Abstract
Rationale: Macrophage elastase (matrix metalloproteinase [MMP]-12) is a potent protease that contributes to the lung destruction that accompanies cigarette smoking; it simultaneously inhibits lung tumor angiogenesis and metastasis by catalyzing the formation of antiangiogenic peptides. Recent studies have revealed novel nonproteolytic functions of MMP12, including antimicrobial activity through a peptide within its C-terminal domain (CTD).
Objectives: To determine whether the MMP12 CTD contributes to its antitumor activity in lung cancer.
Methods: We used recombinant MMP12 peptide fragments, including its catalytic domain, CTD, and a 20 amino acid peptide within the CTD (SR20), in an in vitro system to delineate their effects on non–small cell lung cancer cell proliferation and apoptosis. We translated our findings to two murine models of lung cancer, including orthotopic human xenograft and KrasLSL/G12D mouse models of lung cancer.
Measurements and Main Results: We show that SR20 triggers tumor apoptosis by up-regulation of gene expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) and its receptor, death receptor 4, sensitizing cells to an autocrine loop of TRAIL-mediated cell death. We then demonstrate the therapeutic efficacy of SR20 against two murine models of lung cancer.
Conclusions: The MMP12 CTD initiates TRAIL-mediated tumor cell death through its conserved SR20 peptide.
Keywords: matrix metalloproteinase-12, tumor necrosis factor–related apoptosis-inducing ligand, proteases, lung cancer, apoptosis
At a Glance Commentary
Scientific Knowledge on the Subject
Matrix metalloproteinase-12 (MMP12) is believed to impede lung tumor growth and metastasis through the proteolytic generation of antiangiogenic peptides. Recent studies have shown that MMP12 has functions beyond proteolysis, but their roles in lung cancer remain unclear.
What This Study Adds to the Field
This study demonstrates that the SR20 peptide within the carboxy-terminal domain of MMP12 triggers tumor necrosis factor–related apoptosis-inducing ligand–mediated apoptosis in human and mouse tumor cells. Furthermore, SR20 is therapeutic in two in vivo models of lung cancer.
Lung cancer is the leading cause of cancer mortality in the United States, and despite recent progress in the management of many cancers, death rates among patients with lung cancer remain alarmingly high (1). Lung cancer is strongly correlated to a history of cigarette smoking (2), which is accompanied by damage and remodeling cycles that underlie the pathogeneses of other smoking-related diseases (3). Matrix metalloproteinases (MMPs) are among the key endogenous mediators of these alterations in lung structure and function (4–6), and MMPs also play critical roles in tumor biology. Although the overall effect of MMPs is to promote tumor progression (7), some MMPs, particularly MMP12, seem to work for the host in inhibiting tumor progression (8, 9).
The MMPs constitute a family of 24 members with many common functional and structural characteristics, including an amino-terminal proenzyme domain and a zinc-containing catalytic domain. Most MMPs also contain a carboxy-terminal hemopexin-like domain, whereas some possess additional features, such as a transmembrane domain (10). In the context of malignancies, historical studies have focused on the MMPs’ abilities to penetrate basement membranes and clear routes for tumor invasion (11, 12). More recent evidence has shown an increasingly diverse role for MMPs in cancer progression encompassing the release of matrix-bound growth factors (13), generation of chemotactic gradients (14), and modulation of tumor angiogenesis (8, 15, 16). Hence, MMPs have garnered significant attention as potential targets for anticancer treatment (10, 17). However, although MMP inhibitors showed therapeutic promise in murine models of cancer (18, 19), their efficacies in clinical trials have been surprisingly disappointing (20). The failure of these drugs in human cancers is likely caused by the diversity of MMPs, whereby certain MMPs consistently promote tumorigenesis, whereas others exhibit both protumorigenic and antitumorigenic properties depending on the tumor type, disease stage, and cellular source (10, 21).
Macrophage elastase (MMP12) is one of the most highly up-regulated genes in the lungs of cigarette smokers (22), yet its role in lung cancer remains controversial. Gene expression studies have shown significant associations between increased MMP12 expression and risk of local recurrence and metastasis in non–small cell lung cancer (NSCLC) (23, 24). In contrast, promoter polymorphisms causing increased MMP12 expression have been linked to prolonged survival in a cohort of patients with lung cancer (25). Meanwhile, murine models have shown a protective role for MMP12 against lung tumor growth (9) and metastasis (8) attributed to its ability to generate the antiangiogenic peptides endostatin (from type XVIII collagen) and angiostatin (from plasmin[ogen]) (26, 27). Taken together, the anticancer effects of MMP12 may impede the development or progression of lung cancer in human smokers.
We recently demonstrated a role for MMP12 that extends beyond its protein-cleaving function, because the conserved SR20 peptide in its C-terminal domain (CTD) directly enhances bacterial killing (28). We hypothesized that MMP12 may also modulate cancer cell growth independent of its catalytic function. To explore the extraproteolytic roles of MMP12 in lung cancer, we subjected both lung cancer cells and primary lung cells to full-length MMP12 and fragments of both its catalytic domain and CTD. Through this in vitro model, we were able to delineate a novel mechanism by which the CTD of MMP12, through the activity of the SR20 peptide, suppresses tumor growth while sparing noncancerous lung cells. Furthermore, we provide initial evidence supporting the efficacy of SR20 as a peptide chemotherapeutic in two murine models of lung cancer.
Methods
Cells
A549 (ATCC #CCL-185; human NSCLC; KRAS-G12S), H1650 (ATCC #CRL-5883; human NSCLC; KRAS–wild type; PTEN-null), mouse lung epithelial (ATCC #CRL-2110), and LL47 (ATCC #CCL-135; human lung fibroblast) cells were obtained from ATCC (Manassas, VA). 91T (human NSCLC; KRAS-G12V) and 201T (human NSCLC; KRAS–wild type) cells were kindly provided by Dr. Jill Siegfried (29). Murine KW-857 cells (mouse adenocarcinoma; KRAS-G12D; LKB−/−) were kindly provided by Dr. Kwok Wong (30). Human bronchial epithelial cells were a kind donation from Dr. Michael Myerberg. Primary murine fibroblasts were isolated as described previously (31). The previously mentioned cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA) with l-glutamine supplementation, 10% fetal bovine serum (Hyclone, Logan, UT), and 50 U/ml of penicillin/streptomycin (Invitrogen). Human microvascular endothelial cells were purchased from Lonza and grown in EGM2-MV culture media (Lonza, Basel, Switzerland).
Peptides
Human SR20 and SR20-GFP were synthesized at the University of Pittsburgh’s protein core as described (28). Purity of SR20 was determined by HPLC analysis (see Figure E1A in the online supplement). Human (IgG1 Fc-FLAG)-MMP12 CTD was synthesized by GenScript (Piscataway, NJ) and purity was determined by densitometric analysis (ImageJ, https://imagej.nih.gov/ij/; data not shown) of Coomassie blue (VWR International, Radnor, PA) staining of sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (see Figure E1B) per the manufacturer’s instructions. Recombinant human MMP12 catalytic domain (CAT) was synthesized as described previously (32). MMP9 CTD was synthesized as described previously (28). Peptide sequences are provided in Table E1.
Cell Culture
All cells were maintained in a humidified incubator at 37°C and 5% CO2. Cells were treated with 20–50 μg/ml SR20 (in 10% dimethyl sulfoxide; Sigma-Aldrich, St. Louis, MO), 50 μg/ml CTD, 50 μg/ml MMP9 CTD, 100 μg/ml CAT, or 30–100 ng/ml recombinant human (rh) tumor necrosis factor–related apoptosis-inducing ligand (TRAIL; R&D Systems, Minneapolis, MN) for 1 hour in serum-free Dulbecco’s modified Eagle medium and then incubated in serum-free media for 24–72 hours. For inhibitor experiments, cells were preincubated with 20 μM Z-IETD-FMK caspase-8 blocking peptide (BD Pharmingen, San Jose, CA), 20 μM Z-LEHD-FMK caspase-9 blocking peptide (BD Pharmingen), 3 ng/ml recombinant human TRAIL-R1 Fc chimera (rhDR4:Fc; R&D Systems), or 0.25 μg/ml mouse monoclonal (2E5) anti-TRAIL antibody (Abcam, Cambridge, UK) for 30 minutes before the addition of SR20/CTD/CAT/rhTRAIL and remained for the hour of treatment. The anti-TRAIL antibody was reapplied to the media at 0.25 μg/ml after the 1-hour treatment period.
Murine Lung Cancer Models
For orthotopic lung A549 xenografts, pathogen-free NU/J mice were intratracheally xenotransplanted at age 7–9 weeks with 107 A549 cells in 35 μl Dulbecco’s modified Eagle medium with 0.01M ethylenediaminetetraacetic acid. A total of 35 μl of 20 μg/μl SR20 or phosphate-buffered saline was intratracheally instilled twice weekly for 10 weeks. For oncogenic Kras induction, tumors were induced in 8-week old KrasLSL/G12D and KrasLSL/G12D:Mmp12−/− mice by administration of 5 × 106 plaque-forming units adenoviral Cre recombinase (AdCre; University of Iowa). Seven weeks after tumor induction, mice received twice weekly dosing of 35 μl of 20 μg/μl SR20 or phosphate-buffered saline by intratracheal instillation for 10 weeks. At the completion of treatment, mice were asphyxiated by CO2 inhalation and lungs were inflated with 10% buffered formalin (Sigma-Aldrich) at 25 cm H2O for 10 minutes.
Statistics
Statistical analyses for all experiments were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). Data are presented as mean ± SD. Data were checked for normality by the Shapiro-Wilk test with an α of 0.05. Statistical comparisons between groups were made using two-sided independent-sample Student’s t test. P values less than 0.05 were considered statistically significant.
Additional details on these methods are provided in the online supplement.
Results
MMP12 Suppresses [3H]-Thymidine Incorporation by Tumor Cells through the SR20 Peptide in Its CTD
To examine whether MMP12 directly modulates cellular proliferation, we cocultured A549 lung cancer cells with peritoneal macrophages from C57BL/6J wild-type or Mmp12 null mutant (Mmp12−/−) mice and measured [3H]-thymidine incorporation. Coculture with wild-type macrophages led to a dose-dependent reduction in A549 [3H]-thymidine incorporation at early (1–3 h) and late (72 h) incubation periods (Figure 1A), likely corresponding to the release of preformed and newly synthesized mediators, respectively (33). Meanwhile, A549s cocultured with Mmp12−/− macrophages had no change in [3H]-thymidine uptake compared with untreated control animals (Figure 1B), suggesting that MMP12 is necessary for the reduced titrated thymidine uptake by A549 cells seen in macrophage coculture.
Figure 1.
Matrix metalloproteinase (MMP)-12 C-terminal domain (CTD) blunts [3H]-thymidine incorporation by A549 cells. (A) A549 cells were cultured alone (0) (n = 4 replicates per time point) or cocultured with 50,000, 100,000, and 150,000 peritoneal macrophages (n = 3 replicates per group per time point) from wild-type (WT) mice for 1, 3, and 72 hours, and [3H]-thymidine incorporation was measured. (B) Time course from 1 to 72 hours of [3H]-thymidine incorporation in A549 cells in the absence of macrophages (vehicle) or coincubated with 150,000 WT or Mmp12−/− macrophages (n = 3 replicates per group per time point). P values are Mmp12−/− versus vehicle. (C) Primary structure of MMP12 consists of an N-terminal prodomain, catalytic domain (CAT), and CTD containing a conserved SR20 peptide. (D) A549 cells were treated for 1 hour with 100 μg/ml CAT, 50 μg/ml CTD, or 50 μg/ml MMP9 CTD or (E) dimethyl sulfoxide or 20 μg/ml SR20 and [3H]-thymidine incorporation was measured in the 48 hours after treatment (n = 3–4 replicates per treatment). Data are mean ± SD. P values were calculated by two-sided independent sample Student’s t test. DMSO = dimethyl sulfoxide; DPM = disintegrations per minute.
MMP12 is translated as a 470 amino acid protein consisting of a 9-kD amino-terminal prodomain that is cleaved on activation, a 22-kD catalytic domain, and a 23-kD carboxy-terminal hemopexin-like domain (Figure 1C) (34). To localize the effects of MMP12 on thymidine incorporation, we quantified the [3H]-thymidine uptake of A549 cells treated with either the CAT or CTD of recombinant human MMP12. CTD caused a pronounced decrease in [3H]-thymidine uptake compared with vehicle-treated control animals, whereas neither CAT nor the CTD of the similarly elastolytic human MMP9 (35) had a significant effect on uptake (Figure 1D). Furthermore, the antiproliferative effect of CTD was mimicked with the highly conserved CTD fragment SR20 that has previously been shown to enhance bacterial killing by macrophages (Figure 1E). Taken together, these data localize a stunting effect on thymidine incorporation by CTD and SR20 that may be unique to MMP12.
To examine the breadth of this effect beyond A549 cells, we exposed a variety of primary and adenocarcinoma cells to CTD or SR20 and quantified [3H]-thymidine uptake. Adenocarcinoma cell lines included human A549 and 91T (29) cells as well as murine KW-857 cells (30). Nontransformed cells of both murine and human origin included primary murine fibroblasts, murine lung epithelial cells, human lung fibroblasts (LL47), human microvascular endothelial cells, and human bronchial epithelial cells. Consistently, CTD and SR20 stunted the [3H]-thymidine uptake of tumor cells but had no effects on nontransformed cells (see Figures E2A and E2B).
MMP12 CTD and SR20 Induce Apoptosis in Multiple Cancer Cell Lines but Do Not Affect Cell Cycle Progression
Because the attenuation of [3H]-thymidine incorporation by CTD and SR20 could be attributable to either cell death or reduced transit through the cell cycle, we quantified the effects of these peptides on apoptosis and cell cycle progression in multiple NSCLC cell lines harboring either oncogenic (A549 and 91T) or wild-type (H1650 and 201T) KRAS alleles. In each cell line, treatment with CTD (Figure 2A) or SR20 (Figures 2B and 2C; see Figures E3A–E3C) led to a significant increase in the percentage of apoptotic cells as measured by Annexin V positivity. In contrast, neither CTD nor SR20 impacted the proportion of cells in the G0-G1 versus G2-M phases of the cell cycle (Figures 2D–2H; see Figures E4A–E4C) as assessed by propidium iodide staining for DNA content.
Figure 2.
C-terminal domain (CTD) and SR20 induce non–small cell lung cancer cell line apoptosis but do not affect cell cycle progression. A549, 91T, H1650, and 201T cells were untreated (vehicle) or treated with CTD, dimethyl sulfoxide (DMSO), or SR20 (20 μg/ml or 50 μg/ml). Cells were labeled with Annexin V after (A) CTD (n = 2–15 replicates per group) or (B) SR20 (n = 4 replicates per group) treatment, and the percentages of Annexin V+ cells were quantified by flow cytometry. (C) Representative flow cytometry plots of A549 cells labeled with Annexin V and 7-AAD after SR20 treatment (plots are representative of four independent experiments). (D) A549, (E) 91T, (F) H1650, and (G) 201T cells were stained with propidium iodide after CTD (n = 2–4 replicates per group) or SR20 (n = 3–4 replicates per group) treatment, and the percentages of cells in the G0-G1 or G2-M phases of the cell cycle were quantified by flow cytometry. (H) Representative histograms of A549 cells labeled with propidium iodide after DMSO or SR20 treatment (plots are representative of four independent experiments). Values represent the percentages of cells in the respective phases of the cell cycle (G0-G1 or G2-M). The bars delineate the propidium iodide levels associated with the cell cycle phases. Data are mean ± SD. P values were calculated by two-sided independent sample Student’s t test. 7-AAD = 7-aminoactinomycin D.
MMP12 CTD Traffics to the Nucleus and Initiates the Transcription of TRAIL
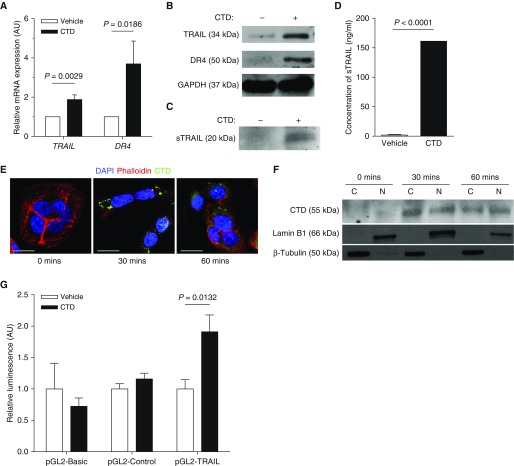
Because CTD and SR20 induced apoptosis while their effects on [3H]-thymidine uptake were limited to cancerous cells, we suspected that they exert their apoptotic effects through TRAIL (also known as TNFSF10), which has also been shown to have specific effects on cancer cell viability (36). TRAIL exists as both a type II transmembrane (36) and soluble TRAIL polypeptide (37) whose binding to death receptors 4 (DR4) or 5 triggers apoptotic cell death (38, 39). Incubation of A549 cells with CTD or SR20 led to a significant increase in TRAIL and DR4 mRNA (Figure 3A; see Figure E5A) and protein (Figure 3B; see Figure E5B) expression, and an 80-fold increase in soluble TRAIL protein (Figures 3C and 3D) after 48 hours incubation. In agreement with a recent study (40), we found that CTD and SR20 traffic to the nucleus of A549 cells (Figures 3E and 3F; see Figure E5C), suggesting that CTD may activate an apoptotic transcriptional program much like the antiviral response triggered by MMP12’s catalytic domain (40). Indeed, CTD, but not IgG Fc, bound to multiple DNA fragments directly upstream of the TRAIL gene Tnfsf10 in electrophoretic mobility shift assays (see Figures E6A–E6C). To confirm the CTD-responsiveness of the TRAIL promoter, we transfected A549 cells with firefly luciferase-encoding plasmids pGL2-Basic, pGL2-Control, and pGL2-TRAIL, in which the luciferase gene is flanked at its 5′ end by no promoter, the CMV promoter, or the 1,523 base-pair region upstream of the Tnfsf10 gene (41), respectively. CTD treatment significantly increased the luciferase activity of pGL2-TRAIL-transfected cells, whereas it had no effect on those transfected with either pGL2-Basic or pGL2-Control (Figure 3G).
Figure 3.
C-terminal domain (CTD) induces the expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) and death receptors 4 (DR4). A549 cells were treated with 50 μg/ml CTD for 1 hour. (A) Relative mRNA expression of TRAIL and DR4 normalized to glyceraldehyde phosphate dehydrogenase (GAPDH) control at 5 hours after CTD treatment (n = 4 means of independent experiments). (B) Representative Western blot of TRAIL, DR4, and GAPDH endogenous control protein 48 hours after CTD treatment. (C) Western blot representative of three independent experiments and (D) quantitative ELISA for soluble TRAIL (sTRAIL) in culture media at 48 hours after CTD treatment (n = 3 means of independent experiments). (E) Confocal microscopy of CTD-treated A549 cells stained with rhodamine-phalloidin (red), 4′,6-diamidino-2-phenylindole (DAPI; blue), and anti–matrix metalloproteinase-12 CTD (green). Images are representative of three independent experiments. Images were captured at ×100; scale bars are 10 μm. (F) A549 cytoplasmic and nuclear extracts were prepared at 0, 30, and 60 minutes after 50 μg/ml CTD treatment. Western blots are representative of three independent experiments. (G) A549 cells were cotransfected with pRL-CMV encoding Renilla luciferase and either pGL2-Basic, pGL2-Control, or pGL2-TRAIL encoding firefly luciferase and treated with 50 μg/ml CTD for 24 hours. Firefly luciferase activity was normalized to Renilla luciferase activity and expressed relative to vehicle-treated control animals (n = 6 replicates per group). Data are mean + SD. P values were calculated by two-sided independent sample t test. AU = arbitrary units; C = cytoplasmic; N = nuclear.
MMP12 CTD Sensitizes Tumor Cells to TRAIL-mediated Apoptosis
To determine whether activation of the TRAIL-DR4 axis was responsible for the induction of apoptosis in A549 cells after CTD treatment, we inhibited TRAIL signaling with a recombinant human DR4:IgG Fc chimera (rhDR4:Fc) during [3H]-thymidine incorporation assays. rhDR4:Fc completely abolished the effects of CTD on [3H]-thymidine uptake (Figure 4A), and we confirmed rhDR4:Fc similarly abolished those effects by SR20 (see Figure E7A). As expected, treatment of A549 cells with CTD (Figure 4B) or SR20 (see Figure E7B), but not CAT, led to cleavage and activation of the apoptotic effector caspase (Casp)-3, which was inhibited by pretreatment with a neutralizing antibody to TRAIL (Figure 4C). Likewise, CTD treatment increased the percentage of TUNEL-positive cells (Figures 4D and 4E), further indicating that CTD causes A549 cell apoptosis. Mirroring its effects on A549 cell proliferation, rhDR4:Fc blunted Casp3 cleavage induced by CTD (Figure 4F). TRAIL-bound DR4 initiates apoptotic cell death through Casp8 (42), which can trigger apoptosis by direct cleavage of Casp3 (43) or intrinsic pathway signaling leading to Casp9 activation (44–46) and subsequent Casp3 cleavage. Previous reports indicated that type II pneumocytes, which share many properties with A549 cells (47), require the activation of both pathways to overcome antiapoptotic mechanisms (48); similarly, we found that Casp3 cleavage in CTD-treated A549 cells was blunted by antagonistic blocking peptides to either Casp8 or Casp9 (Figure 4G).
Figure 4.
C-terminal domain (CTD) induces tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)-dependent apoptosis and TRAIL sensitization. A549 cells were treated with 50 μg/ml CTD for 1 hour, and experiments were conducted 48 hours later. (A) A549 cells were preincubated in the absence or presence of recombinant human DR4: IgG Fc chimera (rhDR4:Fc) for 30 minutes, continuing into CTD incubation, and [3H]-thymidine incorporation was measured (n = 3–4 replicates per treatment). (B) A549 cells were treated with CTD or 100 μg/ml catalytic domain. Representative Western blot of caspase (Casp) 3 and glyceraldehyde phosphate dehydrogenase (GAPDH). (C) A549 cells were preincubated with or without anti-TRAIL antibody, continuing into CTD incubation. Representative Western blot of Casp3 and GAPDH. (D) Representative images of vehicle and CTD-treated cells stained for TUNEL (scale bars are 100 μm). (E) TUNEL-positive cells were quantified as a percentage of total cells (n = 3 means of independent experiments). (F) Representative Western blot for Casp3 and GAPDH in CTD-treated cells plus or minus rhDR4:Fc. (G) Representative Western blot for Casp3, -8, -9 and GAPDH protein in cells treated with CTD in the presence or absence of Casp8 blocking peptides or Casp9 blocking peptides. (H) A549 cells were treated with CTD, 100 ng/ml rhTRAIL, or CTD plus 30 ng/ml rhTRAIL. Representative Western blot for phospho-Bad (pBad), Bad, Bcl-xL, Bcl-xS, XIAP, and GAPDH. (I) Representative Western blot for AKT, NFκB p50, p44/42 MAPK, and GAPDH. (J) Representative Western blot for c-FLIP and Casp3 in cells treated with CTD. Western blots are representative of three to six independent experiments. Data are mean + SD. P values were calculated by two-sided independent sample Student’s t test. Bad = Bcl2-associated death promoter; Bcl-xL = B-cell lymphoma–extra large; CAT = catalytic domain; c-FLIP = cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein; DPM = disintegrations per minute; DR4 = death receptor 4; MAPK = mitogen-activated protein kinase; NFκB = nuclear factor κ-light-chain-enhancer of activated B cells; PBS = phosphate-buffered saline; XIAP = X-linked inhibitor of apoptosis.
Resistance to TRAIL-mediated apoptosis has been a major obstacle in the therapeutic use of TRAIL, and A549 cells have been reported to be largely insensitive to recombinant human TRAIL (rhTRAIL) treatment (49). To interrogate the effects of the MMP12 CTD on TRAIL-resistance pathways, we compared apoptotic mediators in A549 cells treated with either CTD alone, 100 ng/ml rhTRAIL alone, or CTD plus 30 ng/ml rhTRAIL. CTD treatment caused reduced levels of antiapoptotic proteins phospho-Bcl-2-associated death promoter (50) (pBad), B-cell lymphoma-extra-large (Bcl-xL), and X-linked inhibitor of apoptosis (51) (XIAP), along with increases in the proapoptotic proteins Bad and Bcl-xS (52), all of which have been linked to TRAIL sensitivity (53–55) and were unaffected by rhTRAIL alone (Figure 4H). Likewise, CTD treatment decreased the levels of prosurvival proteins nuclear factor κ-light-chain-enhancer of activated B cells, AKT, and p44/42 mitogen-activated protein kinase linked to alternative TRAIL signaling in resistant cells (49, 56), whereas their levels were unchanged by rhTRAIL (Figure 4I). Meanwhile, CTD treatment decreased the expression of cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein in A549 cells (Figure 4J), which functions in both TRAIL-resistance and the acquisition of prosurvival TRAIL-signaling phenotypes (49). Taken together, these data and the up-regulation of DR4 by CTD suggest that CTD sensitizes A549 cells to TRAIL-mediated apoptosis.
SR20 Is Therapeutic In Vivo in Murine Models of Lung Cancer
To confirm the importance of these findings in vivo and examine the feasibility of CTD as a chemotherapeutic, we instilled cancerous mice intratracheally with SR20, which contains the antitumor activity of CTD. The therapeutic efficacy of SR20 in vivo was first tested on an orthotopic A549 lung xenograft in athymic nude mice (NU/J). One week after A549 cell implantation, mice were given intratracheal instillations of SR20 or vehicle control two times per week for 10 weeks, and mice given SR20 experienced a 60% reduction in lung tumor area compared with vehicle-treated mice (Figures 5A and 5B). Furthermore, the lungs of SR20-treated mice had significantly reduced tumor multiplicity, defined as the number of observed tumors per unit of cross-sectional lung area, compared with vehicle-treated control animals (see Figure E8A), suggesting that SR20 eliminated previously established tumors or prevented the development of new tumors during the treatment period. In a second murine model of endogenous lung cancer (KrasLSL/G12D), tumors were induced by intratracheal delivery of AdCre to trigger oncogenic Kras expression (57). Seven weeks after AdCre delivery, mice were begun on a regimen of either SR20 or vehicle control by twice weekly intratracheal instillation for 10 weeks. Interestingly, we observed that concurrent deletion of Mmp12 (KrasLSL/G12D:Mmp12−/−) led to a significant increase tumor burden (Figures 5C and 5D) adding to evidence of a physiologic role for Mmp12 in tumor surveillance (8, 9). Treatment of KrasLSL/G12D:Mmp12−/− mice with SR20 significantly reduced the overall tumor area by greater than 80% (Figures 5C and 5D) while also significantly reducing the tumor multiplicity (see Figure E8B). In addition, tumors in SR20-treated mice harbored a greater percentage of apoptotic cells, as measured by TUNEL staining (Figures 5E and 5F), while having fewer dividing cells as measured by Ki67 positivity (Figures 5G and 5H). In summary, these data suggest that SR20 is an important physiologic inhibitor of tumorigenesis with therapeutic potential in lung cancer.
Figure 5.
SR20 inhibits tumor growth. Athymic nude mouse (NU/J) lungs were instilled with 107 cells and 1 week later were instilled with vehicle control or SR20 twice weekly for 10 weeks. Mice were killed at the end of the 10-week treatment period, lungs were inflated, and hematoxylin and eosin (H&E) sections were prepared. (A) Representative ×10 H&E images of vehicle- and SR20-treated lungs. Boxes show inset area. Insets are ×60 with scale bars of 20 μm. (B) Tumor area was measured as a percentage of total lung area (n = 8 mice per group). Tumorigenesis was initiated in KrasLSL/G12D and KrasLSL/G12D:Mmp12−/− by AdCre instillation. Seven weeks after tumor initiation, mice were treated intratracheally with vehicle control or SR20 twice weekly for 10 weeks. Mice were killed at the end of the 10-week treatment period, lungs were inflated, and H&E sections were prepared. (C) Representative ×10 H&E images of vehicle- and SR20-treated lungs. (D) Tumor area was calculated as a percentage of total lung area (n = 7–14 mice per group). (E) Representative ×60 images of apoptotic cells stained with TUNEL. (F) Apoptotic cells were quantified as the number of TUNEL+ cells per unit of tumor area (n = 3–5 mice per group). (G) Representative ×60 images of dividing cells stained with Ki67. (H) Dividing cells were quantified as the number of Ki67+ cells per unit of tumor area (n = 3–5 mice per group). Data are mean + SD. P values were calculated by two-sided independent sample Student’s t test. AU = arbitrary units.
Discussion
In this study, we present novel evidence for an endogenous mechanism of tumor defense. Using multiple lung cancer cell lines, we show that SR20, a conserved 20 amino acid region in the CTD of MMP12, invokes a potent tumoricidal program. By up-regulating DR4 and its ligand, TRAIL, SR20 initiates an autocrine signaling loop leading to in vitro apoptosis of several lung cancer cell lines. Moreover, as seems to be the case with TRAIL, SR20 is cytotoxic to the tumor cell lines only while leaving noncancerous lung cells intact. SR20 is effective in inhibiting lung tumor growth in vivo hence raising the possibility of a novel and greatly needed therapeutic agent for lung cancer.
Previously, studies from our laboratory (8) and others (9) showed that MMP12 inhibits tumor progression and metastasis, attributing this effect to MMP12’s ability to generate the angiostatic peptides angiostatin and endostatin. Interestingly, although our previous work found that tumor blood vessel density was decreased in Mmp12−/− mice, the levels of antiangiogenic peptides assayed were no different than those of wild-type control animals (8). In identifying a direct tumoricidal role for MMP12 that is independent of its proteolytic activity, this study sheds new light on those earlier investigations. Although it is impossible to distinguish the actions of the MMP12 catalytic and CTDs in a targeted gene deletion model, these results suggest that the absence of SR20 may be either wholly or partially responsible for the increased growth of tumor metastases observed in Mmp12−/− mice (8).
The mechanism elucidated by this study shows a direct tumoricidal role for the SR20 fragment of the MMP12 CTD via TRAIL-mediated apoptosis. This region has previously been shown to mediate intracellular killing of bacteria by macrophages via disruption of lipid membrane integrity (28), whereas full-length MMP12 has recently been shown to possess transcription-modulatory activity residing in its catalytic domain (40). As we confirmed, the MMP12 CTD also traffics to the nucleus and binds DNA regions upstream of the TRAIL gene, Tnfsf10. As CTD sensitizes TRAIL-resistant cells to its apoptotic signaling effects, it is likely that CTD regulates an entire set of target genes that may assist therapeutic efforts to overcome the characteristic resistance to TRAIL (49). Interestingly, a nonsynonymous 1082A/G polymorphism (N357S) in the SR20 domain of human MMP12 is associated with significantly worse outcomes in stage I human NSCLC (58), suggesting that the efficacy of TRAIL induction may be diminished in patients harboring the 1082G allele.
Our study is limited in that, although SR20 seems efficacious against primary murine lung cancers and human lung carcinoma cell lines, the efficacy of CTD and SR20 against primary human lung adenocarcinomas is not assessed. Human lung cancers are known to have varying sensitivities to TRAIL-mediated apoptosis that may include the activation of alternative progrowth signaling pathways (59). However, the ability of CTD and SR20 to initiate cell death in A549 cells, which are known to be resistant to TRAIL-mediated apoptosis (49), is promising in that it may have similar effects on resistant primary tumor cells. Furthermore, in light of preliminary evidence suggesting a transcriptional role for the MMP12 CTD, further benchtop studies are required to fully understand the gene networks whose expression is modulated by CTD, networks that may or may not work in synergy with the mechanism described in this paper.
In this study, the concentration of SR20 delivered to the lungs, assuming efficient homogeneous delivery to a mouse lung volume of 1 ml, is several orders of magnitude higher than the concentration of MMP12 measured in the sputum of human smokers and nonsmokers (60). In this way, the natural infiltration of macrophages to the tumor microenvironment is unlikely to produce enough MMP12 to replicate the antitumor effects that can be achieved with high, therapeutic doses of SR20 peptide. Although well-tolerated by mice, additional studies are required to address the safety of administration of high-dose SR20 to patients. Nearly 25 years ago, the discovery of TRAIL brought great hope because of its ability to induce apoptosis in cancer cells while sparing primary nontransformed cells (61). The discovery of the MMP12 CTD TRAIL-inducing and TRAIL-sensitizing effects suggests that SR20 may represent an opportunity to harness the actions of TRAIL in a potent new chemotherapeutic agent.
Footnotes
Supported by grants 5P01 HL103455 (S.D.S.) and 5T32 HL094295 (N.J.K.) from NHLBI, 5T32 GM008208 (N.J.K.) from the National Institute of General Medical Sciences, and 5P50 CA090440 (A.D.G.) from the National Cancer Institute of the National Institutes of Health; and by the Flight Attendant Medical Research Institute (A.D.G.).
Author Contributions: N.D., N.J.K., A.D.G., and S.D.S. designed the research. N.D., N.J.K., J.P.W., and A.D.G. performed the in vitro experiments. N.D., N.J.K., J.P.W., A.D.G., C.L.B., and A.S.L. performed the in vivo experiments. N.D., N.J.K., J.E.R., and S.D.S. analyzed the data. N.D. and N.J.K. prepared the figures and wrote the paper. A.D.G. and J.E.R. assisted with research design and edited the paper. S.D.S. and A.D.G. supervised the project and edited the paper.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201606-1150OC on March 27, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parks WC, Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res. 2001;2:10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro SD. Proteinases in chronic obstructive pulmonary disease. Biochem Soc Trans. 2002;30:98–102. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro SD. Proteolysis in the lung. Eur Respir J Suppl. 2003;44:30s–32s. doi: 10.1183/09031936.03.00000903a. [DOI] [PubMed] [Google Scholar]
- 7.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 9.Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, Pozzi A, Carbone DP, Schwartz DR, Moin K, et al. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66:7968–7975. doi: 10.1158/0008-5472.CAN-05-4279. [DOI] [PubMed] [Google Scholar]
- 10.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 11.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 12.Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11:143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 14.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci USA. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 18.Kondraganti S, Mohanam S, Chintala SK, Kin Y, Jasti SL, Nirmala C, Lakka SS, Adachi Y, Kyritsis AP, Ali-Osman F, et al. Selective suppression of matrix metalloproteinase-9 in human glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res. 2000;60:6851–6855. [PubMed] [Google Scholar]
- 19.Yonemura Y, Endo Y, Fujita H, Kimura K, Sugiyama K, Momiyama N, Shimada H, Sasaki T. Inhibition of peritoneal dissemination in human gastric cancer by MMP-7-specific antisense oligonucleotide. J Exp Clin Cancer Res. 2001;20:205–212. [PubMed] [Google Scholar]
- 20.Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B, et al. National Cancer Institute of Canada Clinical Trials Group. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296–3302. doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 21.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–6650. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 22.Woodruff PG, Koth LL, Yang YH, Rodriguez MW, Favoreto S, Dolganov GM, Paquet AC, Erle DJ. A distinctive alveolar macrophage activation state induced by cigarette smoking. Am J Respir Crit Care Med. 2005;172:1383–1392. doi: 10.1164/rccm.200505-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann HS, Bartling B, Simm A, Murray R, Aziz N, Hansen G, Silber RE, Burdach S. Identification and classification of differentially expressed genes in non-small cell lung cancer by expression profiling on a global human 59.620-element oligonucleotide array. Oncol Rep. 2006;16:587–595. [PubMed] [Google Scholar]
- 24.Hofmann HS, Hansen G, Richter G, Taege C, Simm A, Silber RE, Burdach S. Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non-small cell lung cancer patients. Clin Cancer Res. 2005;11:1086–1092. [PubMed] [Google Scholar]
- 25.Scherf DB, Dally H, Müller P, Werle-Schneider G, Jäger B, Edler L, Tuengerthal S, Fischer JR, Drings P, Bartsch H, et al. Single nucleotide polymorphisms in matrix metalloproteinase genes and lung cancer chemotherapy response and prognosis. Eur Respir J. 2010;35:381–390. doi: 10.1183/09031936.00125608. [DOI] [PubMed] [Google Scholar]
- 26.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaissé J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 27.Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- 28.Houghton AM, Hartzell WO, Robbins CS, Gomis-Rüth FX, Shapiro SD. Macrophage elastase kills bacteria within murine macrophages. Nature. 2009;460:637–641. doi: 10.1038/nature08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 30.Gandhi L, McNamara KL, Li D, Borgman CL, McDermott U, Brandstetter KA, Padera RF, Chirieac LR, Settleman JE, Wong KK. Sunitinib prolongs survival in genetically engineered mouse models of multistep lung carcinogenesis. Cancer Prev Res (Phila) 2009;2:330–337. doi: 10.1158/1940-6207.CAPR-08-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuoka H, Zhou Z, Pilewski JM, Oury TD, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltration. Am J Pathol. 2006;169:1633–1642. doi: 10.2353/ajpath.2006.060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nénan S, Planquois JM, Berna P, De Mendez I, Hitier S, Shapiro SD, Boichot E, Lagente V, Bertrand CP. Analysis of the inflammatory response induced by rhMMP-12 catalytic domain instilled in mouse airways. Int Immunopharmacol. 2005;5:511–524. doi: 10.1016/j.intimp.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Raza SL, Nehring LC, Shapiro SD, Cornelius LA. Proteinase-activated receptor-1 regulation of macrophage elastase (MMP-12) secretion by serine proteinases. J Biol Chem. 2000;275:41243–41250. doi: 10.1074/jbc.M005788200. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993;268:23824–23829. [PubMed] [Google Scholar]
- 35.Mecham RP, Broekelmann TJ, Fliszar CJ, Shapiro SD, Welgus HG, Senior RM. Elastin degradation by matrix metalloproteinases. Cleavage site specificity and mechanisms of elastolysis. J Biol Chem. 1997;272:18071–18076. doi: 10.1074/jbc.272.29.18071. [DOI] [PubMed] [Google Scholar]
- 36.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 37.Mariani SM, Matiba B, Armandola EA, Krammer PH. Interleukin 1 beta-converting enzyme related proteases/caspases are involved in TRAIL-induced apoptosis of myeloma and leukemia cells. J Cell Biol. 1997;137:221–229. doi: 10.1083/jcb.137.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 39.Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 40.Marchant DJ, Bellac CL, Moraes TJ, Wadsworth SJ, Dufour A, Butler GS, Bilawchuk LM, Hendry RG, Robertson AG, Cheung CT, et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014;20:493–502. doi: 10.1038/nm.3508. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Ji Y, Wang X, Evers BM. Isolation and molecular characterization of the 5′-upstream region of the human TRAIL gene. Biochem Biophys Res Commun. 2000;276:466–471. doi: 10.1006/bbrc.2000.3512. [DOI] [PubMed] [Google Scholar]
- 42.Hao C, Song JH, Vilimanovich U, Kneteman NM. Modulation of TRAIL signaling complex. Vitam Horm. 2004;67:81–99. doi: 10.1016/S0083-6729(04)67006-3. [DOI] [PubMed] [Google Scholar]
- 43.Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 45.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 46.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 47.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 48.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song JH, Tse MC, Bellail A, Phuphanich S, Khuri F, Kneteman NM, Hao C. Lipid rafts and nonrafts mediate tumor necrosis factor related apoptosis-inducing ligand induced apoptotic and nonapoptotic signals in non small cell lung carcinoma cells. Cancer Res. 2007;67:6946–6955. doi: 10.1158/0008-5472.CAN-06-3896. [DOI] [PubMed] [Google Scholar]
- 50.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 51.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 52.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 53.Zhu H, Guo W, Zhang L, Davis JJ, Wu S, Teraishi F, Cao X, Smythe WR, Fang B. Enhancing TRAIL-induced apoptosis by Bcl-X(L) siRNA. Cancer Biol Ther. 2005;4:393–397. doi: 10.4161/cbt.4.4.1616. [DOI] [PubMed] [Google Scholar]
- 54.Taghiyev AF, Guseva NV, Harada H, Knudson CM, Rokhlin OW, Cohen MB. Overexpression of BAD potentiates sensitivity to tumor necrosis factor-related apoptosis-inducing ligand treatment in the prostatic carcinoma cell line LNCaP. Mol Cancer Res. 2003;1:500–507. [PubMed] [Google Scholar]
- 55.Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11:915–923. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- 56.Kataoka T, Tschopp J. N-terminal fragment of c-FLIP(L) processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-kappaB signaling pathway. Mol Cell Biol. 2004;24:2627–2636. doi: 10.1128/MCB.24.7.2627-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heist RS, Marshall AL, Liu G, Zhou W, Su L, Neuberg D, Lynch TJ, Wain J, Christiani DC. Matrix metalloproteinase polymorphisms and survival in stage I non-small cell lung cancer. Clin Cancer Res. 2006;12:5448–5453. doi: 10.1158/1078-0432.CCR-06-0262. [DOI] [PubMed] [Google Scholar]
- 59.Lemke J, von Karstedt S, Zinngrebe J, Walczak H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014;21:1350–1364. doi: 10.1038/cdd.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhuri R, McSharry C, Brady J, Donnelly I, Grierson C, McGuinness S, Jolly L, Weir CJ, Messow CM, Spears M, et al. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: relationship to disease severity. J Allergy Clin Immunol. 2012;129:655–663 e658. doi: 10.1016/j.jaci.2011.12.996. [DOI] [PubMed] [Google Scholar]
- 61.Gajewski TF. On the TRAIL toward death receptor-based cancer therapeutics. J Clin Oncol. 2007;25:1305–1307. doi: 10.1200/JCO.2006.09.9804. [DOI] [PubMed] [Google Scholar]