Abstract
Posttraumatic stress disorder (PTSD) and its comorbidities are endemic among injured trauma survivors. Previous collaborative care trials targeting PTSD after injury have been effective, but they have required intensive clinical resources. The present pragmatic clinical trial randomized acutely injured trauma survivors who screened positive on an automated electronic medical record PTSD assessment to collaborative care intervention (n = 60) and usual care control (n = 61) conditions. The stepped measurement-based intervention included care management, psychopharmacology, and psychotherapy elements. Embedded within the intervention were a series of information technology (IT) components. PTSD symptoms were assessed with the PTSD Checklist at baseline prerandomization and again, 1-, 3-, and 6-months postinjury. IT utilization was also assessed. The technology-assisted intervention required a median of 2.25 hours (interquartile range = 1.57 hours) per patient. The intervention was associated with modest symptom reductions, but beyond the margin of statistical significance in the unadjusted model: F(2, 204) = 2.95, p = .055. The covariate adjusted regression was significant: F(2, 204) = 3.06, p = .049. The PTSD intervention effect was greatest at the 3-month (Cohen’s effect size d = 0.35, F(1, 204) = 4.11, p = .044) and 6-month (d = 0.38, F(1, 204) = 4.10, p = .044) time points. IT-enhanced collaborative care was associated with modest PTSD symptom reductions and reduced delivery times; the intervention model could potentially facilitate efficient PTSD treatment after injury.
Physical injury trauma constitutes a major public health problem for U.S. civilian and veteran patient populations (Institute of Medicine, 2012). Each year approximately 30 million American civilians are seen in emergency departments after injury and 1.5 to 2.5 million require hospitalization for the treatment of more severe injuries including traumatic brain injury (TBI; National Center for Injury Prevention, 2012). The symptoms of posttraumatic stress disorder (PTSD) and its comorbid conditions (e.g., depression) are common in physically injured youth and adults (Bryant et al., 2010; O’Donnell et al., 2008; Shalev et al., 1998; Zatzick et al., 2007). After injury, PTSD and its comorbidities are associated with a broad profile of functional impairment (Agency for Healthcare Research & Quality, 2013; O’Donnell, Creamer, Elliott, Atkin, & Kossmann, 2005).
Some, but not all, previous intervention trials have suggested that injured trauma survivors with PTSD symptoms may respond to early cognitive behavioral therapy (CBT) and pharmacologic interventions (Agency for Healthcare Research & Quality, 2013; Bryant et al., 2008; Kassam-Adams et al., 2011; Kearns, Ressler, Zatzick, & Rothbaum, 2012; O’Donnell et al., 2012; Rothbaum et al., 2012). Epidemiologic data, however, have suggested that substantial barriers to accessing evidence-based treatments exist (Geiss Trusz, Wagner, Russo, Love, & Zatzick, 2011; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Shalev, Ankri, Peleg, Israeli-Shalev, & Freedman, 2011). Barriers previously described included multiple competing postinjury demands such as physical health, work and finance, and other major postinjury concerns, as well as active substance-use problems (Geiss Trusz et al., 2011). Effective intervention models that serve to initially engage injured trauma survivors, use care management to address postinjury concerns, address substance use problems, and then deliver evidence-based PTSD services are therefore a crucial element of the early mental health response to trauma exposure (Agency for Healthcare Research & Quality, 2013; Kearns et al., 2012; Roberts, Kitchiner, Kenardy, & Bisson, 2009).
Large-scale randomized trials have established the effectiveness of collaborative care models that integrate care management, pharmacotherapy, and CBT in the treatment of primary care patients with depressive and anxiety disorders (Gilbody, Bower, Fletcher, Richards, & Sutton, 2006). Previous collaborative care investigations have effectively targeted PTSD and comorbidity in injured patients presenting to acute care medical settings (Zatzick et al., 2004, 2013, 2014); these previous treatments have required on average over 10 hours of interventionist time for full protocol implementation. Although the American College of Surgeons has adopted policy mandates for alcohol screening and brief intervention at trauma centers, the inability to briefly and efficiently screen and intervene may present a barrier to implementation of similar policy mandates for PTSD (American College of Surgeons Committee on Trauma, 2006, 2014).
Technologic innovation has begun to impact the development of PTSD screening and intervention procedures within trauma care systems (Price et al., 2014; Ranney et al., 2012; Van Eaton et al., 2014). Population-based automated screening procedures now exist that can enhance the efficient detection of injury survivors at high risk for the development of PTSD (Russo, Katon, & Zatzick, 2013). Similarly, web-based, cell, and smartphone applications have been developed for PTSD screening and intervention (Bush, Bosmajian, Fairall, McCann, & Ciulla, 2011; Mouthaan et al., 2013; Price et al., 2014; Ranney et al., 2012; Ruzek et al., 2011).
This investigation was a randomized effectiveness trial designed to assess whether injured patients participating in a technology-enhanced stepped collaborative care protocol would demonstrate reductions in PTSD symptoms when compared to patients assigned to a usual care control condition. The investigation also explored whether technologic innovation could enhance the efficiency and acceptability of care delivery for PTSD and its comorbidities.
Method
Participants
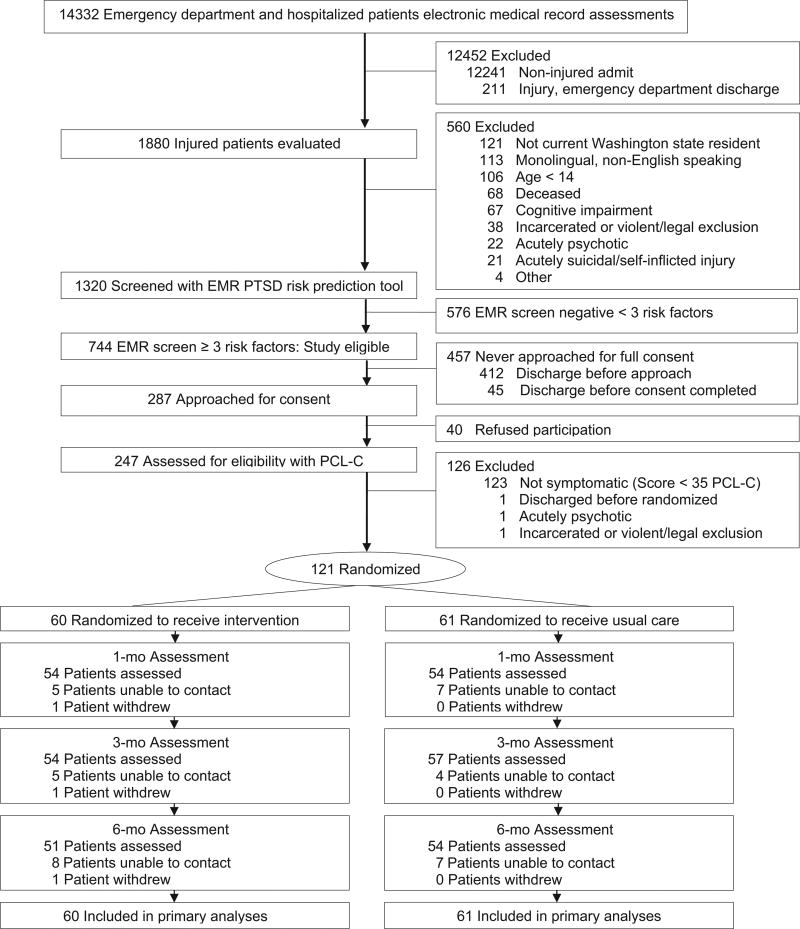
Patients included in the study were ≥ 14 years old, female and male survivors of intentional and unintentional injuries who were admitted to the University of Washington’s Harborview Medical Center Level I trauma center inpatient surgical ward or emergency department for ≥ 24 hours (Figure 1). Consenting patients scoring ≥ 3 on an electronic medical record (EMR) PTSD screen were subsequently evaluated with the 17-item PTSD Checklist-Civilian Version (PCL-C; see further description below) and those with a score of ≥ 35 were included. Prior investigation with the PCL-C in general medical settings informed the decision to use the cutpoint of ≥ 35 on the measure for study inclusion (Bliese et al., 2008; Walker et al., 2003; Zatzick et al., 2013).
Figure 1.
Flow of participants through the trial. EMR = electronic medical record; PCL-C = PTSD Checklist-Civilian version; PTSD = posttraumatic stress disorder.
The 121 injured patients recruited and randomized into the investigation were predominantly publically insured or uninsured patients with histories of multiple prior traumatic life events before the index injury admission (Table 1). At baseline, prior to randomization, 87 (71.9%) patients reported having access to a cell phone. Although 48 (39.7%) patients reported that they owned or had access to a smartphone at baseline prior to randomization, less than 10.0% of patients had a smartphone available for the download of applications.
Table 1.
Baseline Patient Characteristics
| Variable | All (N = 121)
|
Intervention (n = 60)
|
Usual care (n = 61)
|
|||
|---|---|---|---|---|---|---|
| n or M | % or SD | n or M | % or SD | n or M | % or SD | |
| Age, years | 43.17 | 14.69 | 42.80 | 14.65 | 43.52 | 14.84 |
| Female | 43 | 35.5 | 21 | 35.0 | 22 | 36.1 |
| Race/ethnicity | ||||||
| White | 55 | 45.4 | 24 | 40.0 | 31 | 50.9 |
| Black | 26 | 21.5 | 18 | 30.0 | 8 | 13.1 |
| American Indian | 19 | 15.7 | 6 | 10.0 | 13 | 21.3 |
| Asian | 6 | 5.0 | 3 | 5.0 | 3 | 4.9 |
| Hispanic | 15 | 12.4 | 9 | 15.0 | 6 | 9.8 |
| Education, years | 12.43 | 2.22 | 12.83 | 2.62 | 12.02 | 1.67 |
| Marital status | ||||||
| Married/living with partner | 27 | 22.3 | 13 | 21.7 | 14 | 23.0 |
| Employed | 45 | 37.2 | 24 | 40.0 | 21 | 34.4 |
| Insurance | ||||||
| Private | 19 | 15.7 | 8 | 13.3 | 11 | 18.0 |
| Public | 76 | 62.8 | 39 | 65.0 | 37 | 60.7 |
| None | 26 | 21.5 | 13 | 21.7 | 13 | 21.3 |
| Acute care injury & medical | ||||||
| Injury severity category | ||||||
| 0–8 | 36 | 29.7 | 20 | 33.3 | 16 | 26.2 |
| 9–15 | 33 | 27.3 | 15 | 25.0 | 18 | 29.5 |
| ≥ 16 | 52 | 43.0 | 25 | 41.7 | 27 | 44.3 |
| Traumatic brain injury | ||||||
| None | 78 | 64.5 | 37 | 61.6 | 41 | 67.2 |
| Mild | 20 | 16.5 | 13 | 21.7 | 7 | 11.5 |
| Moderate/severe | 23 | 19.0 | 10 | 16.7 | 13 | 21.3 |
| Intentional injurya | 34 | 28.1 | 19 | 31.7 | 15 | 24.6 |
| Comorbid medical conditions | ||||||
| 0 | 43 | 35.5 | 24 | 40.0 | 19 | 31.2 |
| 1 | 30 | 24.8 | 14 | 23.30 | 16 | 26.2 |
| 2 | 26 | 21.5 | 10 | 16.70 | 16 | 26.2 |
| ≥ 3 | 22 | 18.2 | 12 | 20.0 | 10 | 16.4 |
| Days in ICU | ||||||
| 0 | 45 | 37.2 | 24 | 40.0 | 21 | 34.4 |
| 1 | 20 | 16.5 | 12 | 20.0 | 8 | 13.1 |
| ≥ 2 | 56 | 46.3 | 24 | 40.0 | 32 | 52.5 |
| Days in hospital | 12.45 | 15.10 | 10.98 | 14.87 | 13.89 | 15.31 |
| Clinical | ||||||
| Prior traumas before admission | 5.86 | 3.10 | 5.68 | 2.97 | 6.04 | 3.23 |
| Preinjury PTSD symptoms | 57 | 47.1 | 25 | 41.7 | 32 | 52.5 |
| Baseline PCL-C totalb | 46.98 | 10.47 | 46.97 | 10.16 | 47 | 10.85 |
| Baseline PHQ-9 depression totalb | 14.66 | 5.36 | 14.32 | 5.68 | 15 | 5.04 |
| Preinjury AUDIT-C total | 4.34 | 3.65 | 4.45 | 3.38 | 4.23 | 3.92 |
| Blood alcohol + on admission | 43 | 35.5 | 25 | 41.7 | 18 | 29.5 |
| Screen + for stimulants | 20 | 16.5 | 11 | 18.3 | 9 | 14.80 |
| Screen + for marijuana | 13 | 10.7 | 5 | 8.3 | 8 | 13.1 |
Note. No comparisons were statistically significant. PTSD = posttraumatic stress disorder; PCL-C = PTSD Checklist–Civilian version; PHQ-9 = Patient Health Questionnaire; ICU = intensive care unit; AUDIT-C = The Alcohol Use Disorders Identification Test–Consumption items.
Intentional injury includes gunshots, physical assault, and stabbings.
For PCL-C and PHQ-9 baseline assessments, inpatients were asked to report symptoms since the injury event.
Over the course of the 6 months after the injury, 20.7% of usual care patients reported visiting a psychiatrist, 19.0% a psychologist, and 20.7% a mental health counselor. Overall 41.0% of usual care patients reported visits to these mental health providers over the course of the 6 months after injury.
To maximize study generalizability and population impact, the study aimed to minimize exclusion criteria (Koepsell, Zatzick, & Rivara, 2011). Patients were only excluded if they required immediate psychiatric intervention (i.e., self-inflicted injury, active psychosis), were not Washington State residents, or were currently incarcerated.
Procedure
A previously developed EMR screen was used to assess the population of admitted injured trauma survivors at risk for the development of PTSD (Russo et al., 2013). The screen utilized 10 data elements that are both associated with increased risk for PTSD and that are readily available in any robust EMR system. The 10 elements related to PTSD risk were (a) EMR PTSD ICD diagnosis according to the International Classification of Diseases (ICD); (b) any other comorbid ICD psychiatric diagnosis; (c) any ICD substance use disorder, tobacco use, or positive blood alcohol concentration on admission; (d) any chronic ICD medical comorbidities; (e) injury (E) code indicative of an intentional injury; (f) Intensive care unit (ICU) admission during the current hospitalization; (g) any EMR documentation of prior trauma center inpatient hospitalizations; and (h–j) demographic characteristics including female sex, non-White ethnicity, and low income or veterans insurance status. When the 10 data elements were used to predict scores on the PCL-C of ≥ 35, the EMR screen demonstrated adequate sensitivity (.71), specificity (.66), and area under the ROC curve (.72; Russo et al., 2013).
Follow-up interviews were conducted over the telephone at 1-, 3-, and 6-months postinjury and patients were reimbursed $35, $35, and $40, respectively, in addition to receiving $30 for completion of the baseline interview. The University of Washington Institutional Review Board approved all study procedures prior to protocol initiation and written informed consent was obtained from each participant. Study recruitment occurred over an 8-month period from July 1,2012 through February 28, 2013. The 6-month follow-up was completed by August 31, 2013.
Randomization occurred in a 1:1 ratio according to a computer-generated random assignment sequence prepared by the study statistician. Research associates conducting all baseline screening assessments and follow-up interviews were blinded to patient intervention or usual care group status.
Patients in the usual care condition underwent informed consent, both EMR and in-person PTSD screenings, baseline surgical ward evaluation, and follow-up interviews. As an enhancement to usual care, immediately after randomization, patients in the usual care condition were delivered a study laptop. Otherwise these patients received postinjury care as usual; prior investigation suggested that usual postinjury care included routine outpatient surgical, primary care, and emergency department visits, as well as the use of specialty mental health services (Zatzick et al., 2004, 2013, 2014)
Patients randomized to the intervention condition received stepped measurement-based care from a trauma center-based mental health team over the course of the 6 months postinjury. The intervention team included doctoral-level care management and behavioral therapy (S.O.) and the medical pharmacotherapy (D.Z.) interventionists. As in previous trials, stepped collaborative care intervention elements included postinjury care management and pharmacotherapy targeting PTSD and its comorbidities, motivational interviewing (MI), and CBT elements embedded within routine care management (Zatzick et al., 2004, 2013, 2014).
The stepped care intervention began with the delivery of a laptop computer by the bedside to all intervention patients. Patients were instructed to use the computer for whatever purpose they found helpful after their injury including e-mail, social networking, or obtaining informational material on postinjury medical and psychiatric conditions, including PTSD. The laptop computer web browser had a bookmark for the afterdeployment.org website; afterdeployment.org provides trauma-exposed individuals with self-assessments, self-management strategies, videos, and other materials that address a number of topics including physical injury and TBI, PTSD, alcohol and drug use, and resilience after trauma exposure (Bush et al., 2011; Ruzek et al., 2011). LifeArmor is an accompanying smartphone application that contains all the afterdeployment.org materials. The study care manager (S.O.) was trained in the assistance of web-based and smartphone technology use and was available to assist injured patients in reviewing the afterdeployment.org website and LifeArmor smartphone applications. Intervention patients were encouraged to use the afterdeployment.org and LifeArmor applications both in the hospital and after hospital discharge, over the course of the 6 months after injury. These initial technology-related intervention activities aimed to provide educational content regarding PTSD and comorbidity after trauma exposure to the patient; these intervention activities also aimed to enhance the establishment of a therapeutic alliance between the patient and care manager by allowing the patient and care manager to discuss questions regarding presented materials.
As with prior collaborative care intervention protocols, patients randomized to the intervention condition discussed PTSD treatment preferences with the care manager. To engage injured trauma survivors in patient-centered care, care managers first elicited and attempted to ameliorate each patient’s unique constellation of postinjury concerns. Care managers coordinated care across surgical inpatient, primary care, and community service delivery settings.
The interventionist was trained in the delivery of evidence-based MI intervention targeting problematic alcohol use and other behaviors that risk recurrent injury, such as weapon carrying. The interventionist was also trained in the delivery of stepped CBT elements targeting PTSD and depressive symptoms. The MI and CBT elements were designed to be flexibly delivered both during inpatient stays and to outpatients. As with broad reach MI interventions, the CBT elements were designed to be readily deliverable within routine care management. The CBT elements included problem solving, psychoeducation, anxiety reduction techniques such as training in progressive muscle relaxation and breathing, attention to experience, and exposure-based and pleasant activities scheduling interventions targeting anxiety and depressive symptoms (Geiss Trusz et al., 2011). These elements were given in a stepped fashion such that elements with greater ease of delivery such as problem solving and psychoeducation were given initially, followed later by the delivery of more complex elements such as activities scheduling. The medication intervention component aimed to initiate and maximize adherence to psychopharmacological treatments targeting PTSD and related disturbances, such as insomnia.
The intervention was designed as a stepped measurement-based care procedure. Intervention patients’ symptoms were repeatedly measured and higher-intensity care was available for patients with persistent or recurrent symptoms of PTSD and comorbidity. The investigative team developed and refined a computerized decision support tool as part of the stepped measurement-based collaborative care intervention. The decision support tool facilitated real-time workflow integrated screening and intervention procedures targeting the full spectrum of PTSD and its comorbidities (Engel et al., 2008; Unützer, Choi, Cook, & Oishi, 2002). The collaborative care interventionist received 1–2 hours of decision support tool facilitated coaching per week from the principal investigator (D.Z.). For example, the supervising physician would independently review cases in the tool prior to staffing rounds and record recommendations for care in the tool staffing note module; these staffing notes were available to the intervention team in real time and could be reviewed both prior to and during staffing rounds to guide clinical treatment decisions. The appropriateness of cases for the intensified stepping up of medication and CBT intervention elements was discussed during supervision sessions. Preestablished criteria for symptomatic improvement (e.g., < 50% reduction in baseline PTSD and/or depressive symptom levels at 1- and 3-month postinjury time points) were used in team discussions to inform care intensification with medication and CBT elements.
The decision support tool consisted of University of Washington ITS-provided virtual machine server space running Windows Server 2008, IIS, SQL Server 2008, and ASP.NET. Patient data were entered in real time into the web application front end to populate the project’s SQL database. The interventionist used the web application’s front end to display a variety of care management decision support purposes. These included display of scores for the automated EMR screen and longitudinal PTSD and depressive symptom assessments for intervention patients, documentation of inpatient, outpatient, and telephone patient and provider contacts, and the use of supervisory notes to help clarify care plans and coordinate care from the trauma center to primary care and community settings.
Measures
For all hospitalized inpatients, PTSD symptoms were assessed with the PCL-C (Weathers, Keane, & Davidson, 2001). The PCL-C has established reliability and validity across trauma-exposed populations. In the current protocol, the average Cronbach’s α for the PCL-C across the four study time points was .86 (range = .71 to .92).
The Patient Health Questionnaire-9 Item Depression Screen (PHQ-9) was used as a continuous measure to assess depressive symptoms (Kroenke, Spitzer, & Williams, 2001). The questionnaire has established reliability and validity in acute and primary care medical patients (Kroenke et al., 2001). The average Cronbach’s α for the PHQ-9 across the four study time points was .83 (range = .73 to .89).
An abbreviated 3-item version of The Alcohol Use Disorders Identification Test (AUDIT-C), was used to assess alcohol use problems before and after the injury hospitalization (Bradley et al., 2007). Previously developed items assessing postinjury technology, medication, and health service utilization were administered at baseline and at the 1-, 3-, and 6-month follow-up interviews (Bush, Fullerton, Crumpton, Metzger-Abamukong, & Fantelli, 2012; Ranney et al., 2012). Technology items assessed cell and smartphone access and barriers, as well as the use of and satisfaction with web-based and smartphone applications, and the use of e-mail and text message to communicate health information.
Preinjury trauma was assessed with a modified version of the trauma history screen developed for the National Co-morbidity Study (Kessler et al., 1995). Interview items included patient self-report descriptions of current medication usage (i.e., name, dosage, duration). Previously developed items assessing satisfaction with general health care services were included in all interviews (Zatzick et al., 2013).
The investigation determined injury severity at baseline during the index admission from the medical record ICD 9 codes using the Abbreviated Injury Scale and Injury Severity Score (Johns Hopkins Health Services Research and Development Center, 1989). TBI was also prospectively identified in the medical record (Zatzick et al., 2013). Race and ethnicity were assessed through patient self-report. Laboratory toxicology results, insurance status, length of hospital and ICU stays, and other clinical characteristics were abstracted from the EMR.
Data Analysis
The investigation examined PTSD symptoms and other outcomes longitudinally for the intent to treat sample using data for all randomized patients. The primary outcome analysis examined repeated measurements of the PCL-C continuous scale scores at the 1-, 3-, and 6-month postinjury time points. To determine if patients in the intervention and usual care groups manifested different patterns of change in PCL-C scores over the course of the 6 months after injury, the study used mixed effects random coefficient regression models (Gibbons, Hedeker, & DuToit, 2010). Initial models were adjusted only for baseline group differences in PCL-C scores. Subsequent models included additional adjustments for previously established design variables (sex, age, race, and injury severity; Zatzick et al., 2004, 2013). Mixed model regression was also used to deter-mine if intervention patients had differential patterns of depressive symptoms, psychotropic medication usage, or satisfaction with care over the course of the 6 months postinjury. For all dependent variables, models were fit containing time categories, intervention, and intervention × time interactions. The number needed to treat (NNT), defined as the percentage of patients at the 6-month postinjury time point with a ≥ 10 point reduction from baseline in PCL-C, was also assessed. Finally, the study team performed sensitivity analyses that included imputed values for missing outcome data.
Results
Compared to all other patients admitted to the trauma center during the period of the investigation, the 121 randomized study patients were significantly more likely to be intentionally injured (not self-inflicted), younger, blood alcohol positive, admitted to the ICU, and have an overall greater length of hospital stay (Figure 1).
The intervention required a median time of 2.25 hours (interquartile range = 1.57 hours) per patient. Time intensity in the stepped care procedure gradually decreased over the course of the 6 months after injury; approximately 80% of all intervention activity occurred within the first 3 months postinjury. All patients, however, did receive some intervention between 3 and 6 months after the injury.
Of intervention patients, 37 (61.7%) received one or more motivational interviews targeting either substance use or risk behaviors. The willingness and readiness of all patients in the trial to begin pharmacotherapy was assessed. There were 44 (73.3%) intervention patients who expressed adequate readiness to have pharmacotherapy targeting high PTSD and depressive symptom levels; 27 (45.0%) of these patients adhered to their medication regimes during the study. The willingness and readiness of all patients in the trial to enter CBT was assessed. We offered CBT to the 35 (58.3%) patients who expressed an interest in CBT and demonstrated adequate CBT readiness; 14 (23.3%) of those patients received one or more CBT elements delivered during routine care management. Only two individuals entered and completed five CBT sessions.
Over the 6 months after the injury, more than 75.0% of usual care patients and over 85.0% of intervention patients reported either a change in their phone or phone number (comparison not statistically significant, see Table 2). Compared to usual care patients, intervention patients were significantly more likely to report using afterdeployment.org over the course of the weeks and months after the index injury hospitalization (χ2 (1, N = 121) = 7.31, p = .01; Table 2).
Table 2.
Use of Information Technology Care Processes by Group
| Variable | Intervention (n = 60)
|
Usual care (n = 61)
|
||
|---|---|---|---|---|
| n or M | % or SD | n or M | % or SD | |
| Hospital IT use | ||||
| Laptop used | 37 | 61.7 | 33 | 54.1 |
| Total laptop use in minutes | 87.36 | 100.38 | 95.96 | 77.37 |
| afterdeployment.org used | 37 | 61.7 | 32 | 52.4 |
| Total use of afterdeployment.org in minutes | 24.76 | 42.51 | 16.05 | 26.82 |
| Use/barriers of IT over 6 months postinjury | ||||
| afterdeployment.org useda | 19 | 31.7 | 7 | 11.5 |
| LifeArmor smartphone app used | 7 | 11.7 | 3 | 4.9 |
| E-mail used to receive health information | 20 | 33.3 | 13 | 21.3 |
| Internet websites used for health information | 33 | 55.0 | 26 | 42.6 |
| Social networking used for health information | 7 | 11.7 | 7 | 11.5 |
| Text messaging used for health information | 5 | 8.3 | 5 | 8.2 |
| Any reported change (phone and/or phone number) | 51 | 86.4 | 46 | 75.4 |
| Change of physical phone since hospitalization | 43 | 76.8 | 39 | 66.1 |
| Change of phone number since hospitalization | 37 | 62.7 | 37 | 60.7 |
Note. N = 121. IT = information technology.
χ2 yielded p ≤ .05.
Intervention patients were significantly more likely to take antidepressant medications than usual care patients, relative risk (RR) = 1.66, 95% confidence interval (CI) [1.08, 2.57], and were more likely to have received an adequate dosage of antidepressant medication, RR = 2.32, 95% CI [1.31, 4.12]. Intervention patients were also significantly more likely to use PTSD insomnia medications, RR = 2.02, 95% CI [1.02, 4.01]. There were no observed significant group, time, or group × time effects for psychotherapy visits over the course of the 6 months after injury. Over the course of the 6-month postinjury intervention, patients demonstrated greater satisfaction with care relative to usual care patients, F(2, 199) = 3.81, p = .023.
The intervention group demonstrated modest reductions in PTSD symptoms over the course of the 6 months after injury, but beyond the margin of statistical significance in the unadjusted regression, group × time interaction, F(2, 204) = 2.95, p = .055; however, the covariate adjusted regression, group × time interaction was significant, F(2, 204) = 3.06, p = .049. The intervention effect sizes for PTSD symptoms were greatest at the 3-month, d = 0.35, F(1, 204) = 4.11, p = .044, and 6-month postinjury time points, d = 0.38, F(1, 204) = 4.10, p = .044 (Table 3). At the 6-month postinjury time point 45% of intervention patients versus 30% of usual care patients demonstrated a > 10 point reduction from baseline on the PCL-C (NNT = 6.5).
Table 3.
Comparison of Mean PTSD (PCL-C) and Depression (PHQ-9) Total Symptoms by Group at Three Follow-Up Points
| Variable | Baselinea
|
1 Monthb
|
3 Monthb
|
6 Monthb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC M |
95% CI | UC M |
95% CI | CC M |
95% CI | UC M |
95% CI | d | CC M |
95% CI | UC M |
95% CI | d | CC M |
95% CI | UC M |
95% CI | d | |
| PTSD | 46.91 | [43.22, 50.60] | 47.66 | [43.99,51.33] | 44.37 | [40.60,48.14] | 44.64 | [40.92, 48.37] | 0.00 | 41.17 | [37.33, 44.82] | 46.4c | [42.77,50.12] | 0.35 | 42.16 | [38.36, 45.97] | 47.61° | [43.89,51.34] | 0.38 |
| DEP | 14.23 | [12.60, 15.85] | 15.17 | [13.54, 16.79] | 13.39 | [11.80, 14.98] | 14.00 | [12.42, 15.57] | 0.09 | 12.59 | [11.01, 14.17] | 14.11 | [12.56, 15.67] | 0.27 | 12.82 | [11.21, 14.43] | 14.21 | [12.62, 15.79] | 0.24 |
Note. N = 121, collaborative care intervention n = 60, usual care control n = 61. CC = collaborative care intervention; UC = usual care control; PTSD = posttraumatic stress disorder; PCL-C = PTSD Checklist-Civilian version; PHQ-9 = Patient Health Questionnaire.
Adjusted for age, gender, injury severity score, and race.
Adjusted for baseline PCL-C score, age, gender, injury severity score, and race.
t statistic from mixed model regression yielded p ≤ .05.
Although intervention patients showed a pattern of improved depression treatment effects relative to usual care patients, no clinically or statistically significant effects were observed for depressive symptoms over time (Table 3). Sensitivity analyses did not substantially alter the magnitude, pattern, or significance of the observed treatment effects.
Discussion
The results of this investigation suggested that an information technology- (IT-) enhanced stepped collaborative care intervention was associated with modest PTSD symptom reductions and reduced delivery times; the observed reductions in PTSD symptoms were not significant in unadjusted analyses, but were significant in adjusted analyses. Intervention patients had a more favorable course of PTSD recovery as evidenced by diminished PTSD symptom severity at the 3- and 6-month postinjury time points. Intervention patients received higher quality post-traumatic care that included more frequent prescriptions for evidence-based PTSD pharmacotherapy and also greater satisfaction with health care services.
This was one of the first investigations to describe processes of care associated with the introduction of IT-enhanced collaborative care. The investigation introduced a number of innovative web and smartphone applications, and a computerized clinical decision support tool. The time required to deliver the intervention was markedly diminished when compared to prior collaborative care intervention trials that did not incorporate intervention technology enhancements. As an example, a prior stepped collaborative care intervention required a median of 13.2 hours per patient (interquartile range = 13.3 hours) with similar end-of-study treatment effects as the current investigation (Zatzick et al., 2013). Multiple intervention components could have contributed to the observed reductions in intervention time required, including the ability to connect to preinjury social supports through the use of the study laptop and efficiencies introduced by the study decision support tool. Other nontechnological factors such as the introduction of a highly trained doctoral-level care manager could have contributed to the observed reduction in time required to deliver the intervention.
The current investigation was one of a series of studies that attempts to deliver brief effective interventions early on after trauma exposure (Mouthaan et al., 2013; O’Donnell et al., 2012; Rothbaum et al., 2012). Rothbaum and colleagues have developed a brief exposure-based intervention deliverable from emergency departments; the 3 hours required to deliver the exposure-based treatment is comparable to the time required for the IT-enhanced collaborative care treatment.
This study had limitations. To begin, because this was a multifaceted intervention, the investigation did not yield information regarding which components of the treatment were effective. Thus, we cannot conclude that the addition of the IT component to the stepped collaborative care intervention was associated with any observed PTSD treatment effects. Future investigations could test specific intervention components (e.g., pharmacotherapy, CBT elements, IT enhancements) or attempt to dismantle the effects of these individual components of the multifaceted intervention. The study used only patient self-report measures to substantiate PTSD symptom severity. Also, although the majority of intervention activity occurred during the first 3 months after the injury, the intervention extended up until the 6-month injury time point and the investigation did not conduct a formal posttreatment outcome assessment. An additional limitation of the study was the decision to use a usual care control comparison condition; usual care control conditions are frequently employed in effectiveness spectrum randomized clinical trial designs that aim to assess treatment effects and understand the delivery of new treatment models relative to usual practice (Curran, Bauer, Mittman, Pyne, & Stetler, 2012; Flay, 1986; Zatzick, Simon, & Wagner, 2006).
Beyond these considerations, this investigation contributes to an evolving literature on early posttraumatic interventions for individuals treated in real-world nonspecialty mental health settings (Hobfoll et al., 2007; Kassam-Adams et al., 2011). Prior investigation suggests that early PTSD interventions could productively focus on maximizing both treatment effects and breadth of applicability to optimize overall population impact; technology-enhanced stepped collaborative care is associated with the efficient delivery of PTSD treatment which has the potential to enhance intervention breadth of applicability, and ultimately population impact (Koepsell et al., 2011). Future early PTSD intervention studies that combine efficiencies in treatment introduced by IT enhancements with more in-depth understanding of postinjury recovery trajectories (Galatzer-Levy et al., 2013; Osenbach et al., 2014) could simultaneously enhance PTSD treatment effects and early intervention efficiency, thus optimizing overall population impact. The American College of Surgeons has demonstrated the capacity to mandate screening and intervention procedures for alcohol use problems at U.S. trauma centers based on the results of empiric investigations, and now recommends PTSD screening and intervention as a best practice clinical guideline (American College of Surgeons Committee on Trauma, 2006, 2014). Orchestrated investigative and policy efforts could systematically evaluate multisite IT-enhanced screening and intervention procedures for PTSD and comorbidity (American College of Surgeons Committee on Trauma, 2006, 2014).
Acknowledgments
This work was supported by National Institute of Mental Health Grants K24MH086814 and 1UH2MH106338.
Footnotes
Trial Registration: Clinicaltrials.gov identifier-NCT01625416.
References
- Agency for Healthcare Research & Quality. Effective Health Care Program. Vol. 92. Rockville, MD: Author; 2013. Psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD) p. 760. [PubMed] [Google Scholar]
- American College of Surgeons Committee on Trauma. Resources for optimal care of the injured patient. Washington, DC: Author; 2006. [Google Scholar]
- American College of Surgeons Committee on Trauma. Resources for optimal care of the injured patient. Washington, DC: Author; 2014. [Google Scholar]
- Bliese PD, Wright KM, Adler AB, Cabrera O, Castro CA, Hoge CW. Validating the primary care posttraumatic stress disorder screen and the posttraumatic stress disorder checklist with soldiers returning from combat. Journal of Consulting and Clinical Psychology. 2008;76:272–281. doi: 10.1037/0022-006X.76.2.272. [DOI] [PubMed] [Google Scholar]
- Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcoholism, Clinical and Experimental Research. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Mastrodomenico J, Felmingham KL, Hopwood S, Kenny L, Kandris E, Creamer M. Treatment of acute stress disorder: A randomized controlled trial. Archives of General Psychiatry. 2008;65:659–667. doi: 10.1001/archpsyc.65.6.659. [DOI] [PubMed] [Google Scholar]
- Bryant RA, O’Donnell ML, Creamer M, McFarlane AC, Clark CR, Silove D. The psychiatric sequelae of traumatic injury. The American Journal of Psychiatry. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- Bush NE, Bosmajian CP, Fairall JM, McCann RA, Ciulla RP. Afterdeployment.org: A web-based multimedia wellness resource for the postdeployment military community. Professional Psychology Research and Practice. 2011;42:455–462. doi: 10.1037/A0025038. [DOI] [Google Scholar]
- Bush NE, Fullerton N, Crumpton R, Metzger-Abamukong M, Fantelli E. Soldiers’ personal technologies on deployment and at home. Telemedicine Jounal and E-Health. 2012;18:253–263. doi: 10.1089/tmj.2011.0131. [DOI] [PubMed] [Google Scholar]
- Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical Care. 2012;50:217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel CC, Oxman T, Yamamoto C, Gould D, Barry S, Stewart P, Dietrich AJ. RESPECT-Mil: Feasibility of a systems-level collaborative care approach to depression and post-traumatic stress disorder in military primary care. Military Medicine. 2008;173:935–940. doi: 10.7205/MILMED.173.10.935. [DOI] [PubMed] [Google Scholar]
- Flay BR. Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs. Preventive Medicine. 1986;15:451–474. doi: 10.1016/0091-7435(86)90024-1. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M, Shalev AY. Early PTSD symptom trajectories: Persistence, recovery, and response to treatment: Results from the Jerusalem trauma outreach and prevention study (J-TOPS) PloS One. 2013;8:e70084. doi: 10.1371/journal.pone.0070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss Trusz S, Wagner A, Russo J, Love J, Zatzick D. Assessing barriers to care and readiness for cognitive behavioral therapy in early acute care PTSD interventions. Psychiatry. 2011;74:207–223. doi: 10.1521/psyc.2011.74.3.207. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annual Review of Clinical Psychology. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: A cumulative meta-analysis and review of longer-term outcomes. Archives of Internal Medicine. 2006;166:2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- Hobfoll SE, Watson P, Bell CC, Bryant RA, Brymer MJ, Friedman MJ, Ursano RJ. Five essential elements of immediate and mid-term mass trauma intervention: Empirical evidence. Psychiatry. 2007;70:283–315. doi: 10.1521/psyc.2007.70.4.283. discussion 316-269. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Treatment for posttraumatic stress disorder in military and veteran populations: Initial assessment. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- Johns Hopkins Health Services Research and Development Center. Determining injury severity from hospital discharges: A program to map ICD-9-CM diagnoses into AIS and ISS severity scores. Baltimore, MD: The Johns Hopkins University Press; 1989. [Google Scholar]
- Kassam-Adams N, Felipe Garcia-Espana J, Marsac ML, Kohser KL, Baxt C, Nance M, Winston F. A pilot randomized controlled trial assessing secondary prevention of traumatic stress integrated into pediatric trauma care. Journal of Traumatic Stress. 2011;24:252–259. doi: 10.1002/jts.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO. Early interventions for PTSD: A review. Depression and Anxiety. 2012;29:833–842. doi: 10.1002/da.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koepsell TD, Zatzick DF, Rivara FP. Estimating the population impact of preventive interventions from randomized trials. American Journal of Preventive Medicine. 2011;40:191–198. doi: 10.1016/j.amepre.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthaan J, Sijbrandij M, de Vries GJ, Reitsma JB, van de Schoot R, Goslings JC, Olff M. Internet-based early intervention to prevent posttraumatic stress disorder in injury patients: Randomized controlled trial. Journal of Medical Internet Research. 2013;15:e165. doi: 10.2196/jmir.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell ML, Creamer M, Elliott P, Atkin C, Kossmann T. Determinants of quality of life and role-related disability after injury: Impact of acute psychological responses. Journal of Trauma. 2005;59:1328–1334. doi: 10.1097/01.ta.0000197621.94561.4e. [DOI] [PubMed] [Google Scholar]
- O’Donnell ML, Creamer MC, Parslow R, Elliott P, Holmes AC, Ellen S, Bryant RA. A predictive screening index for posttraumatic stress disorder and depression following traumatic injury. Journal of Consulting and Clinical Psychology. 2008;76:923–932. doi: 10.1037/a0012918. [DOI] [PubMed] [Google Scholar]
- O’Donnell ML, Lau W, Tipping S, Holmes AC, Ellen S, Judson R, Forbes D. Stepped early psychological intervention for posttraumatic stress disorder, other anxiety disorders, and depression following serious injury. Journal of Traumatic Stress. 2012;25:125–133. doi: 10.1002/jts.21677. [DOI] [PubMed] [Google Scholar]
- Osenbach JE, Lewis C, Rosenfeld B, Russo J, Ingraham LM, Peterson R, Zatzick DF. Exploring the longitudinal trajectories of posttraumatic stress disorder in injured trauma survivors. Psychiatry Interpersonal and Biological Processes. 2014;77:386–397. doi: 10.1521/psyc.2014.77.4.386. [DOI] [PubMed] [Google Scholar]
- National Center for Injury Prevention. CDC 2012. Atlanta, GA: Center for Disease Control and Prevention; 2012. [Google Scholar]
- Price M, Ruggiero KJ, Ferguson PL, Patel SK, Treiber F, Couillard D, Fahkry SM. A feasibility pilot study on the use of text messages to track PTSD symptoms after a traumatic injury. General Hospital Psychiatry. 2014;36:249–254. doi: 10.1016/j.genhosppsych.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney ML, Choo EK, Wang Y, Baum A, Clark MA, Mello MJ. Emergency department patients’ preferences for technology-based behavioral interventions. Annals of Emergency Medicine. 2012;60:218–227. e248. doi: 10.1016/j.annemergmed.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Systematic review and meta-analysis of multiple-session early interventions following traumatic events. The American Journal of Psychiatry. 2009;166:293–301. doi: 10.1176/appi.ajp.2008.08040590. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, Houry D. Early intervention may prevent the development of posttraumatic stress disorder: A randomized pilot civilian study with modified prolonged exposure. Biological Psychiatry. 2012;72:957–963. doi: 10.1016/j.biopsych.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Katon W, Zatzick D. The development of a population-based automated screening procedure for PTSD in acutely injured hospitalized trauma survivors. General Hospital Psychiatry. 2013;35:485–491. doi: 10.1016/j.genhosppsych.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzek JI, Hoffman J, Ciulla R, Prins A, Kuhn E, Gahm G. Bringing Internet-based education and intervention into mental health practice: Afterdeployment.org. European Journal of Psychotraumatology. 2011;2 doi: 10.3402/ejpt.v2i0.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Ankri YL, Peleg T, Israeli-Shalev Y, Freedman S. Barriers to receiving early care for PTSD: Results from the Jerusalem trauma outreach and prevention study. Psychiatric Services. 2011;62:765–773. doi: 10.1176/appi.ps.62.7.765. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK. Prospective study of posttraumatic stress disorder and depression following trauma. American Journal of Psychiatry. 1998;155:630–637. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- Unützer J, Choi Y, Cook IA, Oishi S. A web-based data management system to improve care for depression in a multicenter clinical trial. Psychiatric Services. 2002;53:671–673. 678. doi: 10.1176/ps.53.6.671. [DOI] [PubMed] [Google Scholar]
- Van Eaton EG, Zatzick D, Gallagher T, Flum D, Tarczy-Hornoch P, Rivara F, Maier R. A nationwide survey of trauma center information technology and electronic medical record leverage capacity. Journal of the American College of Surgeons. 2014;219:505–510. doi: 10.1016/j.jamcollsurg.2014.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Katon W, Russo J, Ciechanowski P, Newman E, Wagner AW. Health care costs associated with postraumatic stress disorder symptoms in women. Archives of General Psychiatry. 2003;60:369–374. doi: 10.1001/archpsyc.60.4.369. [DOI] [PubMed] [Google Scholar]
- Weathers F, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depression and Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Zatzick D, Jurkovich G, Rivara FP, Russo J, Wagner A, Wang J, Katon W. A randomized stepped care intervention trial targeting posttraumatic stress disorder for surgically hospitalized injury survivors. Annals of Surgery. 2013;257:390–399. doi: 10.1097/SLA.0b013e31826bc313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatzick D, Rivara FP, Nathens AB, Jurkovich GJ, Wang J, Fan MY, Mackenzie EJ. A nationwide US study of post-traumatic stress after hospitalization for physical injury. Psychological Medicine. 2007;37:1469–1480. doi: 10.1017/S0033291707000943. [DOI] [PubMed] [Google Scholar]
- Zatzick D, Roy-Byrne P, Russo J, Rivara F, Droesch R, Wagner A, Katon W. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Archives of General Psychiatry. 2004;61:498–506. doi: 10.1001/archpsyc.61.5.498. [DOI] [PubMed] [Google Scholar]
- Zatzick D, Russo J, Lord SP, Varley C, Wang J, Berliner L, Rivara FP. Collaborative care intervention targeting violence risk behaviors, substance use, and posttraumatic stress and depressive symptoms in injured adolescents: A randomized clinical trial. JAMA Pediatrics. 2014;168:532–539. doi: 10.1001/jamapediatrics.2013.4784. [DOI] [PubMed] [Google Scholar]
- Zatzick D, Simon GE, Wagner AW. Developing and implementing randomized effectiveness trials in general medical settings. Clinical Psychology: Science and Practice. 2006;13:53–68. doi: 10.1111/j.1468-2850.2006.00006.x. [DOI] [Google Scholar]