Abstract
Borderline personality disorder (BPD) is a serious condition involving emotion dysregulation. Past research has identified BPD-associated differences within fronto-limbic circuitry during conditions of processing negative emotion. Functional magnetic resonance imaging (fMRI) paradigms that incorporate overt and covert (masked) presentations of emotional stimuli can provide complementary information about neural systems underlying emotion processing (e.g., both slow [overt] and fast [covert; automatic] processing pathways). This study examined brain activation during processing of overt and covert presentations of emotional faces in 12 women with BPD and 12 age-matched healthy controls. To assess a range of emotional valence and arousal, we examined responses to fear, happy and neutral expressions. All participants underwent an fMRI scanning session in which participants passively viewed emotional faces. Scanning sessions consisted of 5 runs including: (1) Overt Fear (OF) versus Neutral (N), (2) Covert Fear (CF) versus Covert Neutral (CN), (3) Overt Happy (OH) versus N, (4) Covert Happy (CH) versus CN, and (5) N versus fixation. We compared whole-brain activation between groups for each run. In response to overt fear, BPD patients showed greater activation both in left amygdala and in several frontal cortical regions. There were no significant differences in brain activation in response to overt happy faces. In response to covert fear and covert happy stimuli, the BPD group also showed greater activation than controls in several regions including frontal and temporal cortical regions, as well as cerebellum and thalamus. These findings add to prior reports suggesting increased amygdala activation in BPD, but we found this only in the overt fear versus fixation condition. In this sample, BPD patients showed hyper-activation, rather than hypo-activation, of cortical regulatory regions during overt fear. Enhanced cortical recruitment in response to covert fear and happy faces in BPD could reflect a more extended response system in which stimuli that typically only activate automatic pathways are additionally tapping into cortical regulatory systems. The observation of this pattern both in response to fear and in response to happy presentations suggests that the effect of arousal may be as or more impactful than the effect of emotional valence.
Keywords: Borderline personality disorder, Amygdala, Emotion, fMRI, Overt, Covert, Backward masking
Introduction
The diagnosis of borderline personality disorder (BPD) describes a complex and heterogeneous set of behaviors which results in significant impairment (Skodol et al. 2002). Functional impairment related to BPD has been described in the context of self, relationships, work and leisure (Levy 2005; Skodol et al. 2002). Additionally, those with BPD engage in dysregulated behaviors, including non-suicidal self-injury, parasuicidal behavior and suicide attempts (Brown et al. 2002), with a suicide completion rate of up to 10 % (Black et al. 2004). Although the prognosis of BPD is considered better than was once thought (Zanarini et al. 2012), the morbidity, high rates of successful suicide, and long-term disturbance in psychosocial functioning dictate the need to delineate the complex interplay of biology and environment underlying the disorder. Specifically, identifying the neurobiological antecedents and correlates of emotion dysregulation is critical to developing enhanced psychotherapeutic and pharmacologic interventions.
Literature describing BPD has focused extensively on the role of emotion dysregulation in symptom development and subsequent impairment (Crowell et al. 2009; Gratz et al. 2009; Linehan 1993). Linehan’s Biosocial Theory (1993) posited that emotion dysregulation results from biological sensitivity in the context of an invalidating environment. In the last two decades, neuroscientific research has contributed to the development of these theories, and emotion regulation is now understood as the complex interaction between various brain networks, with fronto-limbic circuitry taking center stage (Goldsmith et al. 2008; Ochsner et al. 2012; Vuilleumier and Pourtois 2007). Significant differences in functioning of fronto-limbic circuitry in patients with BPD compared to healthy controls have been identified (Johnson et al. 2003; O’Neill et al. 2013; Putnam and Silk 2005). One of the most studied structures in the limbic system is the amygdala, which has been found to be important in emotional learning and is activated in the context of salient perceptual stimuli. Some (but not all) (Ruocco et al. 2012) prior studies have found greater amygdala activation to negative stimuli in patients with BPD compared to control subjects (Donegan et al. 2003; Hazlett et al. 2012; Herpertz et al. 2001; Koenigsberg et al. 2014, 2009; Minzenberg et al. 2007; Mitchell et al. 2014; Schulze et al. 2011). Increased amygdala activation in BPD has also been found during anger induction (Jacob et al. 2013) and during recall of unresolved life events (Beblo et al. 2006). Furthermore, patients with BPD show a failure of the amygdala to attenuate its response to repeated negative stimuli (Hazlett et al. 2012; Kamphausen et al. 2013; Koenigsberg et al. 2014). Relatively less research has been conducted examining the processing of positive emotion using stimuli such as pleasant scenes or happy faces. In one study, amygdala activation in BPD patients differed from controls in response to fear, sad and neutral faces, but there was no amygdala activation difference in response happy faces (Donegan et al. 2003). A more recent study found that patients with BPD showed reduced amygdala activation compared to controls to novel pleasant pictures, but that with repetition, BPD patients showed greater amygdala activation to pleasant pictures than controls (Hazlett et al. 2012).
Emotion regulation circuitry involves multiple pathways (Ledoux 2000). Whereas conscious processing of overt emotion involves interaction between amygdala and cortical areas, automatic processing of unconsciously perceived stimuli involves interaction between amygdala and other subcortical areas such as the thalamus (Ledoux 2000). A common task used for eliciting amygdala activation involves negatively-valenced emotional facial expressions (fearful, angry, disgust, and sad) (Atkinson and Adolphs 2011; Fusar-Poli et al. 2009). By using masking techniques with emotional face stimuli (also referred to as covert presentation of stimuli), researchers can assess the circuitry that mediates unconscious emotion processing (Whalen et al. 1998). Research in healthy adults has shown greater amygdala activation when viewing subliminally compared to consciously viewed fearful face stimuli (Kim et al. 2010; Whalen et al. 1998). We have previously reported that functional connectivity between amygdala and rostral anterior cingulate during overt fear and between amygdala and thalamus during covert fear was greater in BPD patients than healthy comparison subjects (Cullen et al. 2011). However, the whole-brain regional activation findings from that investigation have not yet been reported, and are the focus of the present study.
The goal of the present study was to investigate brain activation during processing of overt and covert emotional stimuli in female patients with BPD compared to age-matched controls. To reduce variability and minimize confounds, we studied only women and set limits on co-morbid diagnoses and medication status for the BPD group. Our primary hypotheses addressed fear processing, with exploratory analyses conducted for happy face processing. Our primary hypothesis was that, similar to previous work, amygdala activation in response to fearful faces would be greater in BPD patients than controls. To gain further understanding of which amygdala networks are activated in response to fear, our fMRI protocol included both overt and covert presentations of emotional face stimuli. We hypothesized that in response to covert fear faces, women with BPD would show greater amygdala activation than controls, and that in response to overt fear, they would show both greater amygdala activation and less frontal activation than controls. We also explored whether BPD patients would show alterations in response to overt and covert happy faces. Finally, we explored whether brain activation in response to emotional faces would be associated with any specific measures of psychopathology in the BPD group.
Method
Participants
The study was approved by the University of Minnesota (UMN) Institutional Review Board. The fMRI data collected from the same study sample has previously been reported (Cullen et al. 2011); in that publication, we focused on brain connectivity, whereas in this paper we focus on regional brain activation. Participants were recruited using community postings and referrals from the UMN student health center and other mental health services. Participants provided informed consent prior to participation. The BPD and healthy comparison groups each included 12 women, rigorously screened and matched one-to-one for age, ethnicity, and handedness. Healthy controls meeting two or more of the nine DSM-IV criteria for BPD were ineligible for this study. A study goal was to reduce confounds associated with diagnostic comorbidity or medication effects. Thus, for the BPD group, exclusion criteria included a history of any psychotic disorder, bipolar disorder, current major depressive disorder (MDD) (meeting criteria within the past 2 months), any history of MDD with psychotic features, obsessive-compulsive disorder, generalized anxiety disorder (GAD), social phobia, and posttraumatic stress disorder (PTSD). Based on previous research reporting elevated amygdala responses in patients with PTSD (Rauch et al. 2000; Shin et al. 2005; St Jacques et al. 2011), GAD (McClure et al. 2007), and social phobia (Birbaumer et al. 1998; Yoon et al. 2007), participants were excluded if they met current criteria for these disorders. It was determined by the study team that due to high rates of lifetime MDD (Gunderson et al. 2008) and PTSD (Shea et al. 2004) among individuals with BPD, exclusion of all subjects with a history of MDD, PTSD, and substance use disorders would result in a sample that was not representative of BPD. Similarly, substance use disorders are very common in BPD (Zanarini et al. 2011). If participants met criteria for substance abuse or dependence, it was required that they be in at least partial remission. Participants were instructed to refrain from abusing substances before the scanning session (1 week of abstinence for illicit substances and 24 h for alcohol). Compliance was assessed at the scanning session by self-report. Finally, we excluded all subjects that were taking medications that were prescribed for a psychiatric diagnosis (however, psychoactive medications were allowed if they were prescribed for other medical purposes; n =1.)
Assessment
Participants were diagnostically assessed using two forms of the Structured Clinical Interview for DSM-IV (SCID). The SCID-I/P, Research Version, Patient Edition (First et al. 2002) was used to screen for major Axis I psychiatric disorders, and the SCID-II (First et al. 1997) was used to confirm the diagnosis of BPD. Structured diagnostic interviews were conducted by a trained graduate student and/or a registered nurse supervised by a psychiatrist. On the day of the assessment, participants completed self-report scales including the Symptom Checklist-90 (SCL-90) (Derogatis 1994), to assess psychiatric symptoms, and the Multidimensional Personality Questionnaire-Brief Form (MPQ) (Patrick et al. 2002), which includes three higher factors of Negative Emotionality, Positive Emotionality and Constraint. Following the fMRI scan, participants completed the State-Trait Anxiety Inventory (STAI) (Spielberger et al. 1983) to examine temporary and dispositional anxiety. These scales were administered to provide continuous measures of individual differences for later comparison with MRI data.
MRI data acquisition
Structural and functional MRI data were acquired on a Siemens 3-Tesla Trio scanner using a single-channel quadrature head coil. High-resolution, T1-weighted, 3-D images were acquired for each participant. Two participants were imaged with an SPGR sequence (TE=6.83 ms, TR=25 ms, field of view=256 mm, matrix: 256×256, slice thickness=1.5 mm, with a 20 % gap, flip angle=25°, 144 coronal slices). All other participants were imaged with a FLASH sequence (TE= 4.7 ms, TR=20 ms, field of view=256 mm, matrix=256×256, slice thickness=1 mm, with a 20 % gap, flip angle=22°, 176 sagittal slices). For each of the fMRI runs, 156 AC- PC aligned functional images were obtained, using an echoplanar imaging (EPI) sequence (TE=28 ms, TR=2000 ms, field of view=200 mm, matrix: 64×64, slice thickness=3.1 mm, no gap, flip angle=90°, 34 interleaved oblique axial slices).
fMRI paradigm
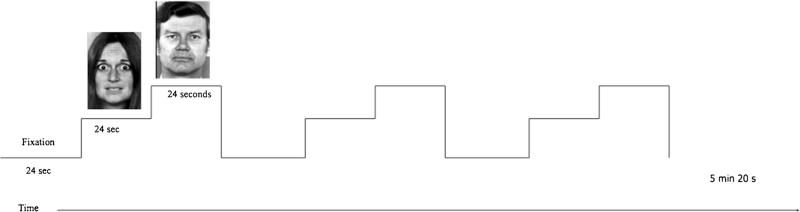
The fMRI task included five runs of a face viewing paradigm used extensively in previous fMRI research (Breiter et al. 1996; Thomas et al. 2001; Whalen et al. 1998). Black and white face photographs (Ekman and Friesen 1976) of 8 individuals (4 women, 4 men) each depicting fearful, neutral, and happy expressions were presented in a block design. Each run contained 13 24-second blocks, which were comprised of a series of facial expressions and a fixation cross (8 face blocks and 5 fixation blocks). The five runs included: overt fear faces and overt neutral faces; covert fear and covert neutral faces; overt happy and overt neutral faces; covert happy and covert neutral faces; and all neutral faces. The order of blocks within each run is shown in Fig. 1. The ordering of runs was as follows: the all neutral run always came first, followed by the two covert runs, and the overt runs came last. Within covert (runs 2 & 3) and overt (runs 4 & 5) conditions, the order of fear versus happy was randomized. We acquired the all neutral run first to assess brain response to neutral faces without potential habituation effects from repeated exposure to these same neutral faces in the other 4 runs. The covert conditions were tested prior to the overt conditions to ensure that the covert emotional stimuli would be less likely to prime emotion processing in the overt runs than vice versa. Finally, we randomized the order of the happy versus fear runs within both covert and overt conditions to minimize the effects of valence order. Each block contained 16 trials. Each trial consisted of a 200 ms presentation period followed by a 1300 ms fixation period. For the overt emotion runs, fear, happy or neutral faces were presented for the entire 200 ms presentation period. For the covert emotion runs, fear, happy or neutral faces were presented for only 26 ms and were immediately followed by a mask of a neutral face for 174 ms. The neutral face presented in the covert condition belonged to a different person than the neutral face it was masking. In all cases, the presentation period was followed by a fixation period. The fixation period stimulus consisted of a gray screen containing a black “+.” To ensure attention to the stimuli, participants were asked to monitor the fixation period for the occurrence of a rare target stimulus (o rather than +), at which time they were to press a button. Each 24-second block contained two pseudo-randomly distributed occurrences of the target “o” fixation stimulus. Stimulus presentation and response collection was acquired using the IFIS-SA system (MRI Devices, Waukesha, WI).
Fig. 1.
The general design of each fMRI run is shown. Each run consisted of a repeating pattern beginning with a 24 s block of fixation, followed by 24 s of the key emotion (e.g., fear, happy), followed by a 24 s presentation of neutral faces
Post-scan ratings of facial expressions
After completing the fMRI task, participants were shown the same faces they had viewed during the scan. Subjects were asked to rate how they thought the person was feeling on a scale of 1 (Very Bad) to 7 (Very Good).
fMRI preprocessing procedures
The FSL software package (v. 4.1.9; Oxford, England; http://www.fmrib.ox.ac.uk/fsl/) was used to conduct preprocessing and analysis steps. Anatomical data were skull-stripped to remove non-brain tissue. Functional data preprocessing included motion correction, spatial smoothing (Gaussian FWHM 6 mm), grand-mean intensity normalization, and high-pass filtering (75 s). Motion correction was based on the first volume in each data set. Volumes were assessed for exclusion based on the following parameters: 1) motion exceeding one voxel in absolute space, 2) motion exceeding one-half voxel from one volume to the next. None of the volumes met the exclusion parameters. The first four volumes at the start of each run were discarded. Functional data were co-registered to their respective anatomical data, and each participant’s anatomical data were co-registered to the Montreal Neurological Institute’s T1 2 mm average brain image.
FMRI activation analysis
First-level, whole-brain statistical analyses were conducted separately for each run for each participant using a general linear model (GLM). The GLM for these analyses included predictors for both emotion and neutral blocks, with fixation blocks used as baseline. These predictors of interest were convolved with the default gamma hemodynamic response function provided by FSL. Six additional motion predictors were also included as confounds. Results of these analyses were then entered into higher-level, group analyses. Group-level comparisons were performed using a whole-brain, random effects analysis for nine contrasts: overt fear minus fixation; overt fear minus neutral; covert fear minus fixation; covert fear minus neutral; overt happy minus fixation; overt happy minus neutral; covert happy minus fixation; covert happy minus neutral; neutral minus fixation. Areas of significant activation were identified using a threshold of z=2.74 (or p<0.0031) per voxel and a minimum contiguous volume equivalent to 10 functional voxels (or 38 voxels in standard 2×2×2 space, 304 mm3). This threshold was chosen to optimize the balance between type I and type II errors (Lieberman and Cunningham 2009), especially considering the high risk of type II error associated with missing a group difference in the amygdala, a small brain region.
Finally, we conducted Pearson correlation analyses between clinical variables and brain activation values within any clusters showing a significant group difference. To limit the number of comparisons, we used summary measures when available. Therefore, we tested correlations with six clinical measures: SCL-90 global symptom index (GSI), STAI state and trait anxiety (STAI-state and STAI-trait), and the MPQ-BF higher-order constructs of Positive Emotionality, Negative Emotionality, and Constraint (Patrick et al. 2002). Correlations were conducted in the BPD group only, using a Bonferroni correction to set the level of significance.
Results
Subjects
Twelve women with BPD and 12 age-matched healthy women completed all procedures. Demographic and clinical characteristics of these groups are listed in Table 1. Although current medications taken for a psychiatric indication were exclusionary, one BPD participant was taking a low dose of gabapentin for a medical condition (neuropathic pain). Symptom levels for the BPD and control groups are shown in Table 2. BPD patients scored higher than controls on all clinical measures, with significant differences in most cases. Overall, the BPD group demonstrated moderate levels of symptomatology. No significant differences were found in the group comparisons of ratings of facial expressions.
Table 1.
Demographic and clinical characteristics of participants with borderline personality disorder (BPD) and healthy controls
| Characteristic | BPD n=12 |
Healthy n = 12 |
|---|---|---|
| Age (mean years ±SD) | 25.17 (4.67) | 24.17 (4.63) |
| Ethnicity – n (%) | ||
| White | 10 (84) | 11 (92) |
| African American | 1 (8) | 1 (8) |
| Hispanic | 1 (8) | 0 |
| Asian | 0 | 0 |
| Other | 0 | 0 |
| Abuse History – n (%) | ||
| Physical abuse | 2 (17) | 0 |
| Sexual abuse | 6 (50) | 0 |
| Emotional abuse | 5 (42) | 2 (17) |
| Past Comorbidity – n (%) | ||
| Post Traumatic Stress Disorder | 4 (33) | 0 |
| Major Depressive Disorder | 10 (83) | 0 |
| Attention Deficit Hyperactivity Disorder | 0 | 1 (8) |
| Substance Use Disorder, early partial remission | 6 (50) | 2 (17) |
| Substance Use Disorder, sustained full remission | 5 (42) | 1 (8) |
| Current medication treatment – n (%) | ||
| Gabapentina | 1 (8) | 0 |
Participants taking medications prescribed for medical indications were not excluded. One BPD participant was taking gabapentin for neuropathic pain
Table 2.
Symptomatology of study participants
| BPD (n=12) Mean (SD) |
Control (n=12) Mean (SD) |
Comparison BPD vs. Control t, p values |
|
|---|---|---|---|
| State and trait anxiety inventorya | |||
| State anxiety | 45.50 (11.94) | 28.00 (6.12) | −4.52, <0.001 |
| Trait anxiety | 56.42 (8.65) | 31.08 (6.19) | −8.25, <0.001 |
| Symptom checklist-90b | |||
| Global severity index | 53.67 (7.88) | 46.33 (7.38) | −2.35, 0.03 |
| Multidimensional personality questionnaire-brief form (MPQ-BF)c | |||
| Positive emotionality | 41.92 (6.34) | 48.25 (4.57) | 1.5,0.01 |
| Negative emotionality | 64.00 (9.33) | 34.92 (7.66) | −8.3, <0.001 |
| Constraint | 36.75 (5.77) | 40.83 (6.24) | −1.7, 0.110 |
BPD borderline personality disorder, SD standard deviation,
fMRI activation
Between-group results for brain activation in all 9 contrasts tested are summarized in Table 3 and displayed in Figs. 2, 3, 4, 5 and 6. Overall, findings indicated greater brain activation in the BPD group compared with the control group.
Table 3.
Brain activation during all runs and contrasts
| Contrast | Group difference | Mean (S.D.) % signal change |
# voxels | Z-max | MNI coordinates of maximum voxel in cluster |
Figure#* | Brain regions | BA |
|---|---|---|---|---|---|---|---|---|
| Overt Fear > Fixation | BPD > Controls | BPD: 0.16 (0.25) | 44 | 3.2 | 58, −58, 24 | 2a | Right superior temporal gyrus | 39 |
| Controls: −0.25 (0.33) | ||||||||
| BPD: 0.33 (0.23) | 68 | 3.5 | 10, 54, 18 | 2b | Right medial frontal gyrus | 9 | ||
| Controls: −0.13 (0.21) | ||||||||
| BPD: 0.41 (0.22) | 60 | 3.17 | −52, −66, 10 | 2c | Left middle temporal gyrus | 39 | ||
| Controls: −0.05 (0.26) | ||||||||
| BPD: 0.27 (0.33) | 44 | 3.07 | 60, −24, −10 | 2d | Right middle temporal gyrus | 21 | ||
| Controls: −0.21 (0.26) | ||||||||
| BPD: 0.35 (0.18) | 59 | 3.27 | 40, 24, −14 | 2e | Right inferior frontal gyrus | 47 | ||
| Controls: 0.01 (0.18) | ||||||||
| BPD: 0.43 (0.45) | 53 | 3.06 | 42, 46, −14 | 2e | Right inferior frontal gyrus/middle frontal gyrus | 47/11 | ||
| Controls: −0.31 (0.42) | ||||||||
| BPD: 0.29 (0.20) | 42 | 3.32 | −28, −6, −24 | 2f | Left amygdala, hippocampus | |||
| Controls: −0.01 (0.13) | ||||||||
| Controls > BPD | No significant clusters | |||||||
| Overt Fear > Neutral | BPD > Controls | No significant clusters | ||||||
| Controls > BPD | No significant clusters | |||||||
| Covert Fear > Fixation | BPD > Controls | BPD: 0.53 (0.60) | 44 | 3.41 | 50, −72, 40 | 3a | Right inferior parietal lobule/angular gyrus | 40/39 |
| Controls: −0.65 (0.83) | ||||||||
| BPD: 0.13 (0.23) | 50 | 3.51 | −54, −10, 6 | 3b | Left superior temporal gyrus | 22 | ||
| Controls: −0.25 (0.27) | ||||||||
| Controls > BPD | No significant clusters | |||||||
| Covert Fear > Neutral | BPD > Controls | BPD: 0.56 (0.78) | 99 | 3.13 | −4, −76, 52 | 4a | Left precuneus | 7 |
| Controls: −0.61 (0.87) | ||||||||
| BPD: 0.06 (0.27) | 49 | 3.26 | −62, −32, 38 | 4b | Left inferior parietal lobule | 40 | ||
| Controls: −0.27 (0.18) | ||||||||
| BPD: 0.13 (0.18) | 112 | 3.25 | −40, 24, 24 | 4c | Left middle frontal cortex | 9 | ||
| Controls: −0.21, (0.21) | ||||||||
| BPD: 0.03 (0.23) | 55 | 3.08 | −44, 44, 4 | 4d | Left inferior frontal gyrus | 45/46 | ||
| Controls: −0.16 (0.25) | ||||||||
| BPD: 0.55 (0.58) | 39 | 3.46 | −14, 38, −26 | 4e | Left orbital gyrus | 47 | ||
| Controls: −0.39 (0.64) | ||||||||
| Controls >BPD | No significant clusters | |||||||
| Overt Happy > Fixation | BPD > Controls | No significant clusters | ||||||
| Controls >BPD | No significant clusters | |||||||
| Overt Happy > Neutral | BPD > Controls | No significant clusters | ||||||
| Controls BPD> | No significant clusters | |||||||
| Covert Happy > Fixation | BPD >Controls | BPD: 0.28 (0.26) | 45 | 3.16 | −44, −50, 28 | 5a | Left supramarginal gyrus | 40 |
| Controls: −0.09 (0.13) | ||||||||
| Controls>BPD | No significant clusters | |||||||
| Covert Happy > Neutral | BPD > Controls | BPD: 0.20 (0.28) | 48 | 3.25 | −4, 44, 28 | 6a | Left medial frontal gyrus | 9 |
| Controls: −0.21 (0.27) | ||||||||
| BPD: 0.23 (0.17) | 129 | 3.67 | −50, −54, 28 | 6a | Left superior temporal gyrus/ supramarginal gyrus | 39/40 | ||
| Controls: −0.18 (0.19) | ||||||||
| BPD: 0.17 (0.12) | 208 | 3.48 | −18, −8, 12 | 6b | Left thalamus | |||
| Controls: −0.13 (0.20) | ||||||||
| BPD: 0.30 (0.31) | 120 | 3.23 | 62, −50, 12 | 6b | Right superior temporal gyrus | 22 | ||
| Controls: −0.14 (0.30) | ||||||||
| BPD: 0.25 (0.21) | 277 | 3.56 | −60, −20, −8 | 6c | Left middle temporal gyrus | 21 | ||
| Controls: −0.16 (0.13) | ||||||||
| BPD: 0.28 (0.28) | 87 | 3.22 | −58, 20, −8 | 6c | Left lateral cerebral sulcus extending to inferior frontal gyrus | 47 | ||
| Controls: −0.25 (0.43) | ||||||||
| BPD: 0.13 (0.19) | 39 | 3.1 | −36, 44, −16 | 6d | Left middle frontal gyrus | 11 | ||
| Controls: −0.19 (0.22) | ||||||||
| BPD: 0.19 (0.18) | 86 | 3.39 | 28, −76, −26 | 6e | Right cerebellum | |||
| Controls: −0.23 (0.58) | ||||||||
| BPD: 0.39 (0.23) | 148 | 3.3 | −24, −84, −28 | 6f | Left cerebellum | |||
| Controls: −0.25 (0.43) | ||||||||
| Controls > BPD | No significant clusters | |||||||
| Neutral > Fixation | BPD > Controls | No significant clusters | ||||||
| Controls > BPD | No significant clusters |
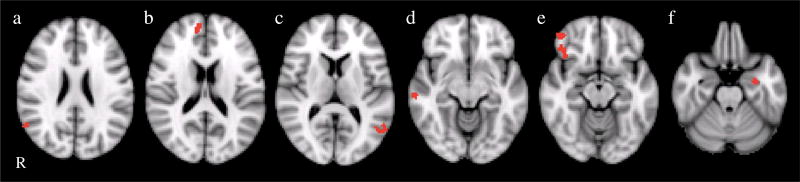
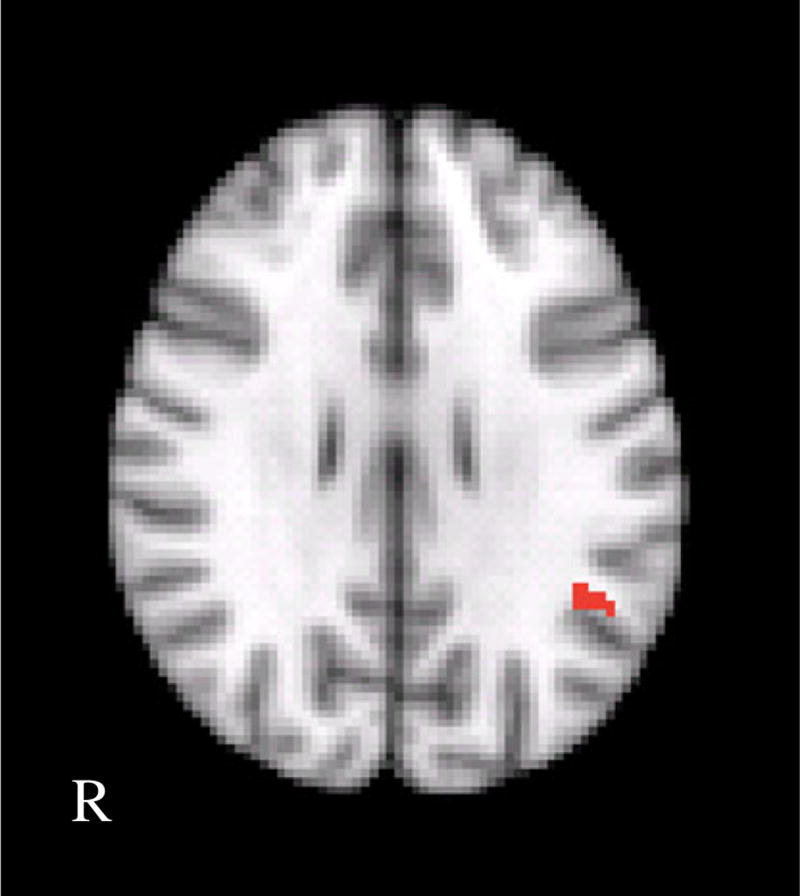
Fig. 2.
Clusters are shown representing the group difference (BPD>controls) for the overt fear>fixation contrast. Cluster locations included the left amygdala/hippocampus, right inferior frontal gyrus (BA 47/11), right medial frontal gyrus (BA9), left middle temporal gyrus, and right superior and middle temporal gyrus. R right
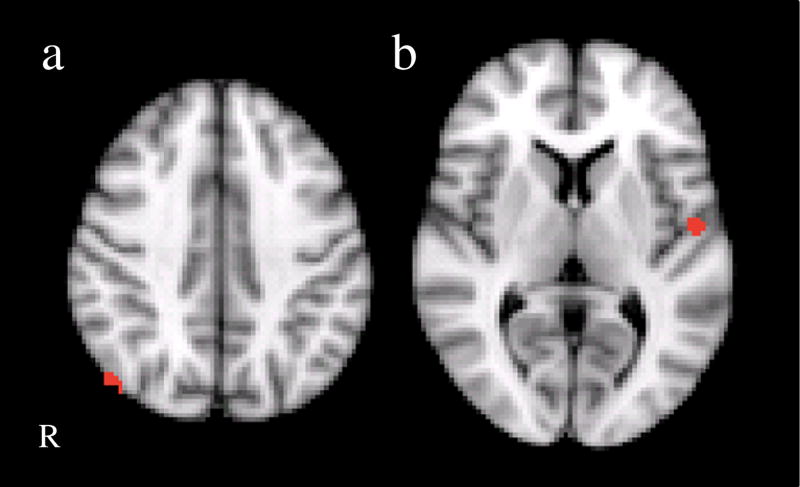
Fig. 3.
Clusters are shown representing the group difference (BPD>controls) for the covert fear>fixation contrast. Cluster locations included left superior temporal gyrus and the right inferior parietal lobule/angular gyrus. R right
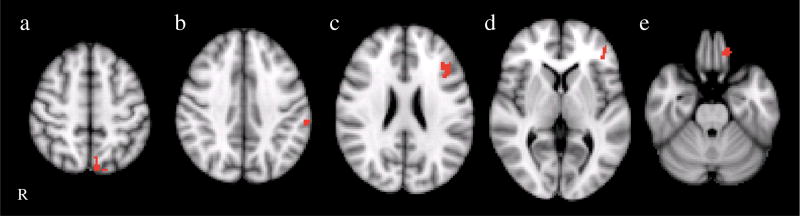
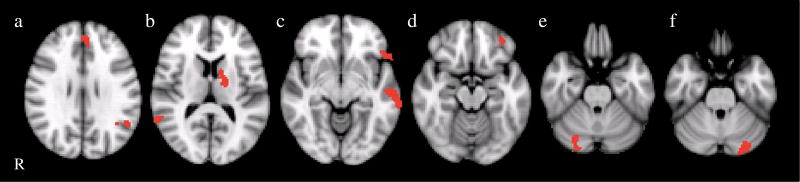
Fig. 4.
Clusters are shown representing the group difference (BPD>controls) for the covert fear>neutral contrast. Cluster locations included left regions of the inferior frontal gyrus (BA 45/46/47), precuneus, and inferior parietal lobule. R right
Fig. 5.
Clusters are shown representing the group difference (BPD>controls) for the covert happy>fixation contrast in the left supramarginal gyrus (BA40). R right
Fig. 6.
Clusters are shown representing the group difference (BPD>controls) for the covert happy>neutral contrast. BPD patients showed increased activation in several left-sided frontal, subcortical and temporal regions, including the medial (BA9) and middle (BA11) frontal gyrus, thalamus, middle temporal gyrus (BA21) as well as bilateral regions of the superior temporal gyrus/supramarginal gyrus (BA39/40, BA22) and cerebellum. R right
Effects for overt fear faces
Consistent with our prediction that amygdala activation would be greater in response to fearful faces in BPD patients compared to controls, we found that BPD patients showed greater activation in the left amygdala (extending into the parahippocampal gyrus and hippocampus) specifically in the overt fear minus fixation contrast (Fig. 2). There were no other contrasts where group differences were found in amygdala activation. In addition to the left amygdala/hippocampus, there were also frontal and temporal areas in the overt fear minus fixation contrast where the BPD group showed greater activation, including the right inferior frontal gyrus (BA 47/ 11), medial frontal gyrus (BA9), left middle temporal gyrus, and right superior and middle temporal gyrus. There were no significant differences found for the overt fear minus neutral contrast.
Effects for covert fear faces
In the covert fear minus fixation contrast, the BPD group showed greater activation than controls in the right inferior parietal lobule/angular gyrus and left superior temporal gyrus (see Fig. 3). To covert fear faces minus neutral faces, BPD patients also demonstrated greater activation in left regions of the inferior frontal gyrus (BA 45/46/47), middle frontal cortex (BA9), precuneus, and inferior parietal lobule in response (see Fig. 4).
Effects for happy and neutral faces
There were no group differences for overt happy minus fixation nor overt happy minus neutral. However, BPD patients showed greater activation in the left supramarginal gyrus (BA40) relative to controls for covert happy minus fixation (see Fig. 5). BPD patients also showed increased activation in response to covert happy minus neutral faces in several leftsided frontal, subcortical and temporal regions, including the medial (BA9) and middle (BA11) frontal gyrus, thalamus, middle temporal gyrus (BA21) as well as bilateral regions of the superior temporal gyrus/supramarginal gyrus (BA39/40, BA22) (see Fig. 6).
There were no significant differences found in the neutral minus fixation contrast.
Individual difference measures
For the correlational analyses conducted within the BPD group, there were 6 clinical tests and 24 significant clusters, with the significance threshold therefore set at p<0.0003. None of the correlations met this threshold.
Discussion
The current study sought to extend previous work on emotion circuitry in BPD, using both overt and covert stimulus techniques to assess both automatic and conscious processing of emotion, and examining activation to fear, happy and neutral faces. Strengths of the current study include the fact that the sample was largely free from medications and comorbidities, and the approach of employing a backward masking paradigm to examine both overt and covert fear processing. Key findings of this study include that in response to overt fear, BPD patients showed greater activation both in left amygdala and in several frontal and temporal cortical regions. In response to covert fear and covert happy stimuli, the BPD group also showed greater activation than controls in several regions including frontal, temporal and occipital cortical regions, as well as cerebellum and thalamus.
Emotion networks have been extensively studied in primates, and these studies have centrally implicated the amygdala. Several amygdala networks have been characterized: the one directly involved in mood involves connections to cortical (ventromedial frontal, rostral insular, and rostral temporal) and subcortical (medial thalamus and ventromedial basal ganglia) areas; a system implicated in modulation of visceral function in relation to emotional stimuli involves connections to the hypothalamus and brain stem; a third system primarily involves sensory regions (olfactory cortex, taste/visceral pathways, sensory association cortex and posterior thalamus) sending information to the amygdala (Price 2003). Within the first system relevant to emotion and mood, LeDoux has proposed that slower, interpretive processing of negative emotion involves amygdala interactions with cortical regions such as the frontal lobe, whereas automatic processing of emotion bypasses the cortex and primarily involves the thalamus (Ledoux 2000). More recently, research has examined hemispheric differences in amygdala emotion processing, and shown that left amygdala is involved to a greater extent in cognitive processing of emotion, whereas the right amygdala is involved to a greater extent in automatic emotion processing (Dyck et al. 2011). This may have relevance to the findings of our study where patients with BPD showed greater amygdala activation than controls in the left amygdala only during processing of overt as opposed to covert fear. Further, recent work has supported a hypothesis that emotion and motor systems are tightly linked during the presence of threatening signals (Grèzes and Dezecache 2014), with evidence for a direct link between amygdala and motor areas (Grèzes et al. 2014). Finally, perception of fear in static and dynamic body expressions has been found to activate not only the amygdala but also widespread cortical regions including frontal, temporal and parietal regions which may represent core components of the emotional response system (Grèzes et al. 2007).
Replicating the findings of several (Donegan et al. 2003; Hazlett et al. 2012; Koenigsberg et al. 2014; 2009; Minzenberg et al. 2007; Mitchell et al. 2014; Niedtfeld et al. 2010; Schulze et al. 2011) but not all (Ruocco et al. 2012) previous studies, and consistent with our hypothesis, in the current study we report greater left amygdala activation in response to overt fear in patients with BPD in comparison to controls. However, we did not find excessive amygdala activation in response to covert fear in the BPD group. Further, there were several cortical regions that were more active in the BPD group than controls during overt and covert fear. The elevated frontal activation displayed by women with BPD during these emotion conditions could reflect increased recruitment to control emotional responses that is unsuccessful in the overt fear condition. A similar finding was reported by Beblo and colleagues (2006), who examined brain activation during recall of unresolved memories in patients with BPD and healthy controls, and found that patients not only showed greater amygdala but also prefrontal activation. They wrote, “The activation of both, the amygdala and prefrontal areas, might reflect an increased effortful but insufficient attempt to control intensive emotions during the recall of unresolved life events in patients with BPD” (Beblo et al. 2006).
Another possible interpretation of our findings that BPD-associated amygdala hyperactivation along with cortical hyperactivation, is that cortical activation during conscious processing of fear may be contributing to increased amygdala reactivity in subjects with BPD. Put another way, the frontal and other cortical regions may be inducing a positive feedback loop of brain activation. Research in healthy adults supports a feed-forward system in which cortical activity could in certain circumstances drive limbic activity. In a study correlating brain activation with trait rumination, participants with a greater tendency to ruminate showed greater activation in left amygdala and left ventrolateral prefrontal cortex when using reappraisal to increase negative responses to neutral images and when passively viewing negative images (Ray et al. 2005). In the emotional cascade model proposed by Selby et al. (2009), dysregulated emotions and behaviors in patients suffering from BPD are a result of intense cycles of extremely painful rumination (Selby et al. 2009, 2013). Taken together, these findings provide preliminary evidence for the hypothesis that maladaptive recruitment of cortical regulatory regions may be contributing to excessive amygdala and emotional responses in BPD.
A recent meta-analysis of 11 fMRI studies of patients with BPD showed that in response to negative versus neutral emotional stimuli, BPD patients showed greater activation in the posterior cingulate and insula but lower activation in the amygdala, subgenual anterior cingulate and dorsolateral pre- frontal cortex (Ruocco et al. 2012). Interestingly, our study did not show any significant differences for the overt fear minus neutral contrast, only for the overt fear minus fixation contrast. Therefore, our findings of increased amygdala activation cannot be said to be specific to overt fear face processing over and above general overt face processing. Prior work has suggested that BPD patients show enhanced amygdala responses even to neutral faces (Donegan et al. 2003). However, the current study did not support this: we found no group differences in the neutral versus fixation contrast for the all-neutral scan. However, in the covert fear minus neutral contrast, we did find that the BPD group relative to controls had greater activation in the precuneus/posterior cingulate area, which is consistent with Ruocco, et al. 2012 study. Regarding the inconsistencies across studies in the direction of amygdala activation (underactivated or overactivated in BPD), it should be noted that Ruocco and colleagues’ meta-analysis included studies that used non-facial stimuli; it may be that excessive amygdala activation is specific to fear faces as opposed to other negative emotion stimuli.
Our findings regarding responses to happy faces add to a small number of studies that have examined responses to positive stimuli. Similar to Donegan and colleagues (2003), who despite finding that adults with BPD had greater amygdala activation to fear, sad and neutral faces, found no group differences in response to happy faces (Donegan et al. 2003), we did not find any group differences in our group comparisons of overt happy versus fixation or overt happy versus neutral contrasts. Our only findings were in the contrasts involving covert happy faces in relation to either fixation or neutral faces, which indicated that patients had greater activation in the left angular gyrus (both contrasts), and in the cerebellum, thalamus, and various frontal and temporal cortical regions. These findings may suggest that covert presentations of highly arousing stimuli such as fearful and happy faces lead to widespread activation in patients with BPD of not only automatic emotion processing systems (e.g., thalamus) but also of cortical systems, to a substantially greater extent than controls. This may reflect an overactive system where pathways normally reserved for conscious processes are activated even with these covert stimuli. Hazlett and colleagues (2012) previously showed that BPD patients showed hypoactivation of the amygdala compared to controls to positive pictures when they were novel; however, over time the BPD group showed hyperactivation to these pictures (Hazlett et al. 2012). Since our study used faces instead of pictures, and since we did not examine attenuation of brain activation to repeated stimuli, these findings are not directly comparable with ours.
Neuroscience research on emotion has long recognized that distinct neural systems underlie different dimensions of emotion, namely valence and arousal (Gerber et al. 2008). Fear and happy emotions are opposite in valence but similar in arousal levels. Although we did not directly compare fear versus happy contrasts, we did find that when comparing fear and happy to fixation or neutral, all our findings of group differences were in the direction of the BPD group showing greater levels of brain activation than the controls. (As shown in Table 3, the controls tended to show deactivation in the identified brain regions.) Thus, our findings may suggest that the effect of emotion arousal on brain activation may be equally or more important than the effect of emotion valence in the enhanced neural responses seen in patients with BPD.
In this study, we did not identify any significant correlations between amygdala activation (either during overt or covert fear) and specific clinical measures within the BPD group alone. These analyses were limited by the small sample, which may have reduced our statistical power to identify significant associations in the large number of potential brain-behavior relationships that we explored.
Although this study had several strengths distinguishing it from previous fMRI research in BPD, several limitations should be considered when interpreting the findings. First, our sample size was small, limiting power and generalizability. Therefore, these findings require replication in larger samples. Second, we included only women; although this decision allowed us to limit heterogeneity, it also prohibits the gener- alizability of the findings to men with BPD. Third, we examined amygdala activation using a face-viewing (emotion processing) paradigm. Since we did not incorporate a task that involved explicit regulation of emotion, we are unable to make any firm conclusions about the role of cortical processing in regulating emotion in this sample. Fourth, despite the considerable care to limit confounds due to medication and co-morbidity, some patients had past diagnoses of relevance (MDD, PTSD), several had substance use disorders, and one was taking a psychotropic medication (gabapentin) that was prescribed for pain. Therefore, the findings could have been influenced by these non-BPD effects. Fifth, the order of runs in the scanner could have influenced the results: the experience of processing earlier stimuli could modify the processing of stimuli later in the scanning session. To address this problem, we acquired the all neutral runs first to assess the brain response to neutral faces without potential habituation effects from repeated exposure to these same neutral faces in the other 4 runs. The covert conditions were tested prior to the overt conditions to further ensure that the covert emotion stimuli would be less likely to prime emotion processing in the overt runs than vice versa. Finally, we randomized the order of the happy versus fear runs within covert and overt conditions to minimize the effects of valence order. However, it is impossible to completely remove potential priming from run order in a within-subject design, and such effects may still have impacted our results. Finally, we did not collect a urine toxicology screen on the day of scanning, so we can not rule out the possibility that illicit substances influenced the results.
In conclusion, we report enhanced brain activation in response to emotional faces in women with BPD with limited psychotropic medications and comorbidity. Our findings that patients had greater amygdala and cortical activation than controls during overt but not during covert fear are supportive of the hypothesis that emotion dysregulation in this group is primarily due to dysfunction in cortical regulation of emotion, and suggestive of the possibility that BPD involves a feedforward system in which frontal input may, in part, drive excessive amygdala activation. Findings of increased cortical and subcortical activation in response to covert presentations of both happy and fear faces in the patient group compared to controls suggests a more extended response system where multiple amygdala circuits are activated in response to highly arousing emotional stimuli, whereas healthy controls only activate subcortical systems. Future work is needed incorporating larger samples, and to directly test how frontal regulatory processes during explicit regulation of emotion may potentially contribute to a feed-forward cycle of emotion dysregulation in BPD.
Footnotes
Conflicts of interest Kathryn R. Cullen, Lori LaRiviere, Nathalie Vizueta, Kathleen M. Thomas, Ruskin H. Hunt, Michael J. Miller, Kelvin O. Lim, and Sellman C. Schultz declare that they have no conflicts of interest.
Informed Consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
References
- Atkinson AP, Adolphs R. The neuropsychology of face perception: beyond simple dissociations and functional selectivity. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2011;366:1726–1738. doi: 10.1098/rstb.2010.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beblo T, Driessen M, Mertens M, Wingenfeld K, Piefke M, Rullkoetter N, Silva-Saavedra A, Mensebach C, Reddemann L, Rau H, Markowitsch HJ, Wulff H, Lange W, Berea C, Ollech I, Woermann FG. Functional MRI correlates of the recall of unresolved life events in borderline personality disorder. Psychological Medicine. 2006;36:845–856. doi: 10.1017/S0033291706007227. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Black DW, Blum N, Pfohl B, Hale N. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. Journal of Personality Disorders. 2004;18:226–239. doi: 10.1521/pedi.18.3.226.35445. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brown MZ, Comtois KA, Linehan MM. Reasons for suicide attempts and nonsuicidal self-injury in women with borderline personality disorder. Journal of Abnormal Psychology. 2002;111:198–202. doi: 10.1037//0021-843x.111.1.198. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Linehan MM. A biosocial developmental model of borderline personality : elaborating and extending linehan’s theory. 2009;135:495–510. doi: 10.1037/a0015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Vizueta N, Thomas KM, Han GJ, Lim KO, Camchong J, Mueller BA, Bell CH, Heller MD, Schulz SC. Amygdala functional connectivity in young women with borderline personality disorder. Brain Connectivity. 2011;1:61–71. doi: 10.1089/brain.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R: Administration, Scoring, and Procedures Manual. Minneapolis: National Computer Systems; 1994. [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashan TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Dyck M, Loughead J, Kellermann T, Boers F, Gur RC, Mathiak K. Cognitive versus automatic mechanisms of mood induction differentially activate left and right amygdala. NeuroImage. 2011;54:2503–2513. doi: 10.1016/j.neuroimage.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of Facial Affect. Palo Alto: Consulting Psychologists Press; 1976. [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) Washington, D.C: American Psychiatric Press, Inc; 1997. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen CID-I/P W/ PSY SCREEN) New York: Biometrics Research Institute; 2002. [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gerber AJ, Posner J, Gorman D, Colibazzi T, Yu S, Wang Z, Kangarlu A, Zhu H, Russell J, Peterson BS. An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces. Neuropsychologia. 2008;46:2129–2139. doi: 10.1016/j.neuropsychologia.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Pollak SD, Davidson RJ. Developmental neuroscience perspectives on emotion regulation. Child Development Perspectives. 2008;2:132–140. doi: 10.1111/j.1750-8606.2008.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Tull MT, Reynolds EK, Bagge CL, Latzman RD, Daughters SB, Lejuez CW. Extending extant models of the pathogenesis of borderline personality disorder to childhood borderline personality symptoms: the roles of affective dysfunction, disinhibition, and self- and emotion-regulation deficits. Development and Psychopathology. 2009;21:1263–1291. doi: 10.1017/S0954579409990150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Dezecache G. How do shared-representations and emotional processes cooperate in response to social threat signals? Neuropsychologia. 2014;55:105–114. doi: 10.1016/j.neuropsychologia.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Pichon S, de Gelder B. Perceiving fear in dynamic body expressions. NeuroImage. 2007;35:959–967. doi: 10.1016/j.neuroimage.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Valabrègue R, Gholipour B, Chevallier C. A direct amygdala-motor pathway for emotional displays to influence action: a diffusion tensor imaging study. Human Brain Mapping. 2014;35:5974–5983. doi: 10.1002/hbm.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson JG, Stout RL, Sanislow CA, Shea MT, McGlashan TH, Zanarini MC, Daversa MT, Grilo CM, Yen S, Skodol AE. New episodes and new onsets of major depression in borderline and other personality disorders. Journal of Affective Disorders. 2008;111:40–45. doi: 10.1016/j.jad.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Zhang J, New AS, Zelmanova Y, Goldstein KE, Haznedar MM, Meyerson D, Goodman M, Siever LJ, Chu K-W. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biological Psychiatry. 2012;72:448–456. doi: 10.1016/j.biopsych.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Jacob GA, Zvonik K, Kamphausen S, Sebastian A, Maier S, Philipsen A, Tebartz van Elst L, Lieb K, Tüscher O. Emotional modulation of motor response inhibition in women with borderline personality disorder: an fMRI study. Journal of Psychiatry & Neuroscience. 2013;38:164–172. doi: 10.1503/jpn.120029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PA, Hurley RA, Benkelfat C, Herpertz SC, Taber KH. Understanding emotion regulation in borderline personality disorder: contributions of neuroimaging. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:397–402. doi: 10.1176/jnp.15.4.397. [DOI] [PubMed] [Google Scholar]
- Kamphausen S, Schröder P, Maier S, Bader K, Feige B, Kaller CP, Glauche V, Ohlendorf S, Tebartz van Elst L, Klöppel S, Jacob GA, Silbersweig D, Lieb K, Tüscher O. Medial prefrontal dysfunction and prolonged amygdala response during instructed fear processing in borderline personality disorder. The World Journal of Biological Psychiatry. 2013;14(307–18):S1–S4. doi: 10.3109/15622975.2012.665174. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Neta M, Davis FC, Oler JA, Mazzulla EC, Whalen PJ. Behind the mask: the influence of mask- type on amygdala response to fearful faces. Social Cognitive and Affective Neuroscience. 2010;5:363–368. doi: 10.1093/scan/nsq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Siever LJ, Lee H, Pizzarello S, New AS, Goodman M, Cheng H, Flory J, Prohovnik I. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Research. 2009;172:192–199. doi: 10.1016/j.pscychresns.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Denny BT, Fan J, Liu X, Guerreri S, Mayson SJ, Rimsky L, New AS, Goodman M, Siever LJ. The neural correlates of anomalous habituation to negative emotional pictures in borderline and avoidant personality disorder patients. The American Journal of Psychiatry. 2014;171:82–90. doi: 10.1176/appi.ajp.2013.13070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levy KN. The implications of attachment theory and research for understanding borderline personality disorder. Development and Psychopathology. 2005;17:959–986. doi: 10.1017/s0954579405050455. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford Press; 1993. [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Research. 2007;155:231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AE, Dickens GL, Picchioni MM. Facial emotion processing in borderline personality disorder: a systematic review and meta-analysis. Neuropsychology Review. 2014;24:166–184. doi: 10.1007/s11065-014-9254-9. [DOI] [PubMed] [Google Scholar]
- Niedtfeld I, Schulze L, Kirsch P, Herpertz SC, Bohus M, Schmahl C. Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biological Psychiatry. 2010;68:383–391. doi: 10.1016/j.biopsych.2010.04.015. [DOI] [PubMed] [Google Scholar]
- O’Neill A, D’Souza A, Carballedo A, Joseph S, Kerskens C, Frodl T. Magnetic resonance imaging in patients with borderline personality disorder: a study of volumetric abnormalities. Psychiatry Research. 2013;213:1–10. doi: 10.1016/j.pscychresns.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the multidimensional personality questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Putnam KM, Silk KR. Emotion dysregulation and the development of borderline personality disorder. Development and Psychopathology. 2005;17:899–925. doi: 10.1017/s0954579405050431. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JDE, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Amirthavasagam S, Zakzanis KK. Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: a metaanalysis of magnetic resonance imaging studies. Psychiatry Research. 2012;201:245–252. doi: 10.1016/j.pscychresns.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Schulze L, Domes G, Krüger A, Berger C, Fleischer M, Prehn K, Schmahl C, Grossmann A, Hauenstein K, Herpertz SC. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biological Psychiatry. 2011;69:564573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Selby EA, Anestis MD, Bender TW, Joiner TE., Jr An exploration of the emotional cascade model in borderline personality disorder. Journal of Abnormal Psychology. 2009;118(2):375–387. doi: 10.1037/a0015711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby EA, Joiner TE, Jr, Thomas E. Emotional cascades as prospective predictors of dysregulated behaviors in borderline personality disorder. Personality Disorders. 2013;4(2):168–174. doi: 10.1037/a0029933. [DOI] [PubMed] [Google Scholar]
- Shea MT, Stout RL, Yen S, Pagano ME, Skodol AE, Morey LC, Gunderson JG, McGlashan TH, Grilo CM, Sanislow CA, Bender DS, Zanarini MC. Associations in the course of personality disorders and axis I disorders over time. Journal of Abnormal Psychology. 2004;113:499–508. doi: 10.1037/0021-843X.113.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: psychopathology, comorbidity, and personality structure. Biological Psychiatry. 2002;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Lushene RE, PR V, Jacobs A. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45:630–637. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Research. 2007;154:93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Frankenbur FR, Weingeroff JL, Reich DB, Fitzmaurice GM, Weiss RD. The course of substance use disorders in patients with borderline personality disorder and Axis II comparison subjects: a 10-year follow-up study. Addiction. 2011;106:342–348. doi: 10.1111/j.1360-0443.2010.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Reich DB, Fitzmaurice G. Attainment and stability of sustained symptomatic remission and recovery among patients with borderline personality disorder and axis II comparison subjects: a 16-year prospective follow-up study. The American Journal of Psychiatry. 2012;169:476–483. doi: 10.1176/appi.ajp.2011.11101550. [DOI] [PMC free article] [PubMed] [Google Scholar]