Abstract
Regenerating tissue must initiate the signaling that drives regenerative growth, and sustain that signaling long enough for regeneration to complete. How these key signals are sustained is unclear. To gain a comprehensive view of the changes in gene expression that occur during regeneration, we performed whole-genome mRNAseq of actively regenerating tissue from damaged Drosophila wing imaginal discs. We used genetic tools to ablate the wing primordium to induce regeneration, and carried out transcriptional profiling of the regeneration blastema by fluorescently labeling and sorting the blastema cells, thus identifying differentially expressed genes. Importantly, by using genetic mutants of several of these differentially expressed genes we have confirmed that they have roles in regeneration. Using this approach, we show that high expression of the gene moladietz (mol), which encodes the Duox-maturation factor NIP, is required during regeneration to produce reactive oxygen species (ROS), which in turn sustain JNK signaling during regeneration. We also show that JNK signaling upregulates mol expression, thereby activating a positive feedback signal that ensures the prolonged JNK activation required for regenerative growth. Thus, by whole-genome transcriptional profiling of regenerating tissue we have identified a positive feedback loop that regulates the extent of regenerative growth.
Author summary
Regenerating tissue must initiate the signaling that drives regenerative growth, and then sustain that signaling long enough for regeneration to complete. Drosophila imaginal discs, the epithelial structures in the larva that will form the adult animal during metamorphosis, have been an important model system for tissue repair and regeneration for over 60 years. Here we show that damage-induced JNK signaling leads to the upregulation of a gene called moladietz, which encodes a co-factor for an enzyme, NADPH dual oxidase (Duox), that generates reactive oxygen species (ROS), a key tissue-damage signal. High expression of moladietz induces continuous production of ROS in the regenerating tissue. The sustained production of ROS then continues to activate JNK signaling throughout the course of regeneration, ensuring maximal tissue regrowth.
Introduction
The capacity to regenerate damaged or lost organs or limbs is significantly greater in some animals than others. The use of model organisms with varying degrees of regenerative capacity, from whole-body regeneration in planaria and hydra, to limb regeneration in amphibians, organ and fin regeneration in zebrafish, and the limited tissue regeneration that occurs in mammalian models, has advanced our understanding of this process [reviewed in 1]. The complementary tools available in different model organisms has enabled identification of conserved mechanisms and signaling pathways that are used in many regeneration contexts, such as WNT signaling [2–8], Receptor Tyrosine Kinase (RTK) signaling [9–16], Hippo signaling [17–22], and Jun N-terminal Kinase (JNK) signaling [23–25], as well as clear differences in regenerative mechanisms among organisms and tissues [26,27].
Assessing changes in gene expression in regenerating tissue is a powerful approach to identifying essential regeneration genes. Model organisms that are amenable to mutagenesis, transgenics, or RNAi-mediated gene knockdown also enable functional studies based on the results of transcriptional profiling. For example, analysis of the transcriptome of the cricket leg blastema identified upregulation of components of the Jak/STAT signaling pathway, which, when knocked down by RNAi, resulted in impaired leg regeneration [28]. The transcriptome from the anterior of the planarian Procotyla fluviatilis, which is capable of regeneration after amputation, was compared to the transcriptome from posterior areas of the planarian body that are incapable of regeneration, identifying upregulation of several WNT ligands and receptors after amputation in the tissue that does not regenerate. RNAi knockdown of the WNT effector β-catenin restored regenerative capacity to the posterior of the animal [29]. In zebrafish, genes regulating anterior-posterior patterning during fin regeneration were identified through transcriptional profiling of anterior and posterior portions of the blastema. Overexpression of one of these genes, hand2 (SO:0000704), affected patterning but not growth during regeneration [30]. Thus, transcriptional profiling followed by functional analysis is an effective approach to identification and validation of regeneration genes.
Drosophila melanogaster is one of the most powerful model organisms for genetic and functional analysis of genes. Furthermore, Drosophila imaginal discs, the epithelial structures in the larva that will form the adult animal during metamorphosis, have been an important model system for tissue repair and regeneration for over 60 years [reviewed in 31]. This structure is a simple epithelium that contains complex patterning and determined cell fates. While classic imaginal disc regeneration experiments involved removal of the tissue from the larva before wounding and culturing in the abdomen of an adult host, the development of systems that use genetic tools to induce tissue ablation in situ has enabled high-throughput experimental approaches such as genetic screens [6,32]. In both methods of inducing damage, the tissue undergoes wound closure and forms a regeneration blastema, or zone of proliferating cells near the wound [6,32–35]. In addition, both methods of inducing damage activate signaling through the Wingless and JNK pathways [6,23,32,36–38].
Previous studies have identified genes differentially expressed during imaginal disc regeneration. Blanco et al. cut imaginal discs and then cultured them in the abdomens of adult female flies, before recovering the discs at various time points during regeneration for microarray analysis [39]. This study used the entire imaginal disc for the microarrays, including tissue not contributing to the blastema. To restrict their analysis to cells near the wound site that were contributing to regeneration, Katsuyama et al. similarly cut and cultured discs, but used GFP-labeling of cells with activated JNK signaling to mark the regeneration blastema for dissection prior to microarray profiling [40]. Together these studies used transcriptional profiling to identify several regeneration genes and mechanisms. However, they used relatively small numbers of cells from few regenerating discs due to the technical challenges inherent in the culture technique. Furthermore, culturing itself may induce high levels of stress in the tissue that may alter the transcriptional profile.
We sought to generate a complete and accurate transcriptional profile of regenerating imaginal disc tissue using deep-sequencing techniques and avoiding ex vivo culture and microdissections. Induction of tissue ablation using genetic tools enables regeneration to proceed in vivo as it would if the tissue were to be damaged by a predator or parasite in the wild. Furthermore, use of a genetic tissue-ablation system facilitates ablation and regeneration of hundreds of imaginal discs simultaneously, enabling collection of sufficient material for mRNA-seq without needing amplification. Finally, functional validation of the differentially expressed genes can be carried out by quantifying the extent and quality of regeneration after in situ tissue ablation in mutants.
Here we report the transcriptional profile of the regeneration blastema after ablation of the wing pouch in the Drosophila wing imaginal disc during the peak of regenerative growth. We have used a method that optimizes our ability to isolate fluorescently labeled blastema cells rapidly and efficiently from the disc [41], enabling collection of material for mRNA-seq. Furthermore, we have functionally validated several of the genes that are differentially expressed during regeneration as novel regulators of regeneration. Importantly, we have identified the mechanism through which regeneration signaling is sustained to ensure regrowth. This mechanism involves a positive feedback loop that requires the DUOX-maturation factor NIP, which is encoded by the gene moladietz (mol) (FBgn0086711) [42]. The JNK signaling pathway, which is essential for regeneration [23] and is activated by ROS at the damage site [43], also upregulates mol, which activates Duox, thereby sustaining production of ROS and JNK signaling. This positive feedback loop sustains the regenerative response for several days after tissue damage. Thus, by whole-genome transcriptional profiling of regenerating tissue we have identified the changes in gene expression that control a key regulatory mechanism of regenerative growth.
Results
Isolation of marked blastema cells
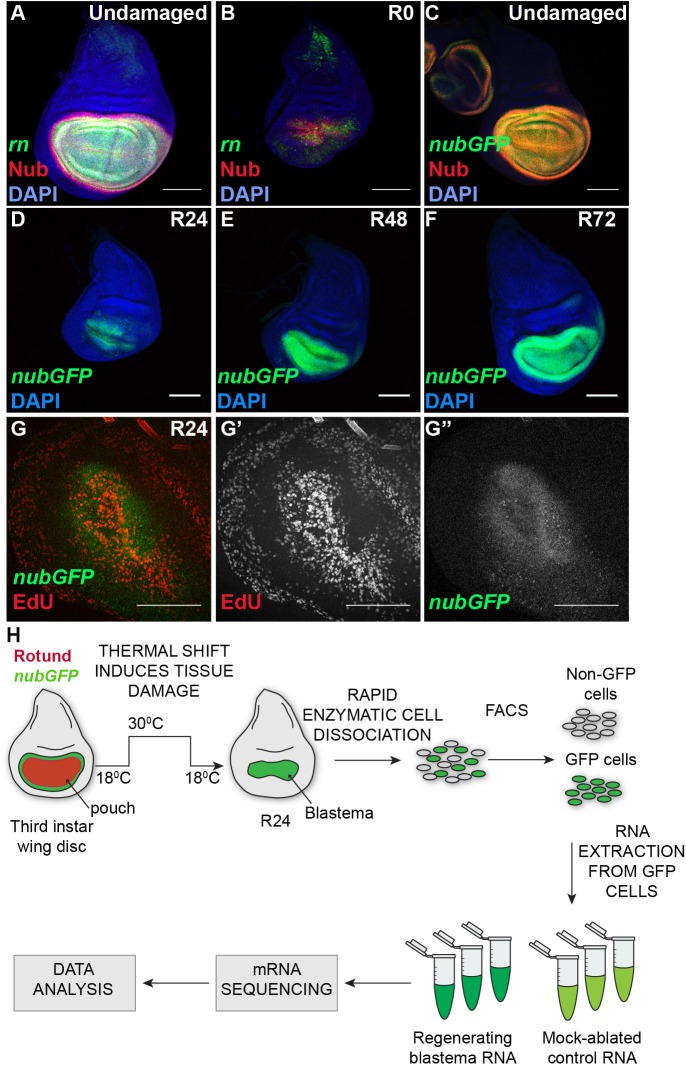
We induced ablation of most of the primordial wing by expressing the pro-apoptotic gene reaper (rpr) (FBgn0011706) [44] in the expression domain of the wing-patterning gene rotund (rn) (FBgn0267337) [45], which comprises most of the wing pouch region of the wing imaginal disc, via rnGAL4, UASrpr [6](Fig 1A and 1B). To control the onset and completion of tissue ablation temporally, we used temperature shifts to regulate the temperature-sensitive repressor Gal80ts [46]. We expressed rpr in the wing primordium for 24 hours at the beginning of the third larval instar, which removed most of the rn-expressing cells by the end of ablation to a reproducible extent (Recovery time 0 hrs or R0) (Fig 1B)(S1 Fig). Wing pouch cells express the wing determinant nubbin (nub) (FBgn0085424) during both normal development and regeneration [6,47]. Thus, nub expression was a convenient way to label blastema cells in these damaged discs as well as control cells in undamaged discs.
Fig 1. Labeling and isolating regeneration blastema cells.
(A-B) Wing imaginal discs that are undamaged (A) or ablated and at 0 hrs recovery (R0) (B). Green = rnGal4, UAS-EGFP. Red = anti-Nub. Blue = DAPI. (C) Wing imaginal disc showing overlap of anti-Nub immunostaining (red) and expression of the nub-GFP MiMIC enhancer trap (green). (D-F) nub-GFP marks the wing pouch at 24 hrs (D) 48 hrs (E) and 72 hrs (F) after ablation. (G) nub-GFP (green) coincides with the regeneration blastema as defined by a zone of high EdU incorporation (red). (H) Schematic of the mRNA-seq procedure, from tissue ablation through cell dissociation and sort to sequencing and data analysis. Scale bars are100 μm.
To label the regeneration blastema cells, we identified a publicly available MiMIC transposon insertion that expresses GFP under the control of the nub locus [48]. Expression of GFP via this insertion occurs in the same cells that are immunostained with an anti-Nub antibody (Fig 1C) [41,47]. The wing primordium continues to express the nub-GFP after ablation and throughout different stages of regeneration (Fig 1D–1F). The GFP-expressing cells also encompass the regeneration blastema at R24 as marked by EdU incorporation (Fig 1G), confirming its suitability as a marker for blastema and control wing pouch cells.
To identify the differentially expressed genes in the blastema, we carried out transcriptional profiling of the GFP-labeled and isolated blastema cell population from R24 wing imaginal discs (Fig 1H). The R24 time point was chosen as it shows a clear blastema, whereas at earlier time points some discs had not yet formed the blastema, and at later time points some discs were beginning to repattern the regrown tissue. Dissociation and fluorescence–activated cell sorting (FACS) of imaginal disc cells is a well-established but lengthy procedure that may affect gene expression and cell viability [49,50]. We therefore optimized our cell dissociation process so that it was rapid and gentle, taking approximately 15 minutes, to minimize changes in transcription and loss of cell viability due to the manipulation of the tissue [41]. We have previously confirmed the accuracy of the sorting by using qPCR to measure expression of pouch and non-pouch genes in the sorted cells [41]. After using this protocol to dissociate and sort regeneration blastema cells and control wing pouch cells, mRNA was prepared and pooled such that each biological replicate produced sufficient mRNA for deep sequencing (Fig 1H).
Identifying differentially expressed genes in the blastema
To identify genes that are differentially expressed during imaginal disc regeneration, we collected three independent samples of nub-GFP-expressing blastema cells from regenerating discs and three independent samples of nub-GFP-expressing cells from undamaged ‘mock-ablated’ control discs after 24 hours of recovery from the thermal shift (R24). While the mock-ablated controls were taken through the thermal shift, they lacked UAS-rpr so did not ablate any tissue. Through deep sequencing we obtained approximately 27 million reads per replicate. Reads were aligned using Tophat2 [51,52] against the Drosophila melanogaster genome (NCBI, build 5.41). A total of 3,798 differentially expressed genes (p<0.05) were identified using Cuffdiff [51], with a false discovery rate of 0.05.
While a log2 fold change of 1.5 is often set as an arbitrary cutoff threshold for differentially expressed genes, our transcriptional profile showed a log2 fold change of 1.3 for the gene puckered (FBgn0243512), which is the phosphatase that is both a target and a negative regulator of JNK signaling in the regeneration blastema [23,53], prompting us to set our cutoff at 1.3. Thus, by selecting a cutoff of log2 fold change ≥ 1.3 or ≤ -1.3, p<0.05, we have identified 660 statistically significant differentially expressed genes, 504 of which are upregulated and 156 of which are downregulated in the regeneration blastema (S1 and S2 Tables).
Several genes previously identified as imaginal disc regeneration genes were upregulated in our transcriptional profile, including dilp8 [54], rgn and mmp1 [55], puckered [23], and myc [6]. In addition, we found some overlap between our gene list and the differentially regulated genes noted in two previously reported transcriptional profiles of regenerating imaginal discs (S2 Fig) [39,40]. We compared the genes that were at least log2 1.3-fold up- or down-regulated in our dataset and those similarly at least 1.3-fold up- or down-regulated in the microarray analysis of posterior regenerating tissue 24 hours after damage presented in Katsuyama et al., in which they cut and cultured imaginal discs, and used GFP-labeling of cells with activated JNK signaling to mark the regeneration blastema for dissection prior to microarray profiling [40]. There were 32 differentially expressed genes in common with this report (S2 Fig). This profile led them to explore the role of JAK-STAT signaling in disc regeneration [40]. Importantly, we also identified the JAK/STAT signaling ligand upd/os (FBgn0004956) as highly upregulated in the regenerating blastema. We also compared the genes that were at least log2 1.3-fold up- or down-regulated in our dataset and those listed as similarly up- or down-regulated in the figures and tables reporting the microarray analysis of whole regenerating discs 24 hours after damage presented in Blanco et al., as the whole list of 1,183 genes they identified as differentially expressed was not published [39]. There were 10 differentially expressed genes in common with this report (S2 Fig). For this analysis, they cut and cultured imaginal discs, and used whole discs for microarray profiling [39]. The minimal overlap with previous studies may be due to several factors, including differences in method of wounding (cut vs. tissue ablation), the discs used (leg vs. wing), regeneration conditions (culture vs. in situ) and method of transcriptional profiling (microarray using few discs/blastemas vs. mRNA-seq using cells isolated from hundreds of blastemas). Furthermore, we have set a 1.3-fold threshold to define differentially expressed genes, and a reduction in this threshold would identify more overlap among these three studies. Strikingly, only one gene was upregulated in all three transcriptional profiles when using the 1.3-fold threshold: yellow-b (FBgn0032601), which is a target of JNK signaling during dorsal closure [56]. Therefore, the current transcriptional profile will enable the study of previously unidentified regeneration genes and pathways.
To confirm that our transcriptional profile identified genes that were indeed differentially regulated in the imaginal disc blastema, we used antibodies, enhancer-trap lines, and protein-trap lines to visualize gene expression in undamaged and damaged wing discs. The “undamaged” control discs depict the expression of these genes during normal development. Of the 22 genes we tested, 16 (73%) were differentially expressed as predicted. Validated upregulated genes were Alkaline phosphatase 4 (Alp4/Aph4) (FBgn0016123) [57], Atf3/A3-3 (FBgn0028550) [58], chronologically inappropriate morphogenesis (chinmo) (FBgn0086758) [59], Ets21C (FBgn0005660) [60], and moladietz (mol) [42](Fig 2A–2E). Other genes had expression patterns that changed from ubiquitous to restricted to the blastema, such as fruitless (fru) (FBgn0004652) [61], LaminC (FBgn0010397) [62], AdoR (FBgn0039747) [63], and kayak (kay) (FBgn0001297) [64] (Fig 2F and 2G, S3 Fig). A third class of genes showed strong upregulation around the blastema and slight upregulation in the blastema including pickled eggs (pigs) (FBgn0029881) [65] and a reporter for Stat92E (FBgn0016917) activity that reflects upd-stimulated signaling [66](Fig 2H and 2I). The genes Thor (FBgn0261560) [67], corto (FBgn0010313) [68], Nlaz (FBgn0053126) [69], twist (twi) (FBgn0003900) [70], and zfh1 (FBgn0004606) [71] showed upregulation in the transcriptional profile but did not show elevated expression with antibody staining (twist) or enhancer-trap expression (zfh1, Thor and Nlaz) or protein-trap expression (corto) (S3 Fig). Some of these genes may be upregulated in the transcriptional profile if, in the course of regeneration, hinge cells convert to pouch cells and begin expressing nub while still expressing some hinge-specific genes such as zfh1. Such hinge-to-pouch conversion has been reported during compensatory proliferation [72,73], and gene expression in these transitioning cells may still be important for regeneration.
Fig 2. Validation of genes identified as upregulated in the regeneration blastema.
Undamaged (A-I) and regenerating (R24) (A’-I’) wing discs. (A-A’) Alp4-lacZ enhancer trap. (B-B’) Atf3-GFP protein trap. (C-C’) chinmo-lacZ enhancer trap. (D-D’) Ets21C-GFP protein trap. (E-E’) mol-lacZ enhancer trap. (F-F’) fru-lacZ enhancer trap. (G-G’) Lamin-GFP protein trap. (H-H’) pigs-GFP enhancer trap. (I-I’) 10xSTAT92E-GFP reporter for STAT activity. Blue dashed line outlines the wing primordium. Scale bars are 100μm.
We also confirmed three of the upregulated genes using qPCR of whole wing discs (S3 Fig). While whole-disc qPCR often fails to detect differences in expression that occur only in the blastema, because the blastema consists of very few cells relative to the rest of the disc, changes in expression of genes that are largely not expressed in the disc prior to damage, such as puckered, can be observed [74]. Thus, we further validated the upregulation of Ets21C, mol, and Nox as representatives of the differentially expressed genes (S3 Fig).
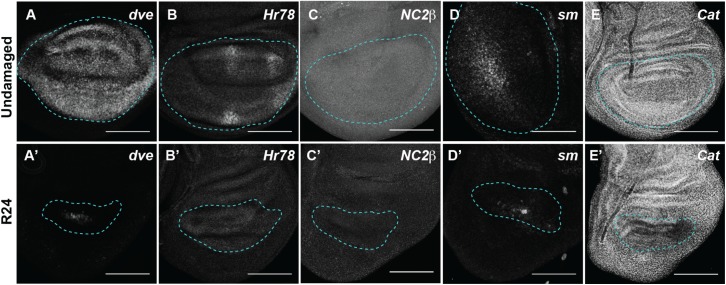
Validated downregulated genes were defective proventriculus (dve) [75], Hormone receptor 78 (Hr78) (FBgn0015239) [76], NC2β (FBgn0028926) [77], smooth (sm) (FBgn0003435) [78], and Catalase (Cat) (FBgn0000261) [79] (Fig 3). Thus, this transcriptional profile successfully identified genes that are differentially expressed in the regeneration blastema that forms after mass tissue ablation.
Fig 3. Validation of genes identified as downregulated in the regeneration blastema.
Undamaged (A-E) and regenerating (R24) (A’-E’) wing discs. (A-A’) dve-lacZ enhancer trap. (B-B’) Hr78-GFP protein trap. (C-C’) NC2β-GFP protein trap. (D-D’) sm-lacZ enhancer trap. (E-E’) Cat-GFP enhancer trap. Blue dashed lines outline the wing primordium. Scale bars are 100 μm.
Identification of novel regeneration genes
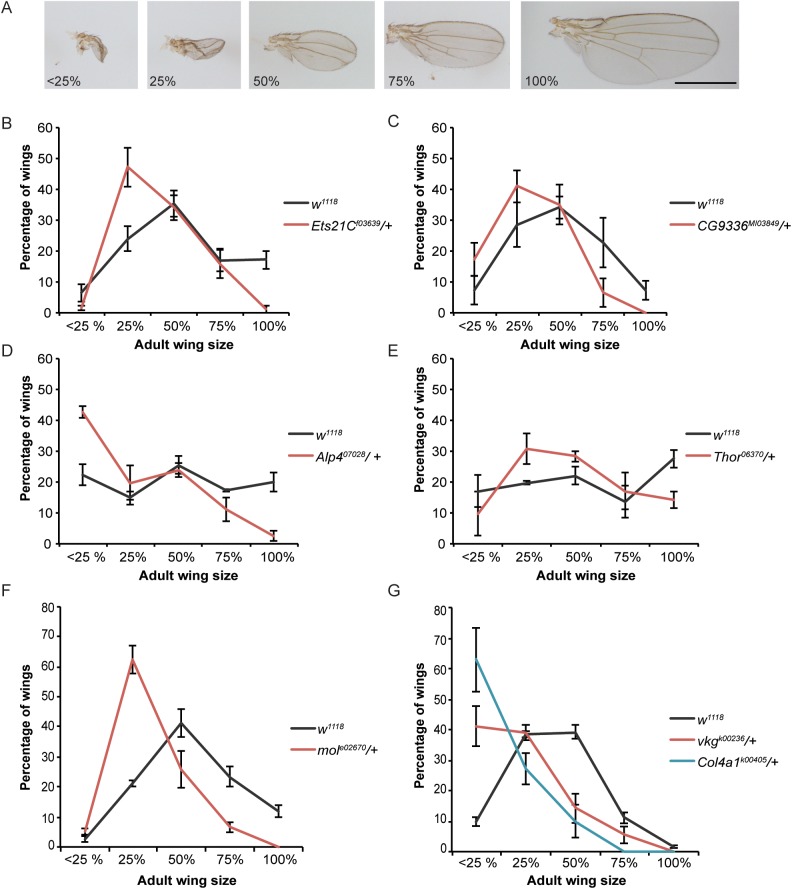
A strong advantage to using a genetically tractable model organism is the ability to assess the functional role of genes of interest that are identified in a transcriptional profile. To assess regenerative capacity in the Drosophila imaginal wing, we induced tissue ablation as described above in animals that were heterozygous mutant for the gene in question. The regenerating animals were then allowed to develop to adulthood, and wing size was measured to assess the extent of regeneration. To measure a population of these wings efficiently, they were sorted into classes that were approximately <25%, 25%, 50%, 75%, and 100% the size of a normal wing (Fig 4A). The distribution of mutant regenerated wings in these classes was then compared to the distribution of regenerated wings generated by control animals. With our system, we observe some heterogeneity in the extent of regeneration within a genotype and also between control experiments conducted at different times. The variation within each genotype was due to variation in each individual animal’s time to pupariation, with animals that had longer to regenerate having larger wings (S1 Fig)[6]. Variation between experiments was due to changes in environmental conditions such as humidity and food quality [74,80,81]. Despite this apparent heterogeneity, we find reproducible differences between mutant and control animals using this method of screening and have successfully identified genes that regulate specific aspects of regeneration [6,74,81,82]. Using this method, we tested available mutants in genes that were strongly upregulated after tissue damage. Twelve out of 16 or 75% of the genes we tested showed a regeneration phenotype, which is unsurprising given that not all important regeneration genes will have a phenotype when only heterozygous mutant, and not all differentially expressed genes will be essential for regeneration. One example of an upregulated gene that was required for regeneration is Ets21c, which encodes a transcription factor that is a known target of JNK signaling and is important for JNK activity in the innate immune response [83], during tumor formation [84,85] and at epidermal wounds [86]. After ablation and regeneration of the imaginal tissue, adult wings in Ets21cf03639/+ animals were smaller than controls (Fig 4B). A second example of an upregulated gene that was required for regeneration is CG9336 (FBgn0032897), which is annotated in the Drosophila genome and has closely related homologs in other Drosophila species but not in vertebrates, and does not appear to have protein domains of known function. After ablation and regeneration of the wing primordium in CG9336MI03849/+ animals, the resulting adult wings were smaller than controls, indicating a requirement for this gene during regeneration (Fig 4C). Additional genes required for regeneration included alkaline phosphatase 4 (Alp-4) [57], the 4E-BP gene Thor [67], moladietz (mol) [42], as well as the collagen components Collagen type IV alpha 1 (Col4a1/Cg25C) (FBgn0000299)[87] and viking (vkg) (FBgn0016075)[88](Fig 4D–4G).
Fig 4. Genetic assays demonstrating that differentially expressed genes have functional roles in regeneration.
(A) Representative examples of wings from damaged discs that are approximately <25%, 25%, 50%, 75%, and 100% of a normal wing. Scale bar is 1mm. (B-G) Sizes of adult wings after regeneration in control (w1118) and heterozygous mutant animals. Three independent experiments each, error bars are SEM. (B) Sizes of adult wings after regeneration in w1118 and Ets21Cf03639/+ animals. w1118 n = 318 wings, Ets21Cf03639/+ n = 255 wings, p<0.0001 using a chi-squared test. (C) Sizes of adult wings after regeneration in w1118 and CG9336MI03849/+ animals. w1118 n = 374 wings, CG9336MI03849/+ n = 215 wings, p<0.0001 by a chi-squared test. (D) Sizes of adult wings after regeneration in w1118 and Alp407028/+ animals. w1118 n = 239 wings, Alp407028/+ n = 217 wings, p<0.0001 by a chi-squared test. (E) Sizes of adult wings after regeneration in w1118 and Thor06270/+ animals. w1118 n = 224 wings, Thor06270/+ n = 146 wings, p = 0.0021 by a chi-squared test. (F) Sizes of adult wings after regeneration in w1118 and mole02670/+ animals. Three independent experiments, w1118 n = 356 wings, mole02670/+ n = 183 wings, p = 0.00001 by a chi-squared test. (G) Sizes of adult wings after regeneration in w1118, Col4a1k00405/+, and vkgk00236/+ animals. w1118 n = 320 wings, Col4a1k00405/+ n = 71 wings, and vkgk00236/+ n = 134 wings, p<0.0001 by a chi-squared test.
Interestingly, several of the mutants tested did not have the predicted effect on regeneration. Rather than leading to poor regeneration, mutations in a subset of upregulated genes enhanced regeneration when heterozygous. These genes included heartless (htl) (FBgn0010389) [89], and fru [61] when assessed in males (S4 Fig). After ablation and regeneration, wing sizes in these mutants were larger than control wings. The mechanisms through which these genes restrict regeneration are not yet understood.
Biological processes affected during regeneration
To identify the biological processes that might be affected during regeneration we carried out gene ontology (GO) enrichment analysis. Transcripts that were significantly upregulated or downregulated were analyzed according to GO categories using DAVID v6.7 [90,91]. Representative GO terms from the most significantly enriched GO clusters describing biological processes are listed in Table 1. Terms that were enriched among the upregulated genes included imaginal disc development and imaginal disc pattern formation, likely because the regenerating tissue was rebuilding what had been ablated. The enrichment of GO terms cell morphogenesis, tissue morphogenesis, cell adhesion, morphogenesis of an epithelium, and cell migration may occur because the cells at the wound edge change shape in order to close the wound [32]. In addition, discontinuity in marked clones in regenerating tissue suggests that cells intercalate and shift relative to each other during imaginal disc regeneration [92]. The GO term regulation of transcription likely contains transcription factors necessary for carrying out the regeneration program, as well as the development and patterning of the regenerating tissue. Interestingly, the GO term open tracheal system development was highly enriched. Two possible reasons for this apparent enrichment include contamination of our sorted cells with tracheal cells, or upregulation in the blastema of the same RTK signaling pathway genes that play critical roles in tracheal system morphogenesis, as has been observed in compensatory proliferation [93]. Another highly enriched GO cluster included the terms negative regulation of cell differentiation, regulation of cell fate commitment, and regulation of cell fate specification. Interestingly, we and others have shown that imaginal disc damage causes a transient loss of markers of cell-fate specification [6,94].
Table 1. Gene ontology (GO) analysis for enrichment of biological process terms.
| GO Term | No. of genes |
|---|---|
| Upregulated genes | |
| Imaginal disc development | 126 |
| Regulation of transcription | 195 |
| Cell morphogenesis | 117 |
| Tissue morphogenesis | 85 |
| Imaginal disc pattern formation | 39 |
| Cell adhesion | 55 |
| Morphogenesis of an epithelium | 73 |
| Cell migration | 52 |
| Open tracheal system development | 54 |
| Negative regulation of cell differentiation | 27 |
| Downregulated genes | |
| Mitochondrial ATP synthesis coupled electron transport | 43 |
| Intracellular transport | 104 |
| Protein catabolic process | 71 |
| Regulation of catabolic process | 17 |
| Vesicle-mediated transport | 108 |
| Cell redox homeostasis | 25 |
| RNA processing | 72 |
| Acetyl-CoA metabolic process | 16 |
| Aerobic respiration | 14 |
| Protein folding | 35 |
Many of the biological process GO terms enriched among the downregulated genes describe general cellular processes, including intracellular transport and vesicle-mediated transport, RNA processing, and catabolism. Interestingly, several classes of genes that affect cellular metabolism were downregulated, including the GO terms mitochondrial ATP synthesis coupled electron transport, acetyl-CoA metabolic process, tricarboxylic acid cycle, and aerobic respiration. While a transcriptional profile of the Xenopus tropicalis tadpole tail regenerative bud has similarly suggested changes in cellular metabolism after tissue damage [95], a broad, functional role for cell-autonomous changes in oxidative phosphorylation, glycolysis or other cellular energetics during regeneration has yet to be demonstrated.
Regulators of reactive oxygen species are differentially expressed during regeneration
An additional downregulated GO category was cell redox homeostasis, suggesting changes in levels of enzymes that regulate Reactive Oxygen Species (ROS) in the regeneration blastema. Indeed, ROS provide important signaling in other model systems of wound healing and regeneration [reviewed in 96]. For example, ROS serve as an attractant for immune cells in larval zebrafish tails after amputation [97] and in Drosophila cuticle wounds [98], and are required for proliferation and regeneration after Xenopus tadpole tail amputation [99] as well as fin and axon regrowth after zebrafish tail amputation [100,101]. Furthermore, ROS stimulate JNK signaling in regenerating zebrafish fins and Drosophila imaginal discs [43,102,103]. During wing imaginal disc regeneration ROS are released by the dying cells, and then taken up by the living cells at the wound edge immediately after physical damage or induction of tissue ablation [43]. However, the extent to which ROS are produced and propagated in the regeneration blastema, as well as the mechanism that underlies ROS production in the regrowing tissue, are unclear.
We examined the expression of genes that regulate ROS production and removal in our transcriptional profile of the imaginal disc regeneration blastema, and found that in addition to the downregulated genes identified by the GO analysis, there were also ROS-regulating factors among the upregulated genes (Table 2). Drosophila has two NADPH oxidases that produce ROS, NADPH Oxidase (Nox) (FBgn0085428) and Dual oxidase (Duox) (FBgn0283531) [104–107]. Interestingly, Nox expression was upregulated, while Duox expression remained unchanged. However, the Duox-maturation factor DUOXA/NIP, which is encoded by the gene moladietz (mol) [42], showed a high level of induction after damage, representing one of the strongest hits in the profile. To reduce ROS, superoxide and hydrogen peroxide are scavenged by superoxide dismutases (Sods) and Catalase (Cat), respectively. Expression of the CuZn-dependent cytoplasmic Sod1 (FBgn0003462)[108] and the Mn-dependent mitochondrial Sod2 (FBgn0010213)[109] was reduced in the regeneration blastema, while the extracellular Sod3 (FBgn0033631)[110] remained unchanged. Furthermore, expression of Cat [79] was strongly reduced. Thus, generation and propagation of ROS in the regeneration blastema could be explained in part by transcriptional upregulation of Nox and mol/NIP, and downregulation of Sod1, Sod2, and Cat.
Table 2. List of reactive oxygen species (ROS) regulating genes.
| Gene symbol | Gene name | log2 Fold Change |
|---|---|---|
| mol | moladietz | 3.48106 |
| Nox | NADPH oxidase | 1.75023 |
| Sod2 | Superoxide dismutase 2 | -0.499425 |
| Sod1 | Superoxide dismutase 1 | -0.649951 |
| Cat | Catalase | -1.62505 |
ROS is required to sustain regeneration signaling
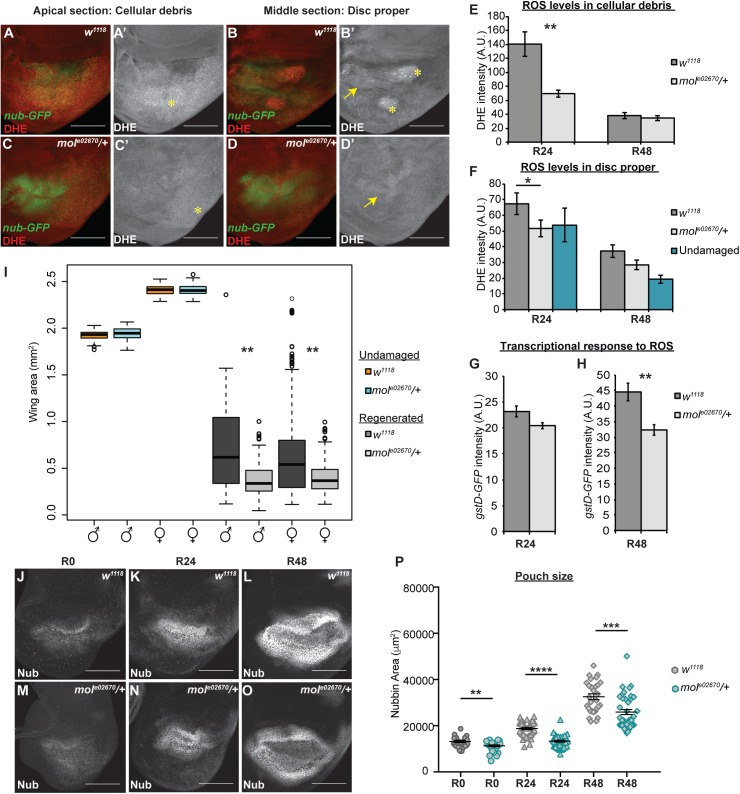
While regenerating zebrafish tails exhibit ROS production for at least 24 hours after amputation [102], and Xenopus tadpole tails produce ROS for days after amputation [99], ROS production in damaged wing discs has only been assessed for 30 minutes after physical damage and 11 hours after induction of tissue ablation [43]. To determine whether ROS persist in regenerating wing discs, we used dihydroethidium (DHE) staining to detect ROS. Importantly, we observed DHE fluorescence in the cellular debris and in the regeneration blastema at R24 (Fig 5A and 5B) and R48 (Fig 6F). We confirmed this finding with the ROS detector H2DCFDA (S6 Fig). Thus, ROS persist in the living, regenerating cells for at least 24 hours after the completion of tissue ablation, suggesting an active mechanism that sustains the production of ROS in the regenerating tissue.
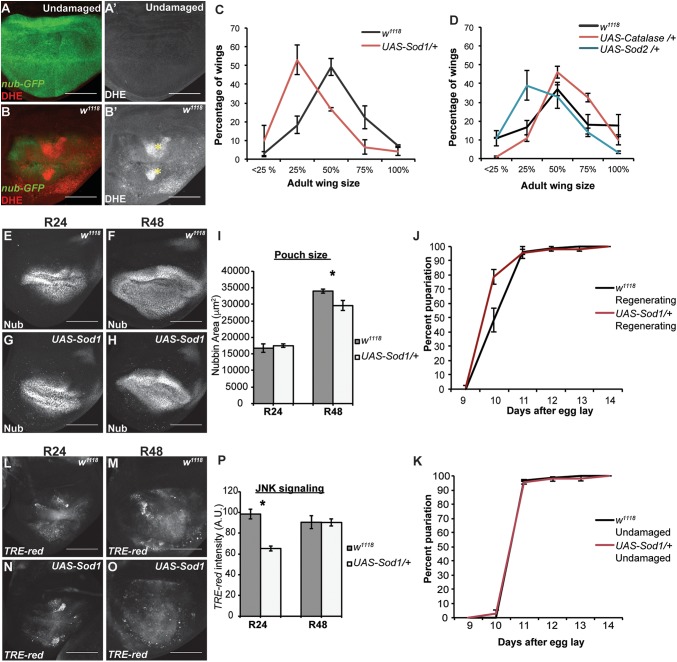
Fig 5. ROS persist in the regeneration blastema and are required for regeneration.
(A-B) DHE staining (red) to detect ROS. The wing pouch is marked with nub-GFP (green). Yellow asterisks mark pockets of cellular debris. (A) Undamaged disc. (B) Regenerating disc at R24. (C-D) Genetic regeneration assays using adult wing size to assess extent of regenerative growth in the imaginal discs. Three independent experiments for each. (C) Sizes of adult wings after regeneration in w1118 and UAS-Sod1/+ animals. w1118 n = 375 wings, UAS-Sod1/+ n = 166 wings, p<0.0001 using a chi-squared test. (D) Sizes of adult wings after regeneration in w1118, UAS-Sod2/+, and UAS-Cat/+ animals. w1118 n = 327 wings, UAS-Sod2/+ n = 332 wings, UAS-Cat/+ n = 361 wings, p<0.0001 using a chi-squared test. (E-H) Anti-Nub marks the wing primordium of w1118 (E,F) and UAS-Sod1 (G,H) regenerating discs at R24 and R48. (I) Quantification of area of the wing primordium as marked by anti-Nub at R24 and R48. w1118 R24 total n = 12 discs, UAS-Sod1 R24 n = 15 discs, w1118 R48 n = 5 discs, UAS-Sod1 R48 n = 10 discs. At R48, p = .0248. (J-K) Pupariation rates. Note that because of the temperature shifts in the ablation protocol the regenerating and undamaged pupariation times cannot be compared to each other. (J) Pupariation timing after regeneration. Three independent experiments, control n = 213 pupae, UAS-Sod1 n = 107 pupae. (K) Pupariation timing during normal development. Three independent experiments, control n = 173 pupae, UAS-Sod1 n = 201 pupae. (L-O) Expression of the TRE-red reporter indicates JNK signaling activity in w1118 (L,M) and UAS-Sod1 (N,O) regenerating discs at R24 and R48. (P) Quantification of TRE-red fluorescence at R24 and R48. *p < .00002. w1118 R24 n = 10 discs, UAS-Sod1 R24 n = 14 discs, w1118 R48 n = 10 discs, UAS-Sod1 R48 n = 14 discs. Scale bars are 100 μm. Error bars are SEM.
Fig 6. moladietz is required for wing disc regeneration.
(A-D) DHE fluorescence (red) indicates the presence of ROS. nub-GFP (green) marks the regenerating wing pouch. (A-B) Confocal slices of a w1118 regenerating disc through the debris field (A,A’) and the disc epithelium (B,B’). Asterisks mark cellular debris in the debris field and in a few folds in the epithelium. Arrow points to the position of the regenerating wing pouch. (C-D) Confocal slices of a mole02670/+ regenerating disc through the debris field (C,C’) and the regenerating epithelium (D,D’). Asterisk and arrow same as above. (E-F) Quantification of DHE fluorescence intensity in the debris fields of w1118 and mole02670/+ regenerating discs (E) and in the regenerating epithelia of w1118 and mole02670/+ regenerating discs and control undamaged discs (F). For R24, three independent experiments, for a total w1118 regenerating n = 12 discs, mole02670/+ regenerating n = 18 discs, w1118 undamaged n = 11 discs. For R48, three independent experiments for a total w1118 regenerating n = 30 discs, mole02670/+ regenerating n = 25 discs, w1118 undamaged n = 10 discs. (G,H) Quantification of GFP fluorescence from a gstD-GFP reporter for ROS-regulated transcription in regenerating w1118 and mole02670/+ discs. For R24, w1118 n = 12 discs, mole02670/+ n = 20 discs. For R48, w1118 n = 12 discs, mole02670/+ n = 10 discs. (I) Adult wing area in w1118 and mole02670/+ male and female wings from undamaged discs and after disc regeneration. Three independent experiments. Undamaged: w1118 females n = 125 wings, w1118 males n = 132 wings, mole02670/+ females n = 82 wings, mole02670/+ males n = 73 wings. Regenerated: w1118 females n = 226 wings, w1118 males n = 134 wings, mole02670/+ females n = 128 wings, mole02670/+ males n = 133 wings. (J-O) Anti-Nub marks the regenerating wing primordium at R0, R24 and R48 in w1118 and mole02670/+ discs. (P) Quantification of the size of the regenerating wing primordium at R0, R24 and R48. R0 w1118 n = 26 and mole02670/+ n = 29, R24 w1118 n = 42 and mole02670/+ n = 41, R48 w1118 n = 29 and mole02670/+ n = 42. Scale bars are 100 μm. Error bars are SEM. **p<0.05, *p<0.005, ***p<0.0002, ****p<0.0001.
To determine the extent to which changes in ROS levels impact regeneration, we overexpressed Sod1, Sod2, or Cat in ablated discs using a UAS-Sod1 [111], UAS-Sod2 [112], or UAS-Cat [113] transgene under the control of rn-GAL4, which induced expression in the ablated tissue as well as in the few surviving rn-expressing cells that contributed to the blastema. This limited overexpression was intended to reduce ROS levels in the debris and partially reduce ROS levels in the blastema, as not all blastema cells expressed the transgenes. According to a prior report, similar overexpression of Sod1 or Cat individually or together reduced the ability of wing discs to recover from tissue ablation [43]. In our ablation system, overexpression of Sod1 or Sod2 during ablation similarly led to smaller adult wings compared to controls, although overexpressing Cat alone did not, confirming that manipulation of levels of ROS-regulating enzymes impact regeneration (Fig 5C and 5D). This reduction in regeneration was likely due to a combination of reduced regenerative growth, as UAS-Sod1 regenerating wing primordia lagged behind controls in size (Fig 5E–5I), and reduced time for regeneration, as UAS-Sod1 regenerating animals failed to delay pupariation in response to the tissue damage as long as the controls (Fig 5J and 5K). The damaged discs with transiently overexpressed Sod1 also had reduced JNK signaling at R24, as observed by expression of the TRE-red transcriptional reporter for JNK pathway activity [114] (Fig 5L–5P).
In damaged eye imaginal discs, ROS recruit hemocytes to the site of damage, which then stimulate JNK signaling in the recovering epithelium [103]. To determine whether hemocytes are recruited to the wing disc in response to ablation of the rn-expressing domain, we observed hemocytes using Hemolectin-RFP (FBgn0029167) [115] and anti-Nimrod (FBgn0259896) [116]. In control discs, small clusters of hemocytes were observed in the folds of 1 of 15 discs (S5 Fig). In damaged and regenerating tissue, clusters of hemocytes were observed in 4 of 15 discs along the peripodial epithelium. These hemocytes were present in close proximity to the debris, but were not in direct contact with the debris, which was trapped between the two epithelial layers (S5 Fig). Thus, in contrast to the eye disc, recruiting hemocytes is unlikely to be the main mechanism through which ROS induce JNK signaling in the wing disc.
The DUOX maturation factor moladietz/NIP is required for ROS production in the regeneration blastema
The striking upregulation of mol in the regeneration blastema by R24 and its continued expression through R48 (Fig 2E, S8 Fig) suggested that its protein product NIP may have an important role in regulating regeneration. Importantly, mol is normally expressed at low levels in the wing disc during development (Fig 2E, S6 Fig). The vertebrate homolog of NIP, DUOX maturation factor (DUOXA) (HGNC:26507), is essential for moving DUOX through the endoplasmic reticulum and the golgi to the cell surface [117]. Once at the cell surface, DUOXA remains in a stable complex with DUOX and enhances the rate and specificity of ROS production [118]. Thus, transcriptional regulation of mol could have a profound effect on ROS production in the regenerating epithelium.
To determine the extent to which the transcriptional upregulation of mol promotes ROS production in the blastema, we assessed ROS levels in heterozygous mol null mutant animals. Duox can produce both hydrogen peroxide and superoxide [118]. As DHE was the reagent that worked best in imaginal discs (Fig 5A and 5B, S6 Fig), we used it as a representative assay for overall ROS levels. Interestingly, production of ROS in both the cellular debris and the regeneration blastema was significantly reduced in the mole02670/+ damaged discs (Fig 6A–6F), indicating that mol is required for overall ROS production after tissue damage. To confirm that the response to ROS is reduced in the mole02670/+ regenerating tissue, we assessed expression of a reporter transgene, gstD1-GFP (FBgn0001149), that responds to ROS-induced activation of transcription [119]. Interestingly, gstD1-GFP expression was significantly reduced in the mutant regeneration blastemas, but not until two days after tissue damage (Fig 6G and 6H; S6 Fig).
Our initial genetic assay showed that NIP was required for regeneration (Fig 4F). To quantify the effect of reduction of NIP further, we measured adult wing size in mole02670/+ females and males after imaginal disc ablation and regeneration. Importantly, while normal wings were the same size in controls and mole02670/+ animals, regenerated mole02670/+ wings were significantly smaller than regenerated controls, indicating that regeneration in these mole02670/+ animals was impaired (Fig 6I). To confirm the requirement for mol we also quantified regeneration using discs expressing molRNAi in the regenerating wing pouch using a UAS-molRNAi transgene. While such RNAi expression was limited temporally and spatially, we have found it to be effective at generating phenotypes in our system [74], possibly due to the propagation of knockdown after limited RNAi expression observed in imaginal discs [120]. Importantly, discs expressing molRNAi also regenerated worse than controls as assessed by adult wing size (S6 Fig).
To understand how reduced expression of mol impairs regeneration, we monitored regrowth of the ablated tissue by measuring the area of the wing primordium at specific times after the completion of ablation. We found that mole02670/+ regenerating discs were slightly smaller than controls beginning in early regeneration, and significantly lagged behind controls in size by two days after tissue damage (Fig 6J–6P).
The DUOX maturation factor moladietz/NIP is required for sustained JNK signaling during regeneration
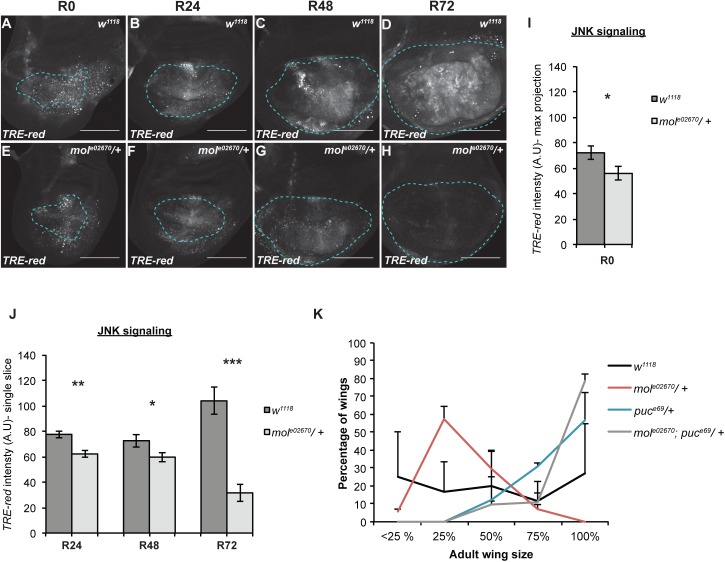
Given that the difference in regrowth was more apparent later in regeneration, we speculated that reduction of NIP levels might be particularly important for the later stages of regeneration. Indeed, expression of the growth-promoter Myc (FBgn0262656), which is important for regenerative growth [6] was comparable to controls at R24 but reduced at R48 (S6 Fig). Because ROS stimulate JNK signaling in damaged imaginal discs [43], we examined JNK signaling levels in mole02670/+ regenerating discs. Importantly, expression of the JNK signaling reporter TRE-red, which reflects the activity of the AP-1 transcriptional complex, was slightly reduced during early and mid regeneration (R0, R24, and R48) and markedly reduced during the late stages of regeneration in mole02670/+ discs (R72) (Fig 7A–7J). To determine whether increasing JNK signaling could compensate for the reduction of NIP levels and ROS production, we examined adult wings after damage and regeneration in animals heterozygous mutant for both mol and the negative regulator of JNK signaling puckered (puc) [53]. These mole02670/+; pucE69/+ regenerated wings were significantly larger than the mole02670/+ regenerated wings, indicating that increased JNK signaling could bypass the requirement for mol and rescue the poor regeneration phenotype of the mole02670/+ mutants (Fig 7K). Thus, upregulation of mol is required for ROS propagation in the regeneration blastema and for sustaining JNK signaling, particularly during the later stages of regeneration.
Fig 7. NIP is required to sustain JNK signaling during late regeneration.
(A-H) Confocal images of fluorescence from the TRE-red reporter for JNK signaling in w1118 (A-D) and mole02670/+ (E-H) regenerating discs at R0 (A,B), R24 (B,F), R48 (C,G) and R72 (D,H). (I) Quantification of fluorescence intensity of the TRE-red reporter in max projections of the confocal images at R0, because at this time point the epithelium cannot be distinguished from the debris. w1118 n = 10 discs, mole02670/+ n = 14 discs. (J) Quantification of fluorescence intensity of the TRE-red reporter in single slices of the confocal images through the regenerating epithelium at R24, R48, and R72. R24 w1118 n = 11 discs, mole02670/+ n = 11 discs. R48 w1118 = 14 discs, mole02670/+ n = 15 discs. R72 w1118 n = 11 discs, mole02670/+ n = 11 discs. (K) Regeneration assays using adult wing size to assess extent of regenerative growth in the imaginal discs in w1118, mole02670/+, pucE69/+, and mole02670/+;pucE69/+ animals. Two independent experiments, thus error bars are SD. w1118 n = 26 wings, mole02670/+ n = 83 wings, pucE69/+ n = 99 wings, and mole02670/+;pucE69/+ n = 95 wings. p<0.0001 for all comparisons using a chi-squared test. Dashed blue line outlines the wing primordium. Scale bars are 100 μm. Error bars are SEM unless otherwise noted. *p<0.05, **p<0.001, ***p<0.0001.
The importance of the Duox-maturation factor in regeneration implies that Duox itself is also important for regeneration, even though it is not transcriptionally upregulated according to our profile. To assess the importance of Duox, we quantified regeneration in UAS-DuoxRNAi animals. Indeed, wing discs expressing DuoxRNAi regenerated poorly compared to control animals (S7 Fig).
Another important regulator of ROS that was upregulated in the transcription profile is the NADPH oxidase Nox. To determine whether Nox is also required for wing disc regeneration, we compared adult wings after damage and regeneration in control animals and animals heterozygous for a Nox mutant (NoxMI15634) or expressing NoxRNAi. Interestingly, both the Nox mutation and the NoxRNAi caused improved regeneration as assessed by adult wing size (S7 Fig). To understand why reduction of Nox led to enhanced regeneration, we assessed pouch size throughout regeneration and rate of pupariation. Interestingly, wing discs with reduced Nox regrew at the same rate as control discs through R48, and pupariation timing was not altered (S7 Fig). Thus, the constraint Nox places on regeneration must occur after R48, possibly during the pupal phase. These results suggest that the ROS produced by Nox and by the Duox/NIP complex are likely functionally, spatially, or temporally different, with Nox-produced ROS acting to inhibit regeneration during the pupal phase.
JNK signaling is required for the upregulation of mol expression after tissue damage
Given that mol upregulation after tissue damage was important for ROS production in the regenerating epithelium and sustained regenerative signaling, we wanted to identify the upstream signal that regulates mol expression. We hypothesized that regeneration signaling itself, specifically JNK signaling, could induce the upregulation of mol. Canonical JNK signaling acts through the transcription factor AP-1, which is a heterodimer of Jun (FBgn0001291) and Fos (FBgn0001297)[121]. Downstream genes are regulated through AP-1 binding to the conserved TPA-responsive element (TRE) sequence (TGAC/GTCA) [122]. Indeed, there are three consensus TRE sites at the mol locus: one 2 Kb upstream of the transcription start site, one in the first intron, and one in the fifth intron (Fig 8A).
Fig 8. Expression of mol is regulated by JNK signaling.
(A) Schematic of the mol locus showing the relative positions of three canonical TRE sites. (B,C) Anti-β-galacosidase immunostaining showing expression of the mol-lacZ (green) reporter in control (B) and UAS-JNKDN (C) R24 discs. (D) Quantification of mol-lacZ fluorescence from the immunostaining. w1118 n = 10 discs, UAS-JNKDN n = 10 discs. (E) Anti-β-galacosidase immunostaining showing expression of the mol-lacZ reporter in a hepr75/+ R24 regenerating disc. (F) Quantification of mol-lacZ fluorescence from the immunostaining. w1118 n = 7 discs, hepr75/+ n = 8 discs. Scale bars are 100 μm. Error bars are SEM. **p<0.002.
To determine the extent to which JNK signaling is required for mol expression after tissue damage, we inhibited JNK signaling by expressing a dominant-negative JNK (UAS-JNKDN) (FBgn0000229)[123] under the control of rn-GAL4 during wing pouch ablation. Interestingly, expression of the reporter mol-lacZ was significantly decreased upon reducing JNK signaling through UAS-JNKDN (Fig 8B–8D), suggesting that JNK signaling is important for mol upregulation after tissue damage. To confirm this finding, we also examined mol-lacZ expression in regenerating wing discs that were heterozygous mutant for hemipterous (hep) (FBgn0010303), which encodes a JNK kinase [124]. Expression of the mol-lacZ reporter was also significantly decreased in female hepr75/+ regenerating wing discs (Fig 8E and 8F). Thus, JNK regulation of mol expression constitutes a positive feedback loop that sustains JNK signaling.
ROS activates both JNK and p38a (FBgn0015765) in the regenerating wing disc [43]. To determine whether p38a signaling also induces a positive feedback loop through mol, we examined mol-lacZ expression in regenerating discs that were heterozygous mutant for p38a1. Interestingly, reduction of p38a did not affect mol-lacZ expression (S8 Fig). Thus, JNK signaling is required for upregulation of mol expression after tissue damage, which is in turn required for sustaining ROS production in the regenerating epithelium and maintaining JNK signaling during late regeneration (Fig 8G).
Discussion
This work has identified a novel mechanism that sustains regeneration signaling and ensures that regrowth of damaged tissue continues beyond the initial burst of damage signaling. While elevated ROS levels are sustained in other regeneration models such as amputated zebrafish fins and Xenopus tails, where they promote signaling and the later stages of regenerative growth [99,100,102], the mechanism through which elevated ROS levels are maintained has remained elusive. Our work has provided insight into this puzzle in Drosophila by discovering a key positive feedback loop that uses JNK-induced upregulation of the Duox-maturation factor encoded by mol to sustain ROS production, JNK signaling, and late regeneration. Similar damage-induced regulation of the Duox-maturation factor may facilitate long-term regeneration signaling in many animals. We were able to identify this mechanism through generation of a transcriptional profile of actively regenerating tissue, made possible by our genetically induced tissue ablation system [6] and our technical advances enabling isolation of sufficient numbers of blastema cells [41].
This is the first report of upregulation of a Duox maturation factor as a key aspect of the regeneration response. Other cellular functions that are regulated by DUOXA/NIP have only recently been identified. For example, DUOXA/NIP affects differentiation in murine skeletal muscle myoblasts [125], murine thyroid hormone production and cerebellar development [126], and the response to bacterial infections in the murine gut [127], as well as development of the exoskeleton in C. elegans [128], and recruitment of hemocytes to wounds in the Drosophila embryo epidermis and neutrophils to airways in mice [129,130]. Here we describe a role for mol during wing disc regeneration and show that while mol is transcriptionally upregulated, Duox levels do not change according to our transcriptional profile, indicating that fine-tuning of ROS levels can be achieved by changes in expression of the maturation factor rather than the enzyme itself. This regulative strategy may be deployed in many other cases in which ROS act as crucial signaling molecules.
In addition to the transcriptional changes observed in regulators of ROS, many of the other changes in gene expression can be combined with our current understanding of tissue regeneration to identify novel and interesting relationships between developmental genes and signals and tissue regeneration. For example, our data indicated downregulation of the hormone receptor Hr78 in regenerating tissue. The expression of Hr78 in the wing disc appeared to be in some of the pro-vein regions (Fig 3B). Tissue damage in the wing disc leads to a transient loss of cell-fate gene expression, including in the pro-veins, during regeneration [6,94]. Thus, Hr78 may be a novel wing vein fate gene whose expression is downregulated along with the other known vein fate genes after tissue damage.
As an additional example, we observed differential regulation of various nuclear hormone receptor genes that are transcriptionally regulated by the hormone ecdysone [131]. Regenerating animals delay metamorphosis to accommodate regrowth of the damaged tissue by regulating ecdysone signaling, which controls developmental transitions [132]. Ecdysone targets that we found downregulated in regenerating wing discs include Hormone receptor 46 (Hr46/Hr3) (FBgn0000448), Hormone receptor 4 (Hr4/CG42527) (FBgn0264562), and Ecdysone-induced protein 78C (Eip78C) (FBgn0004865) (S2 Table). Interestingly, we also see upregulation of Cyp18a1 (FBgn0010383), a cytochrome P450 enzyme that exerts negative feedback regulation on ecdysone signaling by decreasing intracellular levels of ecdysone [133]. Thus, Cyp18a1 may be upregulated to ensure that ecdysone signaling stays low in the regenerating tissue to reinforce the developmental checkpoint induced by tissue damage.
Regeneration involves orchestration of various cellular processes to repair and replace the damaged body part. It requires coordination of proliferation, growth, patterning, and changes in cell architecture and movement in a highly regulated manner. These dramatic changes could be coordinated by key transcription factors. Several transcription factors are differentially expressed in our profile, including chinmo, Ets21C, AP-2/TfAP-2 (FBgn0261953), fru, Atf3/A3-3, dve and Blimp-1 (FBgn0035625). These transcription factors could lie at the center of regulatory networks that bring about key cellular changes. For example, Ets21C is a known downstream target of JNK signaling in wound healing [86], and EGFR signaling in the intestinal stem cells [134], and is also required as a co-factor for the JNK pathway transcription factor AP-1 in regulating transcriptional targets during tumor formation [84,85]. Thus, its expression in the regenerating wing disc could result from integration of multiple signals, and its requirement in regeneration may be due to its role in promoting expression of JNK targets. Further investigation into the mechanisms of these transcription factors will lead to a better understanding of regeneration.
Regeneration is a tightly controlled process, requiring a balance between positive and negative regulators so that growth is stimulated but not deregulated. Indeed, our functional analysis demonstrated that several of the upregulated genes, including heartless and Nox, serve to restrict regeneration, as regeneration improved in heterozygous mutant animals. Therefore, functional analysis is critical for interpretation of gene expression data, as drawing conclusions based on differential expression alone can be misleading. Indeed, it was through functional analysis that we identified mol, and not Nox, as the critical regulator that promotes sustained ROS production and JNK signaling, completing the positive feedback loop that sustains regeneration. Further functional analysis of differentially expressed genes will likely reveal additional mechanisms that control tissue regeneration.
Materials and methods
Ablation system
Tissue ablation was carried out as described previously [6,82] using rnGal4, UAS-rpr, and tubGAL80ts to regulate cell death spatially and temporally, with a thermal shift from 18° to 30° C for 24 hours during the early third larval instar. To synchronize development, eggs were collected for four hours on grape juice plates, first-instar larvae were collected shortly after hatching at two days after egg laying and transferred to vials, and the vials underwent the thermal shift at 7 days after egg laying, which was determined to be just after molting by counting mouth hooks.
Fly lines
Flies were reared on standard molasses medium at 25° C except during regeneration experiments. The following Drosophila lines were obtained from the Bloomington Stock Center or were gifts as noted: w1118; rnGAL4, UAS-rpr, tubGAL80ts/TM6B, tubGAL80 and w1118; rnGAL4, tubGAL80ts/TM6B [6], w1118; y1,w*; Mi{MIC}nubMI05126 (BL37920)[48], y1,w67c23; P{lacW}chinmok13009/CyO (BL10440)[135], y1, w* Mi{MIC}pigsMI11007 (BL56274)[48], P{PZ}Alp407028, ry506 (BL12285)[135], w1118; PBac{Ets21C-GFP.FLAG}VK00033/TM3, Sb1 (BL38639), P{PZ}osp00865; ry506 P{PZ}zfh100856/TM3, ryRK Sb1 Ser1 (BL11515)[136], y1 w67c23; P{lacW}molk11524a/CyO (BL12173)[135], ry506 P{PZ}fru3/MKRS (BL684)[135], w;;pBAC[atf3::EGFP]/TM6B (gift from M. Uhlirova)[137], nlaZ:GFP[R2] (gift from M. Ganfornina)[69], w1118; P{10xStat92E-GFP}1 (BL26197)[66], cn1 P{PZ}dve01738/CyO; ry506 (BL11073)[135], cn1 P{PZ}sm05338/CyO; ry506 (BL11403)[78], y1 w*; P{PTT-GB}LamCCB04957 ttvCB04957/SM6a (BL51528)[138], y1 w*; Mi{PT-GFSTF.1}AdoRMI01202-GFSTF.1/TM6C, Sb1 Tb1 (BL60165)[139], y1 w*; P{lacW}Thork13517 (BL9558)[67], y1 w*; Mi{PT-GFSTF.0}kayMI05333-GFSTF.0 (BL63175)[139], w1118; PBac{corto-GFP.FPTB}VK00037 (BL42268), w1118; PBac{Hr78-GFP.FLAG}VK00037 (BL38653), w1118;PBac{NC2β-GFP.FPTB}VK00033 (BL56157), y1 w*; Mi{PT-GFSTF.0}CatMIO4522-GFSTF.0 (BL60212)[139], HmlΔRFP (gift from K. Bruckner)[115], TRE-red and gstD-GFP (gifts from D. Bohmann)[114], Ets21Cf0369 (BL18678)[140], y1 w*; Mi{MIC}CG9336MI03849 (BL36397)[139], y1 w67c23; P{lacW}Col4a1K00405/CyO (BL10479)[135], y1 w67c23; P{lacW}vkgk00236 (BL10473)[135], P{PZ}Thor06270 cn1/CyO; ry506 (BL11481)[141], w1; P{UAS-Sod1.A}B36 (BL24754), w1; P{UAS-CatA}2 (BL24621), w1; P{UAS-Sod2.M}UM83 (BL24494), w1118; PBac{RB}mole02670/CyO (BL18073)[140], y1 sc* v1; P{TRiP.HMS02560}attP40 (UAS-molRNAi) (BL42867), y1 v1; P{TRiP.GL00678}attP40 (UAS-DuoxRNAi) (BL38907), y1 v1; P{TRIP.GL00678}attP40 (UAS-NoxRNAi) (BL32902), y1 w*; Mi{MIC}NoxMI15634/SM6a (BL61114)[139], pucE69 [53], UAS-JNKDN [142], w* hepr75/FM7C (BL6761)[124], w*; P{neoFRT}82B p38a1 (BL8822)[143].
Immunohistochemistry and microscopy
Immunostaining was carried out as previously described [6].
Anitbodies and dilutions used were Anti-Nubbin (1:500) (gift of S. Cohen) [47], mouse anti-βgal (1:100) (DSHB; 40-1a-s), rabbit anti-βgal (1:500) (MP Biomedicals), mouse anti-GFP (1:10)(DSHB 12E6), rabbit anti-Myc (1:500) (Santa Cruz Biotech d1-717 sc-28207), rabbit anti-PH3 (1:500) (Millipore), mouse anti-Nimrod (1:1000)(gift from I. Ando)[116] and anti-Twist (1:200) (gift from A. Stathopoulos)[144].
Alexa Fluor (AF) secondary antibodies from Molecular Probes were AF488, AF555 and AF633 (used at 1:500). Nuclei were labeled with DAPI (Sigma)(1:5000).
EdU incorporation was carried out using the click-it EdU Alexa Fluor 594 Imaging kit (Molecular Probes) as previously described [145]. Samples were mounted in Vectashield (Vector Labs).
Immunostained samples were imaged on a Zeiss LSM 700 confocal microscope and images were processed using ZenLite, Adobe Photoshop, and Image J software. Bright-field imaging of adult wings was done on an Olympus SZX10 microscope using the CellSens Dimension software, and images were processed using Image J.
ROS detection
ROS were detected in imaginal discs using Dihydroethidium (DHE) (D11347, Molecular Probes) using the protocol described in Owusu-Ansah et al. [146], with slight modifications. Briefly, larvae were dissected in Schneider’s medium (SM). DHE was reconstituted in DMSO and then added to SM at a concentration of 30nM. Samples were incubated in this DHE solution for 5 minutes (mins) on a shaker followed by three quick washes in SM. The samples were then fixed in 7% paraformaldehyde made in 1X phosphate buffer saline (PBS) for 7 mins. Samples were rinsed once in 1X PBS and imaginal discs immediately dissected out to mount in Vectashield with DAPI. The samples were imaged on the confocal immediately to avoid oxidation of the DHE by the environment.
Data quantification and statistical analysis
Fluorescence intensity analysis was performed using single confocal slices. Average intensity was calculated by measuring intensity values in three equal-sized boxes in the pouch region of the wing disc in Image J, except for the gstD-GFP, whose expression was not uniform and thus was quantified by measuring GFP intensity in the entire pouch area. Average intensities of multiple wing discs were combined to calculate the final average intensity plotted in the graphs. For measuring the pouch area, a maximum projection of all the confocal slices was taken and the Nubbin-expressing area measured in Image J. Graphs were plotted in Excel, R, and GraphPad Prism 7.0.
For imaginal disc measurements and immunofluorescence quantifications, the Welch’s t-test was performed using R and GraphPad Prism 7.0. For the adult wing size assay, chi-squared tests were performed using GraphPad online tools. Statistical analyses for adult wing measurements were performed using Welch’s t-test.
Blastema cell isolation and RNA library preparation
The ablated regenerating discs had the genotype nub-GFP/+; rn-Gal4, GAL80ts, UAS-rpr /+, while the mock-ablated controls had the genotype nub-GFP/+; rn-Gal4, GAL80ts/+. Cells were isolated for the transcriptional profile as previously described [41]. Briefly, discs were dissected using teams of 4 researchers dissecting simultaneously to maximize the number of discs obtained per sample. TrypLE Select (Life Technologies) was used to achieve rapid dissociation of the disc cells. The GFP+ cells were sorted via FACS. mRNA from the isolated cells was prepared using an RNeasy Mini Kit (#74104, Qiagen). Multiple days of dissections and RNA preparation were pooled such that each biological replicate consisted of approximately 600 regenerating imaginal discs, 86,000 GFP+ cells, and up to 900ng RNA. The undamaged controls consisted of 120 discs per replicate, which produced approximately 106,500 GFP+ cells and 1000ng RNA. The accuracy of the sorting was previously confirmed [41]. RNA quality was confirmed using a Bioanalyzer (Agilent 2100). Library generation was carried out using Illumina's TruSeq Stranded RNA Sample Prep kit. Sequencing was carried out on a HiSeq2000 using a TruSeq SBS sequencing kit version 3. The Roy J. Carver Biotechnology Center at the University of IIlinois at Urbana-Champaign performed the library preparation and sequencing.
Bioinformatics
Fastq reads were trimmed using FASTQ Quality Trimmer (v.1.0.0) and adaptor sequences were removed using Clip (v.1.0.1) in Galaxy [147]. Reads were aligned through Tophat2 (v.0.6) [51,52] against the Drosophila melanogaster genome (NCBI, build 5.41) with a maximum of 2 mismatches permitted. Intron length was set between 20 and 150,000, and a gene model was provided as GTF (NCBI, build 5.41). FPKM estimation was done using Cufflinks (v.0.0.7) [51], and both bias-correction and multi-read correction were performed. Differential expression analysis was performed using Cuffdiff [51], geometric library normalization was performed and the False Discovery Rate was set at 0.05. Furthermore, aligned reads were counted using HTSeq (v.0.3.2)[51]. All bioinformatics analysis was performed using the Galaxy suite [147]. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus [148] and are accessible through GEO Series accession number GSE101797 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE101797).
Supporting information
(A) Variation in the size of the wing pouch as marked by anti-Nub immunostaining in undamaged and ablated wing discs. Note the minimal variation in pouch size at R0, indicating consistency of ablation. Undamaged n = 8, regenerating n = 28. Error bars are SEM. (B) Quantification of percentage of regenerating animals that had pupariated by each day, showing the asynchronous progression to pupariation that occurred after tissue damage. Undamaged animals were w1118;; rnGAL4, Gal80TS/+, regenerating animals were w1118;; rnGAL4, UAS-reaper, Gal80TS/+, and both experienced the 24-hour temperature shift. Three independent experiments, total undamaged n = 144 pupae, regenerating n = 176 pupae. (C) Percentage of wings of different sizes on w1118;; rnGAL4, UAS-reaper, Gal80TS/+ animals that eclosed on different days after egg laying due the asynchronous development induced by tissue damage. Note that the animals that eclosed first (day 18) had smaller wings than those that eclosed on day 19, and those that eclosed on day 20 had the largest wings. Thus, variation in wing size after disc regeneration is partly determined by length of time for regeneration. Three independent experiments, total n = 371 wings.
(TIF)
(A) Venn diagrams showing genes at least 1.3-fold upregulated or downregulated in three transcription profiles (this work, [39,40]) of regenerating wing discs generated using different methods. Note that the number of genes from the Blanco et al. study is an underrepresentation because the complete list of differentially expressed genes identified was not published. (B) Lists of differentially expressed genes (DEGs) in common between this study and each prior study, along with fold change in this study.
(TIF)
Undamaged (A-G) and regenerating (R24) (A’-G’) wing discs. (A-A’) AdoR-GFP MiMIC enhancer trap. (B-B’) Kayak-GFP protein trap. (C-C’) Thor-lacZ enhancer trap. (D-D’) Corto-GFP protein trap. (E-E’) NLaz-GFP MiMIC enhancer trap. (F-F’) anti-Twist. (G-G’) zfh1-lacZ enhancer trap. Blue dashed line outlines the wing primordium. Scale bars are 100 μm. (H) Quantification of upregulation of mol, Nox, and Ets21C expression using qPCR. Four biological replicates each. Error bars are SEM, *p<0.05.
(TIF)
(A) Adult fru3/+ males had larger wings after regeneration than controls. Three independent experiments, w1118 n = 112 wings, fru3/+ n = 95 wings, p<0.001 by a chi-squared test. (B) Adult htlAB42/+ animals had larger wings after regeneration than controls. Three independent experiments, w1118 n = 316 wings, htlAB42/+ n = 223 wings, p<0.001 by a chi-squared test. Error bars are SEM.
(TIF)
(A-D) Hemolectin-RFP (Hml-RFP) (green) showing hemocytes near undamaged (A,B) and R24 (C,D) wing discs. (E,F) Anti-Nimrod (green) also showing hemocytes near an R24 wing disc, confirming the Hml-RFP results. (B), (D), and (F) are orthogonal slices with the columnar epithelium or the disc proper (DP) toward the bottom and the peripodial epithelium (PE) toward the top of the images. (G) Schematic of an undamaged and a regenerating epithelium showing the location of a hemocyte outside the PE.
(TIF)
(A) Expression of molRNAi caused smaller adult wings after regeneration of the imaginal discs than controls. Three independent experiments, w1118 n = 402 wings, UAS-molRNAi n = 221 wings, p<0.0001 by a chi-squared test. (B-C) Cellular debris is visually distinct from the regenerating epithelium. Regenerating w1118 (B) and mole02670/+ (C) discs at R24, expressing the nub-GFP enhancer trap and stained with DHE. The speckled, grainy GFP tissue is cellular debris, outlined with yellow. The smooth GFP tissue is the intact epithelium, outlined with blue. The side panels are zoomed-in views of debris and epithelium, to show that they are easily distinguished. (D,E) Undamaged and R24 w1118 discs stained with DAPI and the ROS detector H2DCFDA. (F-J) gstD-GFP expression in undamaged w1118 (F), and regenerating w1118 (G,H) and mole02670/+ (I,J) wing discs at R24 (G,I) and R48 (H,J). (K-O) Anti-Myc immunostaining in w1118 (K,L) and mole02670/+ (M,N) regenerating wing discs at R24 (K,M) and R48 (L,N). (O) Quantification of immunofluorescence in Myc staining. R24 w1118 n = 14 discs, mole02670/+ n = 12 discs. R48 w1118 n = 11 discs, mole02670/+ n = 14 discs. Scale bars are 100 μm. Error bars are SEM except where noted. *p<0.02.
(TIF)
Regeneration assays using adult wing size to assess extent of regenerative growth in the imaginal discs. (A) Sizes of adult wings after disc regeneration in w1118 and UAS-DuoxRNAi animals. Three independent experiments. w1118 n = 390 wings, UAS-DuoxRNAi n = 200 wings, p = 0.0005 using a chi-squared test. (B) Sizes of adult wings after disc regeneration in w1118 and UAS-NoxRNAi animals. Two independent experiments, thus error bars are SD. w1118 n = 299 wings, UAS-NoxRNAi n = 257 wings, p<0.0001 using a chi-squared test. (C) Sizes of adult wings after disc regeneration in w1118 and NoxMI15634/+ animals. Three independent experiments. w1118 n = 349 wings, NoxMI15634/+ n = 180 wings, p<0.0001 using a chi-squared test. (D,E) qPCR showing effectiveness of Duox (D) and Nox (E) RNAi. The RNAi was expressed under rn-GAL4 control in the pouch of normally developing wing discs for 24 hours before collecting for qPCR. Three biological replicates each, *p<0.05. (F) Total number of mitotic cells as identified by anti-phospho-Histone H3 staining in the wing pouch as identified by anti-Nub staining in the indicated genotypes. R0 w1118 n = 14 discs, NoxMI15634/+ n = 15 discs, UAS-NoxRNAi n = 15 discs, R24 w1118 n = 12 discs, NoxMI15634/+ n = 17 discs, UAS-NoxRNAi n = 15 discs, R48 w1118 n = 11 discs, NoxMI15634/+ n = 18 discs, UAS-NoxRNAi n = 19 discs. (G) Area of the wing pouch as marked by anti-Nub staining was measured at R0, R24 and R48 for the indicated genotypes. R0 w1118 n = 14 discs, NoxMI15634/+ n = 15 discs, UAS-NoxRNAi n = 15 discs, R24 w1118 n = 12 discs, NoxMI15634/+ n = 17 discs, UAS-NoxRNAi n = 15 discs, R48 w1118 n = 11 discs, NoxMI15634/+ n = 18 discs, UAS-NoxRNAi n = 19 discs. (H) Pupariation timing for regenerating animals of the indicated gentoypes. Three independent experiments. w1118 n = 204, NoxMI15634/+ n = 113, UAS-NoxRNAi n = 206 (I) Pupariation timing for normally developing animals that did not experience the thermal shift and so did not ablate and regenerate the wing primordia. Note that because these animals did not experience the thermal shift, the pupariation timing cannot be compared directly to the pupariation timing in panel H, in which the animals did experience the thermal shift. w1118 n = 97, NoxMI15634/+ n = 49, UAS-NoxRNAi n = 122. Error bars are SEM.
(TIF)
(A-E) Anti-β-galacosidase immunostaining showing expression of the mol-lacZ reporter (green) in w1118 undamaged discs (A), and in regenerating wing discs at R0 (B), R24 (C) and R48 (D). (E) Quantification of mol-lacZ expression changes. Undamaged n = 3, R0 n = 7, R24 n = 10, R48 n = 10. (F,G) Anti-β-galacosidase immunostaining showing expression of the mol-lacZ reporter (green) in w1118 (F) and p38a1/+ (G) R24 discs. (H) Quantification of the fluorescence from the immunostaining. Two independent experiments, for a total w1118 n = 10 discs, p38a1/+ n = 12 discs. Scale bars are 100 μm. Error bars are SEM.
(TIF)
(PDF)
(PDF)
(DOCX)
Acknowledgments
The authors would like to thank A. Brock, K. Schuster, and L. O’Brien for critical reading of the manuscript and helpful discussions, D. Bohmann, K. Bruckner, A. Stathopoulos, I. Ando, M. Uhlirova, M. Ganfornina, and S. Cohen, the Bloomington Drosophila Stock Center (NIH P40OD018537), the TRiP project at Harvard Medical School (NIH/NIGMS R01-GM084947), the Vienna Drosophila Resource Center, and the Developmental Studies Hybridoma Bank (NICHD, University of Iowa) for reagents. The authors would also like to thank B. Pilas at the Flow Cytometry Facility, A. Hernandez at the High-Throughput Sequencing and Genotyping Unit, and R. Khetani at the High Performance Biological Computing Center, all of which are part of the Roy J. Carver Biotechnology Center at the University of Illinois Urbana-Champaign.
Data Availability
All differentially expressed gene files are available from the GEO database (accession number GSE101797). All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by a Roy J. Carver Charitable Trust Young Investigator Award (#12-4041) to RKSB, https://www.carvertrust.org, and a National Institutes of Health, National Institute of General Medical Sciences R01 grant (#R01 GM107140) to RKSB, https://www.nigms.nih.gov. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell. 2011. July 19;21(1):172–85. doi: 10.1016/j.devcel.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobmayer B, Rentzsch F, Kuhn K, Happel CM, Laue von CC, Snyder P, et al. WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature. 2000. September 14;407(6801):186–9. doi: 10.1038/35025063 [DOI] [PubMed] [Google Scholar]
- 3.Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Martí M, Dubova I, et al. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006. December 1;20(23):3232–7. doi: 10.1101/gad.1475106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen CP, Reddien PW. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008. January 18;319(5861):327–30. doi: 10.1126/science.1149943 [DOI] [PubMed] [Google Scholar]
- 5.Petersen CP, Reddien PW. A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci USA. 2009. October 6;106(40):17061–6. doi: 10.1073/pnas.0906823106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev Cell. 2009. June;16(6):797–809. doi: 10.1016/j.devcel.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams JM, Oh S-H, Jorgensen M, Steiger N, Darwiche H, Shupe T, et al. The role of the Wnt family of secreted proteins in rat oval “stem” cell-based liver regeneration: Wnt1 drives differentiation. Am J Pathol. 2010. June;176(6):2732–42. doi: 10.2353/ajpath.2010.080486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehner D, Cizelsky W, Vasudevaro MD, Ozhan G, Haase C, Kagermeier-Schenk B, et al. Wnt/β-catenin signaling defines organizing centers that orchestrate growth and differentiation of the regenerating zebrafish caudal fin. Cell Rep. 2014. February 13;6(3):467–81. doi: 10.1016/j.celrep.2013.12.036 [DOI] [PubMed] [Google Scholar]
- 9.Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, et al. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000. June 15;222(2):347–58. doi: 10.1006/dbio.2000.9722 [DOI] [PubMed] [Google Scholar]
- 10.Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci USA. 2007. October 23;104(43):17081–6. doi: 10.1073/pnas.0704126104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura T, Mito T, Miyawaki K, Ohuchi H, Noji S. EGFR signaling is required for re-establishing the proximodistal axis during distal leg regeneration in the cricket Gryllus bimaculatus nymph. Dev Biol. 2008. July 1;319(1):46–55. doi: 10.1016/j.ydbio.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 12.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009. July 23;138(2):257–70. doi: 10.1016/j.cell.2009.04.060 [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Grenley MO, Bravo M-J, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011. January 7;8(1):84–95. doi: 10.1016/j.stem.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White PM, Stone JS, Groves AK, Segil N. EGFR signaling is required for regenerative proliferation in the cochlea: conservation in birds and mammals. Dev Biol. 2012. March 1;363(1):191–200. doi: 10.1016/j.ydbio.2011.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013. March;19(3):295–304. doi: 10.1038/nm.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015. May;17(5):627–38. doi: 10.1038/ncb3149 [DOI] [PubMed] [Google Scholar]
- 17.Bando T, Mito T, Maeda Y, Nakamura T, Ito F, Watanabe T, et al. Regulation of leg size and shape by the Dachsous/Fat signalling pathway during regeneration. Development. The Company of Biologists Ltd; 2009. June 5;136(13):2235–45. doi: 10.1242/dev.035204 [DOI] [PubMed] [Google Scholar]
- 18.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 2011. February 1;350(1):139–51. doi: 10.1016/j.ydbio.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol. 2011. February 15;350(2):255–66. doi: 10.1016/j.ydbio.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 20.Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014. July 15;307(2):G196–204. doi: 10.1152/ajpgi.00077.2014 [DOI] [PubMed] [Google Scholar]
- 21.Lin AYT, Pearson BJ. Planarian yorkie/YAP functions to integrate adult stem cell proliferation, organ homeostasis and maintenance of axial patterning. Development. 2014. March;141(6):1197–208. doi: 10.1242/dev.101915 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi S, Ochi H, Ogino H, Kawasumi A, Kamei Y, Tamura K, et al. Transcriptional regulators in the Hippo signaling pathway control organ growth in Xenopus tadpole tail regeneration. Dev Biol. 2014. December 1;396(1):31–41. doi: 10.1016/j.ydbio.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 23.Bosch M, Serras F, Martín-Blanco E, Baguñà J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol. 2005. April 1;280(1):73–86. doi: 10.1016/j.ydbio.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 24.Tasaki J, Shibata N, Sakurai T, Agata K, Umesono Y. Role of c-Jun N-terminal kinase activation in blastema formation during planarian regeneration. Dev Growth Differ. 2011. April;53(3):389–400. doi: 10.1111/j.1440-169X.2011.01254.x [DOI] [PubMed] [Google Scholar]
- 25.Wuestefeld T, Pesic M, Rudalska R, Dauch D, Longerich T, Kang T-W, et al. A Direct in vivo RNAi screen identifies MKK4 as a key regulator of liver regeneration. Cell. 2013. April 11;153(2):389–401. doi: 10.1016/j.cell.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 26.Sandoval-Guzmán T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, et al. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell. 2014. February 6;14(2):174–87. doi: 10.1016/j.stem.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 27.Henry JJ, Tsonis PA. Molecular and cellular aspects of amphibian lens regeneration. Prog Retin Eye Res. 2010. November;29(6):543–55. doi: 10.1016/j.preteyeres.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bando T, Ishimaru Y, Kida T, Hamada Y, Matsuoka Y, Nakamura T, et al. Analysis of RNA-Seq data reveals involvement of JAK/STAT signalling during leg regeneration in the cricket Gryllus bimaculatus. Development. 2013. March;140(5):959–64. doi: 10.1242/dev.084590 [DOI] [PubMed] [Google Scholar]
- 29.Sikes JM, Newmark PA. Restoration of anterior regeneration in a planarian with limited regenerative ability. Nature. 2013. August 1;500(7460):77–80. doi: 10.1038/nature12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nachtrab G, Kikuchi K, Tornini VA, Poss KD. Transcriptional components of anteroposterior positional information during zebrafish fin regeneration. Development. 2013. September;140(18):3754–64. doi: 10.1242/dev.098798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worley MI, Setiawan L, Hariharan IK. Regeneration and Transdetermination in Drosophila Imaginal Discs. Annu Rev Genet. 2012. August 29. [DOI] [PubMed] [Google Scholar]
- 32.Bergantixos C, Corominas M, Serras F. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development. 2010. April;137(7):1169–79. doi: 10.1242/dev.045559 [DOI] [PubMed] [Google Scholar]
- 33.Reinhardt CA, Hodgkin NM, Bryant PJ. Wound healing in the imaginal discs of Drosophila. I. Scanning electron microscopy of normal and healing wing discs. Dev Biol. 1977. October 1;60(1):238–57. [DOI] [PubMed] [Google Scholar]
- 34.Reinhardt CA, Bryant PJ. Wound healing in the imaginal discs of Drosophila. II. Transmission electron microscopy of normal and healing wing discs. J Exp Zool. 1981. April;216(1):45–61. doi: 10.1002/jez.1402160107 [DOI] [PubMed] [Google Scholar]
- 35.Kiehle CP, Schubiger G. Cell proliferation changes during pattern regulation in imaginal leg discs of Drosophila melanogaster. Dev Biol. 1985. June;109(2):336–46. [DOI] [PubMed] [Google Scholar]
- 36.Gibson MC, Schubiger G. Hedgehog is required for activation of engrailed during regeneration of fragmented Drosophila imaginal discs. Development. 1999. April;126(8):1591–9. [DOI] [PubMed] [Google Scholar]
- 37.McClure KD, Schubiger G. A screen for genes that function in leg disc regeneration in Drosophila melanogaster. Mech Dev. 2008. January;125(1–2):67–80. doi: 10.1016/j.mod.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schubiger M, Sustar A, Schubiger G. Regeneration and transdetermination: the role of wingless and its regulation. Dev Biol. 2010. November 15;347(2):315–24. doi: 10.1016/j.ydbio.2010.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanco E, Ruiz-Romero M, Beltran S, Bosch M, Punset A, Serras F, et al. Gene expression following induction of regeneration in Drosophila wing imaginal discs. Expression profile of regenerating wing discs. BMC Dev Biol. 2010;10:94 doi: 10.1186/1471-213X-10-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsuyama T, Comoglio F, Seimiya M, Cabuy E, Paro R. During Drosophila disc regeneration, JAK/STAT coordinates cell proliferation with Dilp8-mediated developmental delay. Proc Natl Acad Sci USA. 2015. May 5;112(18):E2327–36. doi: 10.1073/pnas.1423074112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan SJ, Abidi SNF, Tian Y, Skinner A, Smith-Bolton RK. A rapid, gentle and scalable method for dissociation and fluorescent sorting of imaginal disc cells for mRNA sequencing. Fly (Austin). 2016. April 8;:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie X, Hu J, Liu X, Qin H, Percival-Smith A, Rao Y, et al. NIP/DuoxA is essential for Drosophila embryonic development and regulates oxidative stress response. Int J Biol Sci. 2010;6(3):252–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santabárbara-Ruiz P, López-Santillán M, Martínez-Rodríguez I, Binagui-Casas A, Pérez L, Milán M, et al. ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Drosophila Regeneration. PLoS Genet. 2015. October;11(10):e1005595 doi: 10.1371/journal.pgen.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994. April 29;264(5159):677–83. [DOI] [PubMed] [Google Scholar]
- 45.St Pierre SE, Galindo MI, Couso JP, Thor S. Control of Drosophila imaginal disc development by rotund and roughened eye: differentially expressed transcripts of the same gene encoding functionally distinct zinc finger proteins. Development. 2002. March;129(5):1273–81. [DOI] [PubMed] [Google Scholar]
- 46.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004. February 17;2004(220):pl6 doi: 10.1126/stke.2202004pl6 [DOI] [PubMed] [Google Scholar]
- 47.Ng M, Diaz-Benjumea FJ, Cohen SM. Nubbin encodes a POU-domain protein required for proximal-distal patterning in the Drosophila wing. Development. 1995. February;121(2):589–99. [DOI] [PubMed] [Google Scholar]
- 48.Venken KJT, Schulze KL, Haelterman NA, Pan H, He Y, Evans-Holm M, et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Methods. 2011. September;8(9):737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collesano M, Corona DFV. Flow cytometry and karyotype analysis of D.melanogaster eye disc cells. Fly (Austin). 2007. July;1(4):242–4. [DOI] [PubMed] [Google Scholar]
- 50.Neufeld TP, la Cruz de AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998. June 26;93(7):1183–93. [DOI] [PubMed] [Google Scholar]
- 51.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols. Nature Publishing Group; 2012. March 1;7(3):562–78. doi: 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36 doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martín-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998. February 15;12(4):557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012. May 4;336(6081):579–82. doi: 10.1126/science.1216735 [DOI] [PubMed] [Google Scholar]
- 55.McClure KD, Sustar A, Schubiger G. Three genes control the timing, the site and the size of blastema formation in Drosophila. Dev Biol. 2008. July 1;319(1):68–77. doi: 10.1016/j.ydbio.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rousset R, Carballès F, Parassol N, Schaub S, Cérézo D, Noselli S. Signalling crosstalk at the leading edge controls tissue closure dynamics in the Drosophila embryo. PLoS Genet. 2017. February;13(2):e1006640 doi: 10.1371/journal.pgen.1006640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang MY, Wang Z, MacPherson M, Dow JA, Kaiser K. A novel Drosophila alkaline phosphatase specific to the ellipsoid body of the adult brain and the lower Malpighian (renal) tubule. Genetics. 2000. January;154(1):285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekyrova P, Bohmann D, Jindra M, Uhlirova M. Interaction between Drosophila bZIP proteins Atf3 and Jun prevents replacement of epithelial cells during metamorphosis. Development. 2010. January;137(1):141–50. doi: 10.1242/dev.037861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu S, Lin S, Kao C-F, Awasaki T, Chiang A-S, Lee T. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006. October 20;127(2):409–22. doi: 10.1016/j.cell.2006.08.045 [DOI] [PubMed] [Google Scholar]
- 60.Chen T, Bunting M, Karim FD, Thummel CS. Isolation and characterization of five Drosophila genes that encode an ets-related DNA binding domain. Dev Biol. 1992. May;151(1):176–91. [DOI] [PubMed] [Google Scholar]
- 61.Gailey DA, Hall JC. Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Genetics. 1989. April;121(4):773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bossie CA, Sanders MM. A cDNA from Drosophila melanogaster encodes a lamin C-like intermediate filament protein. J Cell Sci. 1993. April;104 (Pt 4):1263–72. [DOI] [PubMed] [Google Scholar]
- 63.Dolezal T, Dolezelova E, Zurovec M, Bryant PJ. A role for adenosine deaminase in Drosophila larval development. PLoS Biol. 2005. July;3(7):e201 doi: 10.1371/journal.pbio.0030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeitlinger J, Kockel L, Peverali FA, Jackson DB, Mlodzik M, Bohmann D. Defective dorsal closure and loss of epidermal decapentaplegic expression in Drosophila fos mutants. EMBO J. 1997. December 15;16(24):7393–401. doi: 10.1093/emboj/16.24.7393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pines MK, Housden BE, Bernard F, Bray SJ, Röper K. The cytolinker Pigs is a direct target and a negative regulator of Notch signalling. Development. 2010. March;137(6):913–22. doi: 10.1242/dev.043224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007. January;7(3):323–31. doi: 10.1016/j.modgep.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 67.Bernal A, Kimbrell DA. Drosophila Thor participates in host immune defense and connects a translational regulator with innate immunity. Proc Natl Acad Sci USA. 2000. May 23;97(11):6019–24. doi: 10.1073/pnas.100391597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kodjabachian L, Delaage M, Maurel C, Miassod R, Jacq B, Rosset R. Mutations in ccf, a novel Drosophila gene encoding a chromosomal factor, affect progression through mitosis and interact with Pc-G mutations. EMBO J. 1998. February 16;17(4):1063–75. doi: 10.1093/emboj/17.4.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez D, Ganfornina MD, Torres-Schumann S, Speese SD, Lora JM, Bastiani MJ. Characterization of two novel lipocalins expressed in the Drosophila embryonic nervous system. Int J Dev Biol. 2000. June;44(4):349–59. [PubMed] [Google Scholar]
- 70.Thisse B, Messal el M, Perrin-Schmitt F. The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987. April 24;15(8):3439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai ZC, Rushton E, Bate M, Rubin GM. Loss of function of the Drosophila zfh-1 gene results in abnormal development of mesodermally derived tissues. Proc Natl Acad Sci USA. 1993. May 1;90(9):4122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herrera SC, Martín R, Morata G. Tissue homeostasis in the wing disc of Drosophila melanogaster: immediate response to massive damage during development. PLoS Genet. 2013;9(4):e1003446 doi: 10.1371/journal.pgen.1003446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verghese S, Su TT. Drosophila Wnt and STAT Define Apoptosis-Resistant Epithelial Cells for Tissue Regeneration after Irradiation. PLoS Biol. 2016. September;14(9):e1002536 doi: 10.1371/journal.pbio.1002536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skinner A, Khan SJ, Smith-Bolton RK. Trithorax regulates systemic signaling during Drosophila imaginal disc regeneration. Development. 2015. October 15;142(20):3500–11. doi: 10.1242/dev.122564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuss B, Hoch M. Drosophila endoderm development requires a novel homeobox gene which is a target of Wingless and Dpp signalling. Mech Dev. 1998. December;79(1–2):83–97. [DOI] [PubMed] [Google Scholar]
- 76.Fisk GJ, Thummel CS. Isolation, regulation, and DNA-binding properties of three Drosophila nuclear hormone receptor superfamily members. Proc Natl Acad Sci USA. 1995. November 7;92(23):10604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willy PJ, Kobayashi R, Kadonaga JT. A basal transcription factor that activates or represses transcription. Science. 2000. November 3;290(5493):982–5. [DOI] [PubMed] [Google Scholar]
- 78.Layalle S, Coessens E, Ghysen A, Dambly-Chaudière C. Smooth, a hnRNP encoding gene, controls axonal navigation in Drosophila. Genes Cells. 2005. February;10(2):119–25. doi: 10.1111/j.1365-2443.2005.00822.x [DOI] [PubMed] [Google Scholar]
- 79.Lubinsky S, Bewley GC. Genetics of Catalase in DROSOPHILA MELANOGASTER: Rates of Synthesis and Degradation of the Enzyme in Flies Aneuploid and Euploid for the Structural Gene. Genetics. 1979. April;91(4):723–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vonesch SC, Lamparter D, Mackay TFC, Bergmann S, Hafen E. Genome-Wide Analysis Reveals Novel Regulators of Growth in Drosophila melanogaster. PLoS Genet. 2016. January;12(1):e1005616 doi: 10.1371/journal.pgen.1005616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brock AR, Seto M, Smith-Bolton RK. Cap-n-collar Promotes Tissue Regeneration by Regulating ROS and JNK Signaling in the Drosophila Wing Imaginal Disc. Genetics. 2017. May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuster KJ, Smith-Bolton RK. Taranis Protects Regenerating Tissue from Fate Changes Induced by the Wound Response in Drosophila. Dev Cell. 2015. June 17;34:119–28. doi: 10.1016/j.devcel.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 83.Radyuk SN, Michalak K, Klichko VI, Benes J, Orr WC. Peroxiredoxin 5 modulates immune response in Drosophila. Biochim Biophys Acta. 2010. November;1800(11):1153–63. doi: 10.1016/j.bbagen.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Külshammer E, Mundorf J, Kilinc M, Frommolt P, Wagle P, Uhlirova M. Interplay among Drosophila transcription factors Ets21c, Fos and Ftz-F1 drives JNK-mediated tumor malignancy. Dis Model Mech. 2015. October 1;8(10):1279–93. doi: 10.1242/dmm.020719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toggweiler J, Willecke M, Basler K. The transcription factor Ets21C drives tumor growth by cooperating with AP-1. Sci Rep. 2016. October 7;6:34725 doi: 10.1038/srep34725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patterson RA, Juarez MT, Hermann A, Sasik R, Hardiman G, McGinnis W. Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila. PLoS ONE. 2013;8(4):e61773 doi: 10.1371/journal.pone.0061773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Monson JM, Natzle J, Friedman J, McCarthy BJ. Expression and novel structure of a collagen gene in Drosophila. Proc Natl Acad Sci USA. 1982. March;79(6):1761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yasothornsrikul S, Davis WJ, Cramer G, Kimbrell DA, Dearolf CR. viking: identification and characterization of a second type IV collagen in Drosophila. Gene. 1997. October 1;198(1–2):17–25. [DOI] [PubMed] [Google Scholar]
- 89.Beiman M, Shilo BZ, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996. December 1;10(23):2993–3002. [DOI] [PubMed] [Google Scholar]
- 90.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 91.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009. January;37(1):1–13. doi: 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sustar A, Bonvin M, Schubiger M, Schubiger G. Drosophila twin spot clones reveal cell division dynamics in regenerating imaginal discs. Dev Biol. 2011. August 15;356(2):576–87. doi: 10.1016/j.ydbio.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan Y, Wang S, Hernandez J, Yenigun VB, Hertlein G, Fogarty CE, et al. Genetic models of apoptosis-induced proliferation decipher activation of JNK and identify a requirement of EGFR signaling for tissue regenerative responses in Drosophila. PLoS Genet. 2014. January;10(1):e1004131 doi: 10.1371/journal.pgen.1004131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Díaz-García S, Baonza A. Pattern reorganization occurs independently of cell division during Drosophila wing disc regeneration in situ. Proc Natl Acad Sci USA. 2013. August 6;110(32):13032–7. doi: 10.1073/pnas.1220543110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Love NR, Chen Y, Bonev B, Gilchrist MJ, Fairclough L, Lea R, et al. Genome-wide analysis of gene expression during Xenopus tropicalis tadpole tail regeneration. BMC Dev Biol. 2011;11:70 doi: 10.1186/1471-213X-11-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van der Vliet A, Janssen-Heininger YMW. Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger? J Cell Biochem. 2014. March;115(3):427–35. doi: 10.1002/jcb.24683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009. June 18;459(7249):996–9. doi: 10.1038/nature08119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moreira S, Stramer B, Evans I, Wood W, Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol. 2010. March 9;20(5):464–70. doi: 10.1016/j.cub.2010.01.047 [DOI] [PubMed] [Google Scholar]
- 99.Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, et al. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol. 2013. February;15(2):222–8. doi: 10.1038/ncb2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoo SK, Freisinger CM, LeBert DC, Huttenlocher A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J Cell Biol. 2012. October 15;199(2):225–34. doi: 10.1083/jcb.201203154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rieger S, Sagasti A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol. 2011. May;9(5):e1000621 doi: 10.1371/journal.pbio.1000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, et al. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep. 2013;3:2084 doi: 10.1038/srep02084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fogarty CE, Diwanji N, Lindblad JL, Tare M, Amcheslavsky A, Makhijani K, et al. Extracellular Reactive Oxygen Species Drive Apoptosis-Induced Proliferation via Drosophila Macrophages. Curr Biol. 2016. March 7;26(5):575–84. doi: 10.1016/j.cub.2015.12.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ha E-M, Lee K-A, Park SH, Kim S-H, Nam H-J, Lee H-Y, et al. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009. March;16(3):386–97. doi: 10.1016/j.devcel.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 105.Ritsick DR, Edens WA, McCoy JW, Lambeth JD. The use of model systems to study biological functions of Nox/Duox enzymes. Biochem Soc Symp. 2004;(71):85–96. [DOI] [PubMed] [Google Scholar]
- 106.Ritsick DR, Edens WA, Finnerty V, Lambeth JD. Nox regulation of smooth muscle contraction. Free Radic Biol Med. 2007. July 1;43(1):31–8. doi: 10.1016/j.freeradbiomed.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009. October;11(10):2607–19. doi: 10.1089/ARS.2009.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seto NO, Hayashi S, Tener GM. Cloning, sequence analysis and chromosomal localization of the Cu-Zn superoxide dismutase gene of Drosophila melanogaster. Gene. 1989. January 30;75(1):85–92. [DOI] [PubMed] [Google Scholar]
- 109.Duttaroy A, Parkes T, Emtage P, Kirby K, Boulianne GL, Wang X, et al. The manganese superoxide dismutase gene of Drosophila: structure, expression, and evidence for regulation by MAP kinase. DNA Cell Biol. 1997. April;16(4):391–9. doi: 10.1089/dna.1997.16.391 [DOI] [PubMed] [Google Scholar]
- 110.Jung I, Kim T-Y, Kim-Ha J. Identification of Drosophila SOD3 and its protective role against phototoxic damage to cells. FEBS Lett. 2011. June 23;585(12):1973–8. doi: 10.1016/j.febslet.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 111.Anderson PR, Kirby K, Hilliker AJ, Phillips JP. RNAi-mediated suppression of the mitochondrial iron chaperone, frataxin, in Drosophila. Hum Mol Genet. 2005. November 15;14(22):3397–405. doi: 10.1093/hmg/ddi367 [DOI] [PubMed] [Google Scholar]
- 112.Missirlis F, Hu J, Kirby K, Hilliker AJ, Rouault TA, Phillips JP. Compartment-specific protection of iron-sulfur proteins by superoxide dismutase. J Biol Chem. 2003. November 28;278(48):47365–9. doi: 10.1074/jbc.M307700200 [DOI] [PubMed] [Google Scholar]
- 113.Missirlis F, Phillips JP, Jäckle H. Cooperative action of antioxidant defense systems in Drosophila. Curr Biol. 2001. August 21;11(16):1272–7. [DOI] [PubMed] [Google Scholar]
- 114.Chatterjee N, Bohmann D. A versatile ΦC31 based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS ONE. 2012. January 1;7(4):e34063–3. doi: 10.1371/journal.pone.0034063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Makhijani K, Alexander B, Tanaka T, Rulifson E, Brückner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011. December;138(24):5379–91. doi: 10.1242/dev.067322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kurucz E, Márkus R, Zsámboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007. April 3;17(7):649–54. doi: 10.1016/j.cub.2007.02.041 [DOI] [PubMed] [Google Scholar]
- 117.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006. July 7;281(27):18269–72. doi: 10.1074/jbc.C600095200 [DOI] [PubMed] [Google Scholar]
- 118.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009. April;23(4):1205–18. doi: 10.1096/fj.08-120006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008. January;14(1):76–85. doi: 10.1016/j.devcel.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bosch JA, Sumabat TM, Hariharan IK. Persistence of RNAi-Mediated Knockdown in Drosophila Complicates Mosaic Analysis Yet Enables Highly Sensitive Lineage Tracing. Genetics. 2016. May;203(1):109–18. doi: 10.1534/genetics.116.187062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perkins KK, Dailey GM, Tjian R. Novel Jun- and Fos-related proteins in Drosophila are functionally homologous to enhancer factor AP-1. EMBO J. 1988. December 20;7(13):4265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987. June 19;49(6):729–39. [DOI] [PubMed] [Google Scholar]
- 123.Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, et al. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999. March;19(3):2322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glise B, Bourbon H, Noselli S. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995. November 3;83(3):451–61. [DOI] [PubMed] [Google Scholar]
- 125.Sandiford SD, Kennedy KA, Xie X, Pickering JG, Li SS. Dual Oxidase Maturation factor 1 (DUOXA1) overexpression increases reactive oxygen species production and inhibits murine muscle satellite cell differentiation. Cell Communication and Signaling 2014 12:1. BioMed Central; 2014. January 11;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Amano I, Takatsuru Y, Toya S, Haijima A, Iwasaki T, Grasberger H, et al. Aberrant Cerebellar Development in Mice Lacking Dual Oxidase Maturation Factors. Thyroid. 2016. May;26(5):741–52. doi: 10.1089/thy.2015.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grasberger H, El-Zaatari M, Dang DT, Merchant JL. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology. 2013. November;145(5):1045–54. doi: 10.1053/j.gastro.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moribe H, Konakawa R, Koga D, Ushiki T, Nakamura K, Mekada E. Tetraspanin is required for generation of reactive oxygen species by the dual oxidase system in Caenorhabditis elegans. PLoS Genet. 2012. September;8(9):e1002957 doi: 10.1371/journal.pgen.1002957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol. 2013. March 4;23(5):424–9. doi: 10.1016/j.cub.2013.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang S, Linderholm A, Franzi L, Kenyon N, Grasberger H, Harper R. Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radic Biol Med. 2013. December;65:38–46. doi: 10.1016/j.freeradbiomed.2013.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.King-Jones K, Thummel CS. Nuclear receptors—a perspective from Drosophila. Nat Rev Genet. 2005. April;6(4):311–23. doi: 10.1038/nrg1581 [DOI] [PubMed] [Google Scholar]
- 132.Halme A, Cheng M, Hariharan IK. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr Biol. 2010. March 9;20(5):458–63. doi: 10.1016/j.cub.2010.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guittard E, Blais C, Maria A, Parvy J-P, Pasricha S, Lumb C, et al. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev Biol. 2011. January 1;349(1):35–45. doi: 10.1016/j.ydbio.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 134.Jin Y, Ha N, Forés M, Xiang J, Gläßer C, Maldera J, et al. EGFR/Ras Signaling Controls Drosophila Intestinal Stem Cell Proliferation via Capicua-Regulated Genes. PLoS Genet. 2015. December;11(12):e1005634 doi: 10.1371/journal.pgen.1005634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, et al. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999. September;153(1):135–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995. March 1;9(5):534–46. [DOI] [PubMed] [Google Scholar]
- 137.Rynes J, Donohoe CD, Frommolt P, Brodesser S, Jindra M, Uhlirova M. Activating transcription factor 3 regulates immune and metabolic homeostasis. Mol Cell Biol. 2012. October;32(19):3949–62. doi: 10.1128/MCB.00429-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007. March;175(3):1505–31. doi: 10.1534/genetics.106.065961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nagarkar-Jaiswal S, Lee P-T, Campbell ME, Chen K, Anguiano-Zarate S, Gutierrez MC, et al. A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. Elife. 2015. March 31;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004. March;36(3):283–7. doi: 10.1038/ng1314 [DOI] [PubMed] [Google Scholar]
- 141.Mendes CS, Levet C, Chatelain G, Dourlen P, Fouillet A, Dichtel-Danjoy M-L, et al. ER stress protects from retinal degeneration. EMBO J. 2009. May 6;28(9):1296–307. doi: 10.1038/emboj.2009.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weber U, Paricio N, Mlodzik M. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development. 2000. August;127(16):3619–29. [DOI] [PubMed] [Google Scholar]
- 143.Craig CR, Fink JL, Yagi Y, Ip YT, Cagan RL. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 2004. November;5(11):1058–63. doi: 10.1038/sj.embor.7400282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trisnadi N, Stathopoulos A. Ectopic expression screen identifies genes affecting Drosophila mesoderm development including the HSPG Trol. G3 (Bethesda). 2014. December 23;5(2):301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gouge CA, Christensen TW. Detection of S Phase in multiple Drosophila tissues utilizing the EdU labeling technique. Dros Inf Serv. Norman, OK; 2010. December 1;93:203–12. [Google Scholar]
- 146.Owusu-Ansah E, Yavari A, Banerjee U. A protocol for in vivo detection of reactive oxygen species. [Internet]. Protocol Exchange. 2008. [cited 2017 Feb 1]. Available from: http://www.nature.com/protocolexchange/protocols/414 doi: 10.1038/nprot.2008.27 [Google Scholar]
- 147.Blankenberg D, Kuster Von G, Coraor N, Ananda G, Lazarus R, Mangan M, et al. Galaxy: a web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol. 2010. January;Chapter 19:Unit19.10.1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002. January 1;30(1):207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Variation in the size of the wing pouch as marked by anti-Nub immunostaining in undamaged and ablated wing discs. Note the minimal variation in pouch size at R0, indicating consistency of ablation. Undamaged n = 8, regenerating n = 28. Error bars are SEM. (B) Quantification of percentage of regenerating animals that had pupariated by each day, showing the asynchronous progression to pupariation that occurred after tissue damage. Undamaged animals were w1118;; rnGAL4, Gal80TS/+, regenerating animals were w1118;; rnGAL4, UAS-reaper, Gal80TS/+, and both experienced the 24-hour temperature shift. Three independent experiments, total undamaged n = 144 pupae, regenerating n = 176 pupae. (C) Percentage of wings of different sizes on w1118;; rnGAL4, UAS-reaper, Gal80TS/+ animals that eclosed on different days after egg laying due the asynchronous development induced by tissue damage. Note that the animals that eclosed first (day 18) had smaller wings than those that eclosed on day 19, and those that eclosed on day 20 had the largest wings. Thus, variation in wing size after disc regeneration is partly determined by length of time for regeneration. Three independent experiments, total n = 371 wings.
(TIF)
(A) Venn diagrams showing genes at least 1.3-fold upregulated or downregulated in three transcription profiles (this work, [39,40]) of regenerating wing discs generated using different methods. Note that the number of genes from the Blanco et al. study is an underrepresentation because the complete list of differentially expressed genes identified was not published. (B) Lists of differentially expressed genes (DEGs) in common between this study and each prior study, along with fold change in this study.
(TIF)
Undamaged (A-G) and regenerating (R24) (A’-G’) wing discs. (A-A’) AdoR-GFP MiMIC enhancer trap. (B-B’) Kayak-GFP protein trap. (C-C’) Thor-lacZ enhancer trap. (D-D’) Corto-GFP protein trap. (E-E’) NLaz-GFP MiMIC enhancer trap. (F-F’) anti-Twist. (G-G’) zfh1-lacZ enhancer trap. Blue dashed line outlines the wing primordium. Scale bars are 100 μm. (H) Quantification of upregulation of mol, Nox, and Ets21C expression using qPCR. Four biological replicates each. Error bars are SEM, *p<0.05.
(TIF)
(A) Adult fru3/+ males had larger wings after regeneration than controls. Three independent experiments, w1118 n = 112 wings, fru3/+ n = 95 wings, p<0.001 by a chi-squared test. (B) Adult htlAB42/+ animals had larger wings after regeneration than controls. Three independent experiments, w1118 n = 316 wings, htlAB42/+ n = 223 wings, p<0.001 by a chi-squared test. Error bars are SEM.
(TIF)
(A-D) Hemolectin-RFP (Hml-RFP) (green) showing hemocytes near undamaged (A,B) and R24 (C,D) wing discs. (E,F) Anti-Nimrod (green) also showing hemocytes near an R24 wing disc, confirming the Hml-RFP results. (B), (D), and (F) are orthogonal slices with the columnar epithelium or the disc proper (DP) toward the bottom and the peripodial epithelium (PE) toward the top of the images. (G) Schematic of an undamaged and a regenerating epithelium showing the location of a hemocyte outside the PE.
(TIF)
(A) Expression of molRNAi caused smaller adult wings after regeneration of the imaginal discs than controls. Three independent experiments, w1118 n = 402 wings, UAS-molRNAi n = 221 wings, p<0.0001 by a chi-squared test. (B-C) Cellular debris is visually distinct from the regenerating epithelium. Regenerating w1118 (B) and mole02670/+ (C) discs at R24, expressing the nub-GFP enhancer trap and stained with DHE. The speckled, grainy GFP tissue is cellular debris, outlined with yellow. The smooth GFP tissue is the intact epithelium, outlined with blue. The side panels are zoomed-in views of debris and epithelium, to show that they are easily distinguished. (D,E) Undamaged and R24 w1118 discs stained with DAPI and the ROS detector H2DCFDA. (F-J) gstD-GFP expression in undamaged w1118 (F), and regenerating w1118 (G,H) and mole02670/+ (I,J) wing discs at R24 (G,I) and R48 (H,J). (K-O) Anti-Myc immunostaining in w1118 (K,L) and mole02670/+ (M,N) regenerating wing discs at R24 (K,M) and R48 (L,N). (O) Quantification of immunofluorescence in Myc staining. R24 w1118 n = 14 discs, mole02670/+ n = 12 discs. R48 w1118 n = 11 discs, mole02670/+ n = 14 discs. Scale bars are 100 μm. Error bars are SEM except where noted. *p<0.02.
(TIF)
Regeneration assays using adult wing size to assess extent of regenerative growth in the imaginal discs. (A) Sizes of adult wings after disc regeneration in w1118 and UAS-DuoxRNAi animals. Three independent experiments. w1118 n = 390 wings, UAS-DuoxRNAi n = 200 wings, p = 0.0005 using a chi-squared test. (B) Sizes of adult wings after disc regeneration in w1118 and UAS-NoxRNAi animals. Two independent experiments, thus error bars are SD. w1118 n = 299 wings, UAS-NoxRNAi n = 257 wings, p<0.0001 using a chi-squared test. (C) Sizes of adult wings after disc regeneration in w1118 and NoxMI15634/+ animals. Three independent experiments. w1118 n = 349 wings, NoxMI15634/+ n = 180 wings, p<0.0001 using a chi-squared test. (D,E) qPCR showing effectiveness of Duox (D) and Nox (E) RNAi. The RNAi was expressed under rn-GAL4 control in the pouch of normally developing wing discs for 24 hours before collecting for qPCR. Three biological replicates each, *p<0.05. (F) Total number of mitotic cells as identified by anti-phospho-Histone H3 staining in the wing pouch as identified by anti-Nub staining in the indicated genotypes. R0 w1118 n = 14 discs, NoxMI15634/+ n = 15 discs, UAS-NoxRNAi n = 15 discs, R24 w1118 n = 12 discs, NoxMI15634/+ n = 17 discs, UAS-NoxRNAi n = 15 discs, R48 w1118 n = 11 discs, NoxMI15634/+ n = 18 discs, UAS-NoxRNAi n = 19 discs. (G) Area of the wing pouch as marked by anti-Nub staining was measured at R0, R24 and R48 for the indicated genotypes. R0 w1118 n = 14 discs, NoxMI15634/+ n = 15 discs, UAS-NoxRNAi n = 15 discs, R24 w1118 n = 12 discs, NoxMI15634/+ n = 17 discs, UAS-NoxRNAi n = 15 discs, R48 w1118 n = 11 discs, NoxMI15634/+ n = 18 discs, UAS-NoxRNAi n = 19 discs. (H) Pupariation timing for regenerating animals of the indicated gentoypes. Three independent experiments. w1118 n = 204, NoxMI15634/+ n = 113, UAS-NoxRNAi n = 206 (I) Pupariation timing for normally developing animals that did not experience the thermal shift and so did not ablate and regenerate the wing primordia. Note that because these animals did not experience the thermal shift, the pupariation timing cannot be compared directly to the pupariation timing in panel H, in which the animals did experience the thermal shift. w1118 n = 97, NoxMI15634/+ n = 49, UAS-NoxRNAi n = 122. Error bars are SEM.
(TIF)
(A-E) Anti-β-galacosidase immunostaining showing expression of the mol-lacZ reporter (green) in w1118 undamaged discs (A), and in regenerating wing discs at R0 (B), R24 (C) and R48 (D). (E) Quantification of mol-lacZ expression changes. Undamaged n = 3, R0 n = 7, R24 n = 10, R48 n = 10. (F,G) Anti-β-galacosidase immunostaining showing expression of the mol-lacZ reporter (green) in w1118 (F) and p38a1/+ (G) R24 discs. (H) Quantification of the fluorescence from the immunostaining. Two independent experiments, for a total w1118 n = 10 discs, p38a1/+ n = 12 discs. Scale bars are 100 μm. Error bars are SEM.
(TIF)
(PDF)
(PDF)
(DOCX)
Data Availability Statement
All differentially expressed gene files are available from the GEO database (accession number GSE101797). All other relevant data are within the paper and its Supporting Information files.