at days 6 and 14 | Histology showed that at day 6 dense and parallel collagen bundles; reduced vascularity and increased type I collagen, better collagen formation and organisation at day 14. | Shon et al105 | |
| Human induced pluripotent stem cells (iPSC)-derived neural crest stem cells (iPSC-NCSCs) | Rat; patellar tendon; standardised full-thickness window defect (1*4 mm); defect filled with fibrin gel with iPSC-NCSCs; analysis at 1, 2 and 4 weeks. | Superior repair performance in macroscopical observation; significantly enhancement in histological and mechanical examinations. | Xu et al106 | |
| Rat tendon-derived stem cells | Rat; patellar tendon; surgical transection 1 mm; TDSCs-fibrin construct was placed in defect; analysis at 1, 2 and 4 weeks. | Better haematoxylin stain in results showing improved histomerphology; The ultimate stress and the Young’s modulus were significantly higher in the TDSCs group at week 4. | Ni et al107 | |
| Biomaterials | Type I collagen sponge | Rat; Achilles tendon; surgical transection; analysis at 1, 2 and 4 wks. | Defects receiving collagen sponges showed improved healing, with significantly stronger and less stiff tendons than control tendons. No inflammatory reaction due to the collagen sponge was found histologically. | Müller et al108 |
| Collagen-elastin scaffold | Rabbit flexor tendon; surgical cut; analysis at 3 and 8 wks. | Greater gapping after 3 wks; enhances both cellular and extracellular inflammation. | Wichelhaus et al109 | |
| Ovine forestomach matrix (OFM) scaffold | Rat; rotator cuff, surgical transection; OFM scaffolds (5 mm × 10 mm) were overlaid longitudinally on the superficial aspect of the tendon-bone insertion; analysis at 6 days and 12 wks. | Improved healing quality was shown by histological analysis, no evidence of excessive inflammatory response, no biomechanical advantage of augmentation. | Street et al110 | |
| Silk and collagen scaffolds | Mice; Achilles tendon; surgical defect; analysis at 10 days and 24 wks. | A thick, cylindrical, greyish fibrous structure and a shiny white tendon appearance were observed which are more clos to native tendons. | Know et al111 | |
| Nanofiber scaffold (6 layers of polyglycolic acid and 5 layers of fibrin) | Dog; flexor tendon; surgical transection; adipose-derived mesenchymal stem cells and growth factors seeded scaffolds were sutured in between the transection; analysis at 0 and 9 days. | Cell viability showed no significant decreases, growth factor release was higher in scaffold treated group; no obvious inflammatory response was observed on histology. | Manning et al112 | |
| Purified Collagen I Oriented Membrane | Rabbit; patellar tendon surgical transection; collagen membrane was sutured to the upper and lower patellar pole and at the paramedian level; analysis at 1 and 6 months. | Histological findings showed satisfactory graft integration with native tendon; histological examination also showed ongoing angiogenesis. | Gigante et al113 | |
| Collagen fibre implant | Sheep; patellar tendon; surgical transection 4 mm; implant was sutured in between the transaction with tension; analysis at 3 and 6 mnths. | Histology showed better integration, mechanical test showed no statistically difference in stress to failure. | Enea et al114 | |
| Growth factors | Rabbit platelet-rich plasma (PRP) | Rabbit; patellar tendon; full-thickness surgical defect; PRP with the gel form were placed in the defect; analysis at 1, 2, 3 and 4 wks. | Stronger and more extensive expression of TGF-b1 was showed at 1 and 2 wks by immunohistochemistry. | Lyras et al115 |
| Basic fibroblast growth factor | Rat; Achilles tendon; surgical defect; analysis at 12 weeks | Biomechanical properties were not significantly improved. | Kraus et al116 | |
| Bone morphogenetic protein 12 (BMP 12) | Dog; flexor digitorum profundus tendon; surgical transection; 5 mm depth, 2.5 mm width; scaffold with adipose derived stromal cells and BMP 12 were placed in the transection; analysis at 28 days. | Tensile properties showed no significantly difference; proteomics analysis showed amplification of inflammation, stress response and matrix degradation. | Gelberman et al117 | |
| Recombinant human platelet-derived growth factor | Rat; rotator cuff; surgical transection; collagen scaffold; analysis at 5 days and 28 days. | A dose-dependent response in cellular proliferation and angiogenesis was observed. | Kovacevic et al118 | |
| Rat PRP | Rat; rotator cuff tendon; surgical transection; analysis at 8 wks. | No complications related to the surgery or PRP application were observed; both failure loads and displacement were significantly higher in the two PRP-treated groups. | Ersen et al119 | |
| Rabbit PRP | Rabbit; patellar tendon; surgical transection; PRP was applied to the repair site; analysis at 2, 4 and 8 wks. | Mechanical properties showed no significant difference, no difference in collagen content or maturity was detected. | Kollitz et al120 | |
| Rat PRP | Rat; rotator cuff tendon; surgical transection; analysis at 3 wks. | Higher maximal load and stiffness, better Bonar score. | Dolkart et al121 |
The development of tissue engineering has brought new hope in the actual treatment of many clinical diseases, but in order to apply the research results of tissue engineering widely in the actual treatment of clinical diseases, there are a lot of difficulties which need to be solved urgently, including optimum resources of seed cells and ideal scaffold materials, and other factors, which are all important research directions for the future. Meanwhile, the mechanism and interplay of natural growth factors in tendon repair is becoming better understood. However, the optimum concentration of exogenous growth factors, as well as possible side effects, have to be carefully considered and can be restricting for clinical application. How to simulate the internal environment’s successful building of tendon tissue outside the body is one of the main directions of future research. More efforts and experiments should be made to research and solve these problems. We believe that with the continuous development of life and materials science, and the increasing deepening of interdisciplinary research, tissue-engineered tendon will become the ideal material and way to repair tendon defects.
Footnotes
ICMJE Conflict of interest statement: None declared.
Funding
Although none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non-profit organisation with which one or more of the authors are associated.
References
- 1. Sharma P, Maffulli N. Tendinopathy and tendon injury: the future. Disabil Rehabil 2008;30:1733-1745. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat 2008;212:211-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jósza L, Lehto MU, Järvinen M, et al. A comparative study of methods for demonstration and quantification of capillaries in skeletal muscle. Acta Histochemica 1993;94:89-96. [DOI] [PubMed] [Google Scholar]
- 4. Magnusson SP, Kjaer M. Region-specific differences in Achilles tendon cross-sectional area in runners and non-runners. Eur J Appl Physiol 2003;90:549-553. [DOI] [PubMed] [Google Scholar]
- 5. James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am 2008;33:102-112. [DOI] [PubMed] [Google Scholar]
- 6. Kvist M, Jozsa L, Kannus P, et al. Morphology and histochemistry of the myotendineal junction of the rat calf muscles. Cells Tissues Organs 1991;141:199-205. [DOI] [PubMed] [Google Scholar]
- 7. Benjamin M, Toumi H, Ralphs JR, et al. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat 2006;208:471-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatolog (Oxford) 2006;45:508-521. [DOI] [PubMed] [Google Scholar]
- 9. Shaw HM, Benjamin M. Structure–function relationships of entheses in relation to mechanical load and exercise. Scand J Med Sci Sports 2007;17:303-315. [DOI] [PubMed] [Google Scholar]
- 10. Benjamin M, McGonagle D. Entheses: tendon and ligament attachment sites. Scand J Med Sci Sports 2009;19:520-527. [DOI] [PubMed] [Google Scholar]
- 11. Fukuta S, Oyama M, Kavalkovich K, Fu FH, Niyibizi C. Identification of types II, IX and X collagens at the insertion site of the bovine achilles tendon. Matrix Biol 1998;17:65-73. [DOI] [PubMed] [Google Scholar]
- 12. Fukushige T, Kanekura T, Ohuchi E, Shinya T, Kanzaki T. Immunohistochemical studies comparing the localization of type XV collagen in normal human skin and skin tumors with that of type IV collagen. J Dermatol 2005;32:74-83. [PubMed] [Google Scholar]
- 13. Hulmes DJ. Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol 2002;137:2-10. [DOI] [PubMed] [Google Scholar]
- 14. Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech 2004;37:865-877. [DOI] [PubMed] [Google Scholar]
- 15. Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cellular Biochem 2006;98:1436-1449. [DOI] [PubMed] [Google Scholar]
- 16. Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn 2000;218:235-259. [DOI] [PubMed] [Google Scholar]
- 17. Pajala A, Melkko J, Leppilahti J, et al. TenascinC and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histol Histopathol 2009;24:1207-1211. [DOI] [PubMed] [Google Scholar]
- 18. Rock MJ, Holden P, Horton WA, Cohn DH. Cartilage oligomeric matrix protein promotes cell attachment via two independent mechanisms involving CD47 and αVβ3 integrin. Mol Cell Biochem 2010;338:215-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paulsson M, Heinegård D. Purification and structural characterization of a cartilage matrix protein. Biochemical Journal 1981;197:367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Svensson L, Aszódi A, Reinholt FP, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 1999;274:9636-9647. [DOI] [PubMed] [Google Scholar]
- 21. Svensson L, Närlid I, Oldberg Å. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS letters 2000;470:178-182. [DOI] [PubMed] [Google Scholar]
- 22. Rees SG, Dent CM, Caterson B. Metabolism of proteoglycans in tendon. Scand J Med Sci Sports 2009;19:470-478. [DOI] [PubMed] [Google Scholar]
- 23. Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact 2005;5:22-34. [PubMed] [Google Scholar]
- 24. Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. GlycoconjJ 2002;19:249-255. [DOI] [PubMed] [Google Scholar]
- 25. Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biology 2006;298:234-247. [DOI] [PubMed] [Google Scholar]
- 26. Kvist M, Hurme T, Kannus P, et al. Vascular density at the myotendinous junction of the rat gastrocnemius muscle after immobilization and remobilization. The American journal of sports medicine 1995;23:359-364. [DOI] [PubMed] [Google Scholar]
- 27. Dex S, Lin D, Shukunami C, Docheva D. Tenogenic modulating insider factor: Systematic assessment on the functions of tenomodulin gene. Gene 2016;587:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu SC, Cheuk YC, Chan KM, Hung LK, Wong MW. Is cultured tendon fibroblast a good model to study tendon healing? J Orthop Res 2008;26:374-383. [DOI] [PubMed] [Google Scholar]
- 29. Mienaltowski MJ, Adams SM, Birk DE. Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon. Tissue Eng Part A 2013;19:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kohler J, Popov C, Klotz B, et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 2013;12:988-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 2007;13:1219-1227. [DOI] [PubMed] [Google Scholar]
- 32. Tempfer H, Wagner A, Gehwolf R, et al. Perivascular cells of the supraspinatus tendon express both tendon-and stem cell-related markers. Histochem Cell Biol 2009;131:733-741. [DOI] [PubMed] [Google Scholar]
- 33.Thorpe CT, Clegg PD, Birch HL.A review of tendon injury: why is the equine superficial digital. [DOI] [PubMed]
- 34. Carr AJ, Norris SH. The blood supply of the calcaneal tendon. J Bone Joint Surg [Br] 1989;71-B:100-101. [DOI] [PubMed] [Google Scholar]
- 35. Reynolds NL, Worrell TW. Chronic Achilles peritendinitis: etiology, pathophysiology, and treatment. J Orthop Sports Phys Ther 1991;13:171-176. [DOI] [PubMed] [Google Scholar]
- 36. Zantop T, Tillmann B, Petersen W. Quantitative assessment of blood vessels of the human Achilles tendon: an immunohistochemical cadaver study. Arch Orthop Trauma Surg 2003;123:501-504. [DOI] [PubMed] [Google Scholar]
- 37. Stein V, Laprell H, Tinnemeyer S, Petersen W. Quantitative assessment of intravascular volume of the human Achilles tendon. Acta Orthopaedica Scandinavica 2000;71:60-63. [DOI] [PubMed] [Google Scholar]
- 38. Benjamin M, Kumai T, Milz S, et al. The skeletal attachment of tendons—tendon ‘entheses’. Comp Biochem Physiol Part A: Mol & Integr Physiol 2002;133:931-945. [DOI] [PubMed] [Google Scholar]
- 39. Chen TM, Rozen WM, Pan WR, et al. The arterial anatomy of the Achilles tendon: anatomical study and clinical implications. Clin Anat 2009;22:377-385. [DOI] [PubMed] [Google Scholar]
- 40. Åström M. Laser Doppler flowmetry in the assessment of tendon blood flow. Scand J Med Sci Sports 2000;10:365-367. [DOI] [PubMed] [Google Scholar]
- 41. Milz S, Tischer T, Buettner A, et al. Molecular composition and pathology of entheses on the medial and lateral epicondyles of the humerus: a structural basis for epicondylitis. Ann Rheum Dis 2004;63:1015-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ackermann PW, Ahmed M, Kreicbergs A. Early nerve regeneration after Achilles tendon rupture—a prerequisite for healing? A study in the rat. J Orthopaedic Res 2002;20:849-856. [DOI] [PubMed] [Google Scholar]
- 43. Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper's knee. J Orthop Res 2001;19:881-886. [DOI] [PubMed] [Google Scholar]
- 44. Andersson G, Danielson P, Alfredson H, Forsgren S. Presence of substance P and the neurokinin-1 receptor in tenocytes of the human Achilles tendon. Regul Pept 2008;150:81-87. [DOI] [PubMed] [Google Scholar]
- 45. Bjur D, Danielson P, Alfredson H, Forsgren S. Immunohistochemical and in situ hybridization observations favor a local catecholamine production in the human Achilles tendon. Histol Histopathol 2008;23:197-208. [DOI] [PubMed] [Google Scholar]
- 46. Docheva D, Müller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev 2015;84:222-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kvist M. Achilles tendon injuries in athletes. Sports Med 1994;18:173-201. [DOI] [PubMed] [Google Scholar]
- 48. Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique–no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand 2000;71:475-479. [DOI] [PubMed] [Google Scholar]
- 49. Paavola M, Kannus P, Järvinen TA, et al. Achilles tendinopathy. J Bone Joint Surg [Am] 2002;84-A:2062-2076. [DOI] [PubMed] [Google Scholar]
- 50. Heckman DS, Gluck GS, Parekh SG. Tendon disorders of the foot and ankle, part 2: Achilles tendon disorders. Am J Sports Med 2009;37:1223-1234. [DOI] [PubMed] [Google Scholar]
- 51. Mazzone MF, McCue T. Common conditions of the achilles tendon. Am Fam Physician 2002;65:1805-1811. [PubMed] [Google Scholar]
- 52. Zafar MS, Mahmood A, Maffulli N. Basic science and clinical aspects of achilles tendinopathy. Sports Med Arthrosc 2009;17:190-197. [DOI] [PubMed] [Google Scholar]
- 53. Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec 2010;3:29-32 [DOI] [PubMed] [Google Scholar]
- 54. Knobloch K, Yoon U, Vogt PM. Acute and overuse injuries correlated to hours of training in master running athletes. Foot Ankle Int 2008;29:671-676. [DOI] [PubMed] [Google Scholar]
- 55. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med 2005;15:133-135. [DOI] [PubMed] [Google Scholar]
- 56. Suchak AA, Bostick G, Reid D, Blitz S, Jomha N. The incidence of Achilles tendon ruptures in Edmonton, Canada. Foot Ankle Int 2005;26:932-936. [DOI] [PubMed] [Google Scholar]
- 57. Leppilahti J, Orava S. Total Achilles tendon rupture. Sports Med 1998;25:79-100. [DOI] [PubMed] [Google Scholar]
- 58. Riley G. Tendinopathy—from basic science to treatment. Nat Clin Pract Rheumatol 2008;4:82-89. [DOI] [PubMed] [Google Scholar]
- 59. Åström M, Rausing A. Chronic Achilles tendinopathy: a survey of surgical and histopathologic findings mats. Clin Orthop Relat Res 1995;316:151-164. [PubMed] [Google Scholar]
- 60. Alfredson H, Lorentzon M, Bäckman S, Bäckman A, Lerner UH. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthopaed Res 2003;21:970-975. [DOI] [PubMed] [Google Scholar]
- 61. Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131-142. [DOI] [PubMed] [Google Scholar]
- 62. Kjær M, Magnusson P, Krogsgaard M, et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 2006;208:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kjær M, Langberg H, Heinemeier K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports 2009;19:500-510. [DOI] [PubMed] [Google Scholar]
- 64. Järvinen M, Jozsa L, Kannus P, et al. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports 1997;7:86-95. [DOI] [PubMed] [Google Scholar]
- 65. Smith R, Birch H, Goodman S, Heinegård D, Goodship A. The influence of ageing and exercise on tendon growth and degeneration—hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp Biochem and Physiol A Mol Integr Physiol 2002;133:1039-1050. [DOI] [PubMed] [Google Scholar]
- 66. Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 2007;8:703-713. [DOI] [PubMed] [Google Scholar]
- 67. Tuite DJ, Renström PAFH, O’Brien M. The aging tendon. Scand J Med Sci Sports 1997;7:72-77. [DOI] [PubMed] [Google Scholar]
- 68. Rando TA. Stem cells, ageing and the quest for immortality. Nature 2006;441:1080-1086. [DOI] [PubMed] [Google Scholar]
- 69. Sahin E, DePinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010;464:520-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baxter MA, Wynn RF, Jowitt SN, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004;22:675-682. [DOI] [PubMed] [Google Scholar]
- 71. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev 2008;129:163-173. [DOI] [PubMed] [Google Scholar]
- 72. Kasper G, Mao L, Geissler S, et al. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 2009;27:1288-1297. [DOI] [PubMed] [Google Scholar]
- 73. Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell 2010;9:911-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yu JM, Wu X, Gimble JM, et al. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell 2011;10:66-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hoffmann A, Gross G. Tendon and ligament engineering: from cell biology to in vivo application. Regen Med 2006;1:563-574. [DOI] [PubMed] [Google Scholar]
- 76. Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. J Bone Joint Surg [Am] 2010;92-A:2604-2613. [DOI] [PubMed] [Google Scholar]
- 77. Oakes DA, McAllister DR. Failure of heat shrinkage for treatment of a posterior cruciate ligament tear. Arthroscopy 2003;19:e1-e4. [DOI] [PubMed] [Google Scholar]
- 78. Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg [Am] 2005;87-A:187-202. [DOI] [PubMed] [Google Scholar]
- 79. Sharma P, Maffulli N. Basic biology of tendon injury and healing. Surgeon 2005;3:309-316. [DOI] [PubMed] [Google Scholar]
- 80. Martin DE, Severns AE, Kabo JM. Determination of mechanical stiffness of bone by pQCT measurements: correlation with non-destructive mechanical four-point bending test data. J Biomech 2004;37:1289-1293. [DOI] [PubMed] [Google Scholar]
- 81. Kajikawa Y, Morihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cellular Physiol 2008;215:837-845. [DOI] [PubMed] [Google Scholar]
- 82. Obaid H, Connell D. Cell therapy in tendon disorders what is the current evidence? American Journal of Sports Medicine 2010;38:2123-2132. [DOI] [PubMed] [Google Scholar]
- 83. Jones GC, Corps AN, Pennington CJ, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum 2006;54:832-842. [DOI] [PubMed] [Google Scholar]
- 84. Kong D, Xu L, Yu Y, et al. Regulation of Ca2+-induced permeability transition by Bcl-2 is antagonized by Drp1 and hFis1. Mol Cellular Biochem 2005;272:187-199. [DOI] [PubMed] [Google Scholar]
- 85. Nagase H, Brew K. Designing TIMP (tissue inhibitor of metalloproteinases) variants that are selective metalloproteinase inhibitors. Biochem Soc Symp 2003;70:201-212. [DOI] [PubMed] [Google Scholar]
- 86. Watabe T, Miyazono K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res 2009;19:103-115. [DOI] [PubMed] [Google Scholar]
- 87. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283-289. [DOI] [PubMed] [Google Scholar]
- 88. Dahlgren LA, van der Meulen MC, Bertram JE, Starrak GS, Nixon AJ. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res 2002;20:910-919. [DOI] [PubMed] [Google Scholar]
- 89. Wang XT, Liu PY, Tang JB. Tendon healing in vitro: genetic modification of tenocytes with exogenous PDGF gene and promotion of collagen gene expression. J Hand Surgery Am 2004;29:884-890. [DOI] [PubMed] [Google Scholar]
- 90. Moulin V, Tam BY, Castilloux G, et al. Fetal and adult human skin fibroblasts display intrinsic differences in contractile capacity. J Cell Physiol 2001;188:211-222. [DOI] [PubMed] [Google Scholar]
- 91. Hankemeier S, Keus M, Zeichen J, et al. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng 2005;11:41-49. [DOI] [PubMed] [Google Scholar]
- 92. Chan BP, Fu SC, Qin L, et al. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthopaedica Scandinavica 2000;71:513-518. [DOI] [PubMed] [Google Scholar]
- 93. Boyer MI, Watson JT, Lou J, et al. Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model. J Orthop Res 2001;19:869-872. [DOI] [PubMed] [Google Scholar]
- 94. Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest 1997;100:321-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors 2001;19:115-126. [DOI] [PubMed] [Google Scholar]
- 96. Virchenko O, Fahlgren A, Skoglund B, Aspenberg P. CDMP-2 injection improves early tendon healing in a rabbit model for surgical repair. Scand J Med Sci Sports 2005;15:260-264. [DOI] [PubMed] [Google Scholar]
- 97. Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. Journal of Bioscience and Bioengineering 2005;100:418-422. [DOI] [PubMed] [Google Scholar]
- 98. Haasters F, Polzer H, Prall WC, et al. Bupivacaine, ropivacaine, and morphine: comparison of toxicity on human hamstring-derived stem/progenitor cells. Knee Surgery, Sports Traumatology, Arthroscopy 2011;19:2138-2144. [DOI] [PubMed] [Google Scholar]
- 99.flexor tendon most at risk? Equine Vet J 2010;42:174-180. [DOI] [PubMed] [Google Scholar]
- 100. Lui PP, Wong OT, Lee YW. Transplantation of tendon-derived stem cells pre-treated with connective tissue growth factor and ascorbic acid in vitro promoted better tendon repair in a patellar tendon window injury rat model. Cytotherapy 2016;18:99-112. [DOI] [PubMed] [Google Scholar]
- 101. Hsieh CF, Alberton P, Loffredo-Verde E, et al. Periodontal ligament cells as alternative source for cell-based therapy of tendon injuries: in vivo study of full-size Achilles tendon defect in a rat model. Eur Cell Mater 2016;32:228-240. [DOI] [PubMed] [Google Scholar]
- 102. Hsieh CF, Alberton P, Loffredo-Verde E, et al. Scaffold-free Scleraxis-programmed tendon progenitors aid in significantly enhanced repair of full-size Achilles tendon rupture. Nanomedicine (Lond) 2016;11:1153-1167. [DOI] [PubMed] [Google Scholar]
- 103. Al-ani MK, Xu K, Sun Y, et al. Study of bone marrow mesenchymal and tendon-derived stem cells transplantation on the regenerating effect of achilles tendon ruptures in rats. Stem Cells Int 2015;2015:984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen X, Yin Z, Chen JL, et al. Scleraxis-overexpressed human embryonic stem cell–derived mesenchymal stem cells for tendon tissue engineering with knitted silk-collagen scaffold. Tissue Eng Part A 2014;20:1583-1592. [DOI] [PubMed] [Google Scholar]
- 105. Kraus T, Imhoff F, Wexel G, et al. Stem cells and basic fibroblast growth factor failed to improve tendon healing:an in vivo study using lentiviral gene transfer in a rat model. J Bone Joint Surg [Am] 2014;96:761-769. [DOI] [PubMed] [Google Scholar]
- 106. Schon LC, Gill N, Thorpe M, et al. Efficacy of a mesenchymal stem cell loaded surgical mesh for tendon repair in rats. J Transl Med 2014;12:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xu W, Wang Y, Liu E, et al. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng Part A 2013;19:2439-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ni M, Lui PPY, Rui YF, et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res 2012;30:613-619. [DOI] [PubMed] [Google Scholar]
- 109. Müller SA, Dürselen L, Heisterbach P, Evans C, Majewski M. Effect of a simple collagen type I sponge for achilles tendon repair in a rat model. Am J Sports Med 2016;44:1998-2004 [DOI] [PubMed] [Google Scholar]
- 110. Wichelhaus DA, Beyersdoerfer ST, Gierer P, Vollmar B, Mittlmeier T. The effect of a collagen-elastin matrix on adhesion formation after flexor tendon repair in a rabbit model. Arch Orthop Trauma Surg 2016;136:1021-1029. [DOI] [PubMed] [Google Scholar]
- 111. Street M, Thambyah A, Dray M, et al. Augmentation with an ovine forestomach matrix scaffold improves histological outcomes of rotator cuff repair in a rat model. J Orthop Surg Res 2015;10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kwon SY, Chung JW, Park HJ, et al. Silk and collagen scaffolds for tendon reconstruction. Proc Inst Mech Eng H 2014;228:388-396 [DOI] [PubMed] [Google Scholar]
- 113. Manning CN, Schwartz AG, Liu W, et al. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomaterialia 2013;9:6905-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gigante A, Busilacchi A, Lonzi B, et al. Purified collagen I oriented membrane for tendon repair: an ex vivo morphological study. J Orthop Res 2013;31:738-745. [DOI] [PubMed] [Google Scholar]
- 115. Enea D, Gwynne J, Kew S, et al. Collagen fibre implant for tendon and ligament biological augmentation. In vivo study in an ovine model. Knee Surg Sports Traumatol Arthroscopy 2013;21:1783-1793. [DOI] [PubMed] [Google Scholar]
- 116. Lyras DN, Kazakos K, Tilkeridis K, et al. Temporal and spatial expression of tgf-b1 in the early phase of patellar tendon healing after application of platelet rich plasma. Arch Bone Jt Surg 2016;4:156-160 [PMC free article] [PubMed] [Google Scholar]
- 117. Kraus T, Imhoff F, Reinert J, et al. Stem cells and bFGF in tendon healing: Effects of lentiviral gene transfer and long-term follow-up in a rat Achilles tendon defect model. BMC Musculoskelet Disord 2016;17:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gelberman RH, Shen H, Kormpakis I, et al. Effect of adipose-derived stromal cells and BMP12 on intrasynovial tendon repair: A biomechanical, biochemical, and proteomics study. J Orthop Res 2016;34:630-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kovacevic D, Gulotta LV, Ying L, et al. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin Orthop Relat Res 2015;473:1644-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ersen A, Demirhan M, Atalar A, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surgery 2014;134:405-411. [DOI] [PubMed] [Google Scholar]
- 121. Kollitz K, Parsons E, Weaver M, Huang JI. Platelet-rich plasma for zone II flexor tendon repair. Hand (NY) 2014;9:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dolkart O, Chechik O, Zarfati Y, et al. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg 2014;134:1271-1277. [DOI] [PubMed] [Google Scholar]