Abstract
Incidence rates of childhood leukemia in the United States have steadily increased over the last several decades, but only recently have disparities in the increase in incidence been recognized. In the current analysis, Surveillance, Epidemiology and End Results (SEER) data were used to evaluate recent trends in the incidence of childhood leukemia diagnosed at age 0–19 years from 1992–2013, overall and by age, race/ethnicity, gender, and histologic subtype. Hispanic White children were more likely than non-Hispanic White, non-Hispanic Black or non-Hispanic Asian children to be diagnosed with acute lymphocytic leukemia (ALL) from 2009–2013. From 1992–2013, a significant increase in ALL incidence was observed for Hispanic White children (annual percent change (APC)Hispanic=1.08, 95%CI:0.59, 1.58); no significant increase was observed for non-Hispanic White, Black or Asian children. ALL incidence increased by about 3% per year from 1992–2013 for Hispanic White children diagnosed from 15–19 years (APC=2.67; 95%CI:0.88, 4.49), and by 2% for those 10–14 years (APC=2.09; 95%CI:0.57, 3.63), while no significant increases in incidence were observed in non-Hispanic White, Black, or Asian children of the same age. Acute myeloid leukemia (AML) incidence increased among non-Hispanic White children under 1 year at diagnosis, and among Hispanic White children diagnosed at age 1–4. The increase in incidence rates of childhood ALL appears to be driven by rising rates in older Hispanic children (10–14, and 15–19 years). Future studies are needed to evaluate reasons for the increase in ALL among older Hispanic children.
Keywords: childhood leukemia, trends, incidence, SEER
INTRODUCTION
Leukemia is the most common cancer in children and adolescents 0 to 19 years of age (hereafter referred to as “children”), and the highest incidence rates of leukemia have been reported for children of Hispanic ethnicity (Age Adjusted Incidence Rate (AAIR) for 2009–2013=6.05 per 100,000)1. While incidence rates for childhood ALL in all races/ethnicities combined have increased approximately 1% per year since 1973, a greater increase in incidence has recently been observed among Hispanic children1, though reasons for this disparity in rates are unknown. In the past 20 years, numerous studies have focused on evaluating the association between childhood leukemia and environmental or lifestyle factors, which are likely to contribute to the observed racial/ethnic disparity in incidence. Ionizing radiation remains the only established causal environmental risk factor for childhood leukemia. Other lifestyle or environmental factors have been suggested as causal agents, with compelling evidence for residential or occupational exposure to pesticides2–5. A series of meta-analyses or pooled analyses also suggest ALL risk may be higher for children with exposure to non-ionizing radiation6–8, paternal tobacco use9, 10, paint 11–13, and high birth weight14, 15, whereas atopic conditions (e.g. allergy, asthma, hay fever)16, 17, day care attendance (as a surrogate for early life immune challenge, addressing the “population mixing” hypothesis of ALL leukomogenesis)18, 19, and maternal folate supplementation20 are associated with a reduced risk. However, few studies18, 21–24 have reported associations between these risk factors and childhood leukemia by Hispanic ethnicity, specifically.
Allelic variants that are believed to be functional and to affect metabolism of environmental agents may also contribute to observed differences in incidence rates between Hispanic and non-Hispanic children. Susceptibility loci have been evaluated in a number of genes, including MTHFR (14 studies), GSTM1 and GSTT1 (13 studies each), GSTP1, CYP1A1, and NQO1 (7 studies each)25. If Hispanic children are more genetically susceptible to some environmental factors (e.g., pesticides), increased prevalence of exposure to that environmental factor may explain, at least in part, the growing disparity in incidence rate trends by ethnicity.
In this analysis of Surveillance, Epidemiology and End Results (SEER) registry data, we expand upon a previous publication1 to evaluate recent trends in the incidence of childhood leukemia (diagnosed at age 0–19 years) in the United States from 1992–2013, and to evaluate differences in leukemia incidence over time by race/ethnicity and other demographic characteristics. We aim to explore potential reasons for the greater increase in incidence among Hispanic children and the disproportionate burden of disease in Hispanic populations.
MATERIALS AND METHODS
Data
Trends in childhood leukemia incidence from 1992–2013 were analyzed using data from the National Cancer Institute’s SEER population-based registries. Overall trends in leukemia incidence from 1992–2013 were analyzed using SEER 13 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco/Oakland, Seattle/Puget Sound, Utah, Alaska, Los Angeles, San Jose-Monterey, and Rural Georgia). Age-adjusted incidence rates (AAIR) for recent years (2009–2013) by race/ethnicity are shown using data available from all 18 SEER registries (Alaska, Atlanta, Connecticut, Detroit, Greater California, Greater Georgia, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Jersey New Mexico, Rural Georgia, San Francisco-Oakland, San Jose-Monterey, Seattle/Puget Sound, and Utah). SEER 13 (1992–2013) and SEER 18 (2000–2013) data includes standard demographic variables, including race (White, Black, American Indian/Alaska Native, and Asian or Pacific Islander) and a variable describing Hispanic ethnicity, which was assigned based on the North American Association of Central Cancer Registries Hispanic Identification Algorithm 26.
All cases of leukemia diagnosed in children aged 19 or younger were included in the analyses. Childhood leukemia was defined according to the International Classification of Childhood Cancer (ICCC) which uses the World Health Organization International Classification of Diseases for Oncology (ICDO-3) histology codes to define diagnosis with childhood leukemia (defined as those diagnosed at age 19 or younger) (see Supplemental Material for detailed information).
Statistical analysis
AAIRs were calculated using SEER*stat 8.0.4 (www.seer.cancer.gov/seerstat) and the 2000 U.S. Standard Population for age standardization, based on population data from 2009–2013. Trends in incidence rates from 1992–2013 by race/ethnicity were evaluated using estimates of annual percent change (APC). APC estimates were calculated by fitting a least squares regression line using the natural log of the rates as the outcome variable, with year of diagnosis as the primary predictor variable. JoinPoint regression program 4.0.4 (http://surveillance.cancer.gov/joinpoint/) was used to determine whether a piecewise linear spline was a better fit for the data than a simple linear estimator for the overall trends. 95% confidence intervals and p-values estimates for AAIR and APC estimates were calculated using SEER*stat.
RESULTS
Overall
From 2009–2013, 5,443 children were diagnosed with leukemia, including 1,999 Hispanic White children (36.7% of childhood leukemia diagnoses), 2,364 non-Hispanic White children (43.4% of childhood leukemia diagnoses), 416 non-Hispanic Black children (7.6% of childhood leukemia diagnoses), 448 non-Hispanic Asian children (8.2% of childhood leukemia diagnoses), and 216 children of another racial/ethnic category. For all leukemia subtypes combined, the AAIR from 2009–2013 was 6.05 per 100,000 persons for Hispanic White children (95%CI: 5.79, 6.32), 4.45 per 100,000 persons for non-Hispanic White children (95%CI: 4.27, 4.63), 2.62 per 100,000 persons for non-Hispanic Black children (95%CI: 2.37, 2.88), and 4.21 per 100,000 for non-Hispanic Asian children (95%CI: 3.83, 4.62; Table 1). In all racial/ethnic groups, incidence rates were higher in males, and highest in children aged 1–4 years. From 1992–2013, the incidence rate for all leukemias increased significantly among both Hispanic White children (APC=0.96; 95%CI: 0.50, 1.41) and non-Hispanic White children (APC=0.63; 95%CI: 0.12, 1.14), but not among non-Hispanic Black children or among non-Hispanic White children.
Table 1.
Age-Adjusted Incidence Rates (AAIR; SEER18, 2009–2013) and Annual Percent Change (APC; SEER13 1992–2013) for any childhood leukemia, ALL, and AML for each race/ethnicity by selected demographic characteristics
| Hispanic-White | Non-Hispanic White | Non-Hispanic Black | Non-Hispanic Asian | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | AAIRa (95%CI) | APCb (95%CI) | N | AAIRa (95%CI) | APCb (95%CI) | N | AAIRa (95%CI) | APCb (95%CI) | N | AAIRa (95%CI) | APCb (95%CI) | |
| Any Leukemia** | 1999 | 6.05 (5.79, 6.32) | 0.96 (0.50, 1.41)* | 2364 | 4.45 (4.27, 4.63) | 0.63 (0.12, 1.14)* | 416 | 2.62 (2.37, 2.88) | 0.25 (−0.56, 1.05) | 448 | 4.21 (3.83, 4.62) | 0.07 (−0.97, 1.12) |
| Gender | ||||||||||||
| Male | 1099 | 6.53 (6.15, 6.93) | 0.72 (0.07, 1.38)* | 1297 | 4.75 (4.50, 5.02) | 0.38 (−0.33, 1.10) | 231 | 2.86 (2.50, 3.25) | 0.54 (−0.65, 1.75) | 253 | 4.66 (4.10, 5.27) | 0.28 (−1.28, 1.87) |
| Female | 900 | 5.55 (5.19, 5.92) | 1.29 (0.42, 2.17)* | 1067 | 4.13 (3.89, 4.39) | 0.91 (0.22, 1.61)* | 185 | 2.37 (2.04, 2.73) | −0.11 (−1.47, 1.27) | 195 | 3.75 (3.24, 4.32) | −0.28 (−1.53, 0.98) |
| Age | ||||||||||||
| <1 | 95 | 5.04 (4.03, 6.24) | 0.60 (−1.75, 3.00) | 85 | 3.87 (3.13, 4.73) | 3.09 (0.79, 5.45)* | 25 | 3.30 (2.13, 4.87) | NA | 31 | 6.15 (4.18, 8.73) | NA |
| 1–4yrs | 770 | 11.4 (10.6, 12.2) | 0.88 (0.08, 1.68)* | 965 | 9.58 (8.98, 10.2) | 0.46 (−0.34, 1.26) | 141 | 4.58 (3.86, 5.41) | 1.15 (−0.62, 2.95) | 170 | 8.15 (6.97, 9.47) | −0.76 (−2.06, 0.55) |
| 5–9yrs | 448 | 5.51 (5.01, 6.05) | 0.00 (−1.02, 1.03) | 571 | 4.35 (4.00, 4.72) | 0.64 (−0.63, 1.94) | 87 | 2.27 (1.82, 2.80) | −0.97 (−2.93, 1.03) | 100 | 3.77 (3.06, 4.58) | −0.25 (−2.04, 1.57) |
| 10–14yrs | 353 | 4.48 (4.03, 4.98) | 1.41 (0.28, 2.56)* | 350 | 2.55 (2.29, 2.83) | 0.22 (−0.79, 1.25) | 106 | 2.63 (2.16, 3.19) | 1.41 (−1.10, 3.98) | 72 | 2.76 (2.16, 3.47) | 0.49 (−1.79, 2.81) |
| 15–19yrs | 343 | 4.28 (3.84, 4.76) | 1.81 (0.20, 3.46)* | 383 | 2.66 (2.40, 2.94) | 0.72 (−0.66, 2.11) | 57 | 1.31 (0.99, 1.69) | NA | 75 | 2.75 (2.16, 3.44) | 0.57 (−1.83, 3.02) |
|
| ||||||||||||
| ALL | 1617 | 4.89 (4.65, 5.13) | 1.08 (0.59, 1.58)* | 1819 | 3.44 (3.28, 3.60) | 0.63 (0.00, 1.26) | 289 | 1.83 (1.62, 2.05) | 0.34 (−1.04, 1.74) | 316 | 2.97 (2.65, 3.32) | 0.07 (−0.93, 1.08) |
| Gender | ||||||||||||
| Male | 879 | 5.22 (4.88, 5.57) | 0.77 (0.04, 1.51)* | 1016 | 3.74 (3.51, 3.97) | 0.33 (−0.46, 1.12) | 166 | 2.06 (1.76, 2.40) | 1.03 (−0.98, 3.08) | 179 | 3.30 (2.83, 3.82) | 0.01 (−1.86, 1.91) |
| Female | 738 | 4.54 (4.21, 4.87) | 1.56 (0.67, 2.46)* | 803 | 3.13 (2.91, 3.35) | 0.97 (0.13, 1.82)* | 123 | 1.59 (1.32, 1.89) | −0.35 (−2.58, 1.92) | 137 | 2.63 (2.21, 3.11) | 0.01 (−1.35, 1.39) |
| Age | ||||||||||||
| <1 | 43 | 2.55 (1.85, 3.44) | −1.14 (−4.15, 1.97) | 41 | 1.67 (1.20, 2.27) | 2.09 (−0.74, 5.00) | 11 | 1.45 (0.72, 2.59) | NA | 14 | 2.78 (1.52, 4.66) | NA |
| 1–4yrs | 669 | 9.89 (9.15, 10.7) | 0.69 (−0.19, 1.58) | 837 | 8.31 (7.75, 8.89) | 0.66 (−0.16, 1.48) | 102 | 3.32 (2.70, 4.03) | 0.22 (−1.82, 2.29) | 137 | 6.57 (5.51, 7.76) | −0.49 (−1.83, 0.87) |
| 5–9yrs | 387 | 4.86 (4.30, 5.26) | 0.07 (−0.95, 1.11) | 485 | 3.69 (3.37, 4.04) | 0.92 (−0.31, 2.18) | 73 | 1.91 (1.49, 2.40) | −0.58 (−2.99, 1.89) | 82 | 3.09 (2.46, 3.83) | −0.36 (−2.31, 1.62) |
| 10–14yrs | 265 | 3.37 (2.97, 3.80) | 2.09 (0.57, 3.63)* | 251 | 1.83 (1.61, 2.07) | −0.09 (−1.19, 1.02) | 75 | 1.86 (1.47, 2.34) | 1.87 (−1.02, 4.85) | 48 | 1.84 (1.36, 2.44) | 1.04 (−1.87, 4.04) |
| 15–19yrs | 253 | 3.16 (2.78, 3.57) | 2.67 (0.88, 4.49)* | 205 | 1.42 (1.24, 1.63) | 0.54 (−1.31, 2.42) | 28 | 0.64 (0.43, 0.93) | NA | 35 | 1.28 (0.89, 1.78) | 1.21 (−2.17, 4.71) |
|
| ||||||||||||
| AML | 318 | 0.97 (0.87, 1.08) | 0.19 (−1.38, 1.79) | 468 | 0.87 (0.79, 0.95) | 0.86 (0.00, 1.73) | 108 | 0.67 (0.55, 0.81) | 0.91 (−1.90, 3.79) | 111 | 1.04 (0.72, 1.09) | −0.15 (−3.81, 1.62) |
| Gender | ||||||||||||
| Male | 174 | 1.04 (0.89, 1.21) | 0.02 (−1.52, 1.59) | 233 | 0.84 (0.73, 0.95) | 0.65 (−0.58, 1.89) | 52 | 0.64 (0.47, 0.83) | 0.04 (−3.31, 3.50) | 62 | 1.14 (0.87, 1.46) | 0.67 (−2.51, 3.95) |
| Female | 144 | 0.90 (0.76, 1.06) | 0.46 (−2.03, 3.01) | 235 | 0.89 (0.78, 1.02) | 1.01 (−0.26, 2.31) | 56 | 0.70 (0.53, 0.92) | 2.03 (−1.33, 5.51) | 49 | 0.95 (0.70, 1.25) | −1.38 (−4.25, 1.58) |
| Age | ||||||||||||
| <1 | 34 | 2.02 (1.40, 2.82) | 4.29 (−0.54, 9.34) | 45 | 1.83 (1.34, 2.45) | 3.87 (0.82, 7.02)* | 13 | 1.71 (1.30, 3.56) | NA | 12 | 2.38 (1.23, 4.16) | NA |
| 1–4yrs | 83 | 1.23 (0.98, 1.52) | 3.13 (0.32, 6.03)* | 110 | 1.09 (0.90, 1.32) | −0.97 (−2.90, 1.00) | 34 | 1.11 (0.77, 1.54) | NA | 27 | 1.29 (0.85, 1.88) | NA |
| 5–9yrs | 45 | 0.55 (0.40, 0.74) | −1.39 (−4.79, 2.14) | 63 | 0.48 (0.37, 0.61) | −1.50 (−4.59, 1.69) | 9 | 0.23 (0.11, 0.45) | NA | 17 | 0.64 (0.37, 1.03) | NA |
| 10–14yrs | 77 | 0.98 (0.77, 1.22) | −0.63 (−3.63, 2.46) | 88 | 0.64 (0.51, 0.79) | 1.35 (−0.86, 3.60) | 26 | 0.65 (0.42, 0.95) | NA | 22 | 0.84 (0.53, 1.28) | NA |
| 15–19yrs | 79 | 0.99 (0.78, 1.23) | −0.95 (−4.35, 2.58) | 162 | 1.12 (0.96, 1.31) | 1.33 (−0.69, 3.40) | 26 | 0.60 (0.39, 0.87) | NA | 33 | 1.21 (0.83, 1.70) | NA |
AAIR per 100,000 persons, diagnoses from 2009–2013, SEER18 data
APC for 1992–2013 using SEER13 data
NA = not reported due to small numbers of cases
Statistically significant increase in incidence from 1992–2013
ALL
In the five most recent years of SEER data (2009–2013), ALL incidence was higher in Hispanic White children than in non-Hispanic White, Black, or Asian children for both genders and among all ages; the incidence of ALL was markedly lower for non-Hispanic Black youth (Table 1). The relative difference in ALL incidence rates between Hispanic White and non-Hispanic White children increased by age (from <1 year to 19 years), with the greatest difference observed among those in the oldest age group.
Differences in the trends in ALL incidence from 1992–2013 (APC) between Hispanic White and non-Hispanic children also were more pronounced among children diagnosed with ALL. From 1992 to 2013, the incidence of ALL increased only among Hispanic White children (APC=1.08, 95%CI: 0.59, 1.58). Among both Hispanic White and non-Hispanic White children, greater increases in ALL incidence were observed for females. Statistically significant increases in incidence were observed for older Hispanic children (aged 10–14 years: APC=2.09; 95%CI: 0.57, 3.63; aged 15–19 years: APC=2.67; 95%CI: 0.88, 4.49); no increases in incidence were observed among older non-Hispanic White children.
AML
The AAIRs for childhood AML from 2009–2013 overall were similar by ethnicity (Table 1). The incidence rates were highest for Hispanic White and non-Hispanic White children under 1 year of age (AAIRHispanic=2.02; 95%CI:1.40, 2.82; AAIRnon-Hispanic=1.83; 95%CI: 1.34, 2.45); the number of cases of AML among non-Hispanic Black and non-Hispanic Asian children were very small. Statistically significant increases in the incidence of AML from 1992–2013 were observed for non-Hispanic White children under the age of 1 at diagnosis (APC=3.87; 95%CI: 0.82, 7.02), and for Hispanic White children diagnosed at age 1–4 (APC=3.13; 95%CI: 0.32, 6.03).
Trends in Incidence of ALL by Race/Ethnicity
Trends in incidence rates of childhood ALL from 1992–2013 by race/ethnicity are presented in Figure 1. The incidence of childhood ALL increased among Hispanic White children, but not among non-Hispanic children, however, the difference in trends was not statistically significant (APC Pinteraction= 0.29).
Figure 1.
Age-Adjusted Incidence Rates of Childhood Acute Lymphocytic Leukemia by Race and Ethnicity, SEER 13, 1992–2013
Incidence Rates by Age
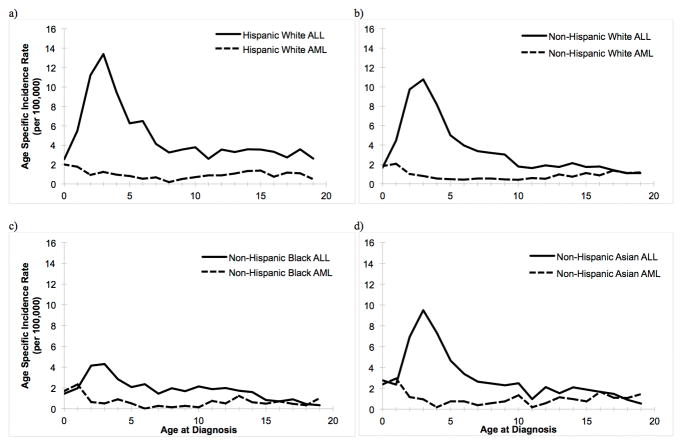
AAIRs from 2009–2013 by leukemia histologic subtype (ALL/AML) for each race/ethnicity are shown in Figure 2. The incidence of ALL peaks at approximately 2–3 years of age for all racial/ethnic groups and decreases until age 8, after which rates appear to stabilize for Hispanics White children, and continue to decline slightly for all non-Hispanic racial groups through age 19. Among non-Hispanic Black children, the peak at this age was substantially lower than for other racial/ethnic groups. In both Hispanic and non-Hispanic children, the rate of AML is highest among infants 0–1 years of age; a decrease in rates is observed from age 2–6, followed by a small, gradual increase in incidence through age 19.
Figure 2.
Age-Specific Incidence Rates of Childhood Acute Lymphocytic Leukemia (ALL) and Acute Myeloid Leukemia (AML) among a) Hispanic White, b) Non-Hispanic White, c) Non-Hispanic Black, and d) Non-Hispanic Asian children, SEER 18, 2009–2013
DISCUSSION
Childhood leukemia incidence rates increased from 1992–2013, an increase that appears largely to have been driven by increasing rates of ALL among Hispanic White children. The largest increase in incidence rates over the time period occurred in Hispanic children 15–19 years of age at diagnosis, among whom the incidence increased by about 3% per year from 1992–2013. In non-Hispanic children of the same age, a much smaller, non-significant increase in the incidence of ALL was observed.
Risk factors which could explain the disparity in increasing rates of childhood ALL would need to increase more rapidly in Hispanic White children than in non-Hispanic White children. Parental smoking during pregnancy is a suspected leukemogen, but the proportion of men and women who smoke has declined over this time period27, 28. Other suspected risk factors, such as pesticide exposure, and maternal and child weight, have increased during this time period, as has the incidence of childhood atopic conditions (a protective factor) but it is unclear whether these factors have increased at a greater rate among Hispanic children, and as such, require further investigation to determine which, if any, may impact trends in childhood ALL incidence. The latest evidence regarding the potential for each of these risk factors to influence trends in leukemia incidence is discussed in detail below.
Exposure to paternal smoking prior to conception, during pregnancy, in early childhood, or while mother was breastfeeding has been studied as a potential risk factor for childhood ALL. A recent meta-analysis, using data from 18 published case-control studies, observed an increased risk of childhood ALL with exposure to paternal smoking overall, prior to conception, and during pregnancy10, while no association was observed for maternal smoking during pregnancy in a meta-analysis based on 20 published studies29. Similarly, no elevated effects were observed in a study in California with a large proportion of Hispanic cases and controls21, 22. In the US, tobacco use has been lower in persons of Hispanic ethnicity than in non-Hispanic White persons (22.2% v. 14.5% in 2009)30 and the percentage of current smokers in the United States decreased from 34.7% in 1970 to 20.6% in 2009 among adults of all ethnicities aged 18 or older30. Parental use of tobacco or alcohol therefore seems an unlikely candidate to explain the increase in incidence of ALL observed in Hispanic children.
Exposure to pesticides may disproportionately affect Hispanic children in the United States, due to higher proportions of families living near farming communities or working in the farming industry. The role of residential, parental occupational, or ambient pesticide exposure to various classes of pesticides (e.g. insecticides, herbicides) early in the child’s life (i.e. in utero, during early childhood) has been investigated in a series of case-control studies. Summary measures for meta-analyses report elevated relative risk estimates for residential and occupational exposure during pregnancy to pesticides, insecticides and herbicides, with stronger effect estimates generally observed for maternal occupational exposure2–5. A study evaluating residential exposure to 6 polychlorinated biphenyl (PCB) congeners and 6 specific chemical pesticides in carpet dust around the time of diagnosis found an association between PCB congeners and childhood leukemia risk in non-Hispanic White children, though levels of PCB congeners were similar in Hispanic and non-Hispanic controls24. A second study evaluating exposure to herbicides in house dust and risk of childhood ALL observed statistically significant associations for the herbicide chlorthal, with a dose response effect observed for non-Hispanic White but not Hispanic children31. The prevalence of exposure is greater in residential agricultural areas with a high proportion of Hispanic families32. However, it is unclear whether the prevalence of pesticide exposure in areas with a large proportion of Hispanic children has increased in recent decades. If so, pesticide exposure increases may in part explain the greater increase in ALL incidence among Hispanic children.
Immune system development, as measured by patterns of infection and immune development are believed to influence ALL development. One proposed mechanism through which early life infections may protect against childhood leukemia is described as the “delayed infection” hypothesis33. The hypothesis proposes that a delay in exposures to common infections during the first year of life may alter the development of the immune system, and heighten the chance for aberrantly strong immune responses to later environmental stresses stimulating mutations necessary for the development of leukemia. Another variant of this hypothesis is the “population mixing” hypothesis, in which entire communities are exposed to new infections based on patterns of high residential mobility or settlement into “new towns”34. Interestingly, such population mixing is an important feature of immigrant Hispanic settlement in the US, with increasingly dispersed suburban or rural enclaves which may impact leukemia risks in the manner proscribed by the population mixing hypothesis35. Day care attendance (as a surrogate for early life infections) has been suggested as a protective factor for childhood ALL 18, 19. However, for Hispanic children no associations were observed23. The authors suggest that the formal variable of “daycare” does not describe the extent of Hispanics childhood contacts adequately, given other family and community-based childhood contacts among Hispanic communities.
Epidemiologic studies have noted a decreased risk of childhood allergies with several surrogate measures of early life infection (including higher birth order and day care attendance), supporting the hypothesis that infection early in life may protect against atopic conditions36. Studies also show inverse associations between these same measures of infection (higher birth order and day care attendance) and childhood leukemia incidence. Overall, inverse associations between childhood atopic conditions, including allergy, asthma, hay fever, eczema and hives were observed with ALL risk. Two meta-analyses of case-control studies found statistically significant protective effects for allergy, hay fever, and eczema, and a protective, non-significant effect for asthma16, 17 with childhood ALL. In recent years, the prevalence of allergic conditions (skin allergies) in children has increased in the United States (1997–2011), with a lower prevalence of allergic conditions in Hispanic children compared to non-Hispanic children37. The incidence of asthma in the United States has also increased in recent years (2001–2010)38. While the increase in allergic conditions and asthma may have prevented increased incidence among White children, such increases are unlikely to explain the observed increase in incidence among Hispanic children.
A recent meta-analysis of case-control studies found that children with high birth weight are at increased risk of both childhood ALL and AML, with evidence of a dose-response trend for ALL (ORper 1Kg increase=1.18; 95%CI: 1.12, 1.23)14, 15. A recent pooled analysis using data from 12 studies participating in Childhood Leukemia International Consortium (CLIC) found that children who were large-for-gestational-age were at an increased risk of childhood leukemia relative to children who were normal-for-gestational-age39. Consistent with these data, Hispanics have been found to experience higher fetal growth rates than whites40 potentially contributing to a higher risk of ALL. However, to our knowledge, data on secular trends in birth weight or weight-for-gestational-age by ethnicity are not readily available, and as such, the influence of increasing birth weight on increasing incidence of ALL in Hispanic children is, at best, speculative.
Another hypothesis is that the increase in childhood leukemia incidence in Hispanic children may be related to the growing proportion of children who are overweight in this population. Data from three nationwide clinical trials of childhood ALL in the UK indicate the prevalence of obesity and overweight at diagnosis among nearly 4,000 ALL cases increased significantly from 1985 to 200241. The prevalence of overweight and obesity at diagnosis in the most recent trial (1997–2002) was 34.5% using the International Obesity Task Force Definition compared to 13.1% and 10.2% for the previous cycles41. Compared to the 1997–2002 trial (34.5%), the average prevalence of overweight and obesity in children in the UK ranged from 13–26%42, suggesting that cases may be more likely than the general population to be overweight or obese prior to diagnosis. Using data from the National Health and Nutrition Examination Survey (NHANES), investigators found that in the United States, the obesity rate has doubled in children aged 2–5 and tripled in adolescents aged 12–18 from 1971–1974 to 2007–200843, with the greatest increase in obesity among Hispanic children. Using NHANES data from 1988–1994 to 2007–2008, investigators also found the prevalence of obesity in Mexican-American boys increased approximately 47% (14.1% to 26.8%), compared to 31% (11.6% to 16.7%) in non-Hispanic White boys43. While all racial/ethnic groups in the United States have experienced rising rates of obesity, Mexican-American children have experienced greater increases than White children at all ages, with the greatest increases occurring in children aged 6 to 11 years and 12 to 17 years from 1971 to 200244.
Although no epidemiologic studies have published findings on the risk of childhood ALL associated with childhood overweight or obesity, four studies have evaluated this association in adults45–48. Two meta-analyses of prospective cohort studies both observed a 60–65% increase in risk of adult ALL associated with obesity (p<0.01)49, 50. Further, mouse models suggest that diet-induced obesity accelerates ALL onset and progression51. The exact mechanism through which obesity in adulthood may lead to ALL in adults, or through which obesity in early childhood may lead to childhood ALL has not been established, but the authors suggest several plausible mechanisms50. Obesity, especially central adiposity, results in the release of excess free fatty acids, cytokines (e.g. IL-6, TNF-α), and a lower release of adiponectin, resulting in the development of insulin resistance and compensatory hyperinsulinaemia52. As a result, production of insulin and insulin-like growth factors (IGF-1) may increase, and production of IGF-binding proteins may decrease, leading to higher levels of bioavailable IGF-152, 53. Insulin and IGF-1 act as mitogens in a variety of tissues including lymphoid cell lines52, 53. The chronic activation of the immune system through the release of cytokines and other inflammatory proteins by adipose also may directly increase the risk of leukemia, or may increase a child’s susceptibility to other factors associated with ALL incidence50, 54.
The increase in rates may instead reflect changes in the diagnosis or classification of leukemia. While histologic classification of leukemia has improved in recent years, it is not clear that improvements in diagnostic practices would identify new cases that previously would have been missed, nor whether such improvements would explain the differential patterns in ALL incidence by ethnicity. We also considered the possibility that the increase in incidence rates observed in Hispanic children may represent a delayed increase, coming decades after an increase in non-Hispanic whites, as Hispanic children become more acculturated and adopt a more Westernized lifestyle. However, when we restricted our analysis to SEER data in regions with data available as early as 1973 and with a historically large proportion of non-Hispanic white populations (e.g. Connecticut, Iowa, Utah), we found only a moderate increase in the incidence rate among non-Hispanic White over this extended time window (1973–2010). Although the data to classify cases as “Hispanic” in SEER was not available until 1992, a greater increase in incidence rates was observed from 1973–2010 when restricting to SEER registries that are likely to include Hispanic white children (e.g. New Mexico, San Francisco-Oakland), than in registry areas with largely non-Hispanic white populations. Finally, classification in SEER as “Hispanic” is based on a surname algorithm26. As such, the differences in the increase in incidence rates of ALL between Hispanic and non-Hispanic children may be artificially driven by changes in the classification of children as Hispanic over time. Alternatively, the population estimates of Hispanic children may underestimate the true number of Hispanic children, resulting in artificially high rates of childhood cancer in Hispanic children. However, the observed difference in incidence rates by ethnicity has only been observed in childhood ALL and is not present in other childhood cancers (e.g. childhood brain tumors), suggesting that changes in ethnic classification are unlikely to be solely responsible for the results reported in this study.
Conclusion
Incidence rates in Hispanic children increased significantly from 1992–2013, with the greatest increases observed in older Hispanic children. Differences in incidence rates by ethnicity may be explained by differential exposure to suspected risk factors for ALL. However, the differential increase in incidence rates by ethnicity is unlikely to be explained by increasing exposure to these same environmental risk factors, as exposure to such factors would have had to increase at a greater rate among Hispanics over the last two decades. Changes in classification of disease, or classification of ethnicity in SEER databases are also unlikely to explain the observed incidence patterns. The large increase in ALL incidence in older Hispanic children may instead be the result of changes in birthweight or childhood obesity. Future studies with data to examine a wide range of potential risk factors by ethnicity, such as the California Childhood Leukemia Study or CLIC55, may be useful in understanding potential causes of the increase in incidence rates of ALL among Hispanic children.
Supplementary Material
What’s New.
In this analysis of Surveillance, Epidemiology and End Results (SEER) registry data, we evaluate recent trends in the incidence of childhood leukemia (diagnosed at age 0–19 years) in the United States from 1992–2013, and evaluate differences in leukemia incidence over time by race and ethnicity, and by other demographic characteristics. We expand upon prior work through an in depth exploration of potential reasons for the greater increase in incidence among Hispanic children and the disproportionate burden of disease in Hispanic populations.
Acknowledgments
This work was supported by the National Institute of Environmental Health (NIEHS) grant T32ES07262 (NIH), NIEHS grant 5R01ES009137, and Alex’s Lemonade Stand Foundation. We would like to acknowledge Adriana Mellor for assisting with updating the trends through 2013.
Footnotes
AUTHORSHIP CONTRIBUTIONS
JB carried out the analysis and drafted the paper. MC, CM, WG, JW, and RM all critically reviewed and contributed to finalizing the paper.
DISCLOSURE OF CONFLICTS OF INTEREST
We declare no conflicts of interest nor financial disclosures for any authors.
References
- 1.Barrington-Trimis JL, Cockburn M, Metayer C, Gauderman WJ, Wiemels J, McKean-Cowdin R. Rising rates of acute lymphoblastic leukemia in Hispanic children: trends in incidence from 1992 to 2011. Blood. 2015;125:3033–4. doi: 10.1182/blood-2015-03-634006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner MC, Wigle DT, Krewski D. Residential Pesticides and Childhood Leukemia: A Systematic Review and Meta-Analysis. Environmental Health Perspectives. 2009 doi: 10.1289/ehp.0900966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigle DT, Turner MC, Krewski D. A Systematic Review and Meta-analysis of Childhood Leukemia and Parental Occupational Pesticide Exposure. Environmental Health Perspectives. 2009;117:1505–13. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey HD, Fritschi L, Infante-Rivard C, Glass DC, Miligi L, Dockerty JD, Lightfoot T, Clavel J, Roman E, Spector LG, Kaatsch P, Metayer C, et al. Parental occupational pesticide exposure and the risk of childhood leukemia in the offspring: findings from the childhood leukemia international consortium. Int J Cancer. 2014;135:2157–72. doi: 10.1002/ijc.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey HD, Infante-Rivard C, Metayer C, Clavel J, Lightfoot T, Kaatsch P, Roman E, Magnani C, Spector LG, Th Petridou E, Milne E, Dockerty JD, et al. Home pesticide exposures and risk of childhood leukemia: Findings from the childhood leukemia international consortium. Int J Cancer. 2015;137:2644–63. doi: 10.1002/ijc.29631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheifets L, Ahlbom A, Crespi CM, Feychting M, Johansen C, Monroe J, Murphy MFG, Oksuzyan S, Preston-Martin S, Roman E, Saito T, Savitz D, et al. A pooled analysis of extremely low-frequency magnetic fields and childhood brain tumors. American Journal of Epidemiology. 2010;172:752–61. doi: 10.1093/aje/kwq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlbom A, Day N, Feychting M, Roman E, Skinner J, Dockerty J, Linet M, McBride M, Michaelis J, Olsen JH, Tynes T, Verkasalo PK. A pooled analysis of magnetic fields and childhood leukaemia. Br J Cancer. 2000;83:692–8. doi: 10.1054/bjoc.2000.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenland S, Sheppard AR, Kaune WT, Poole C, Kelsh MA. A pooled analysis of magnetic fields, wire codes, and childhood leukemia. Childhood Leukemia-EMF Study Group. Epidemiology. 2000;11:624–34. doi: 10.1097/00001648-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Milne E, Greenop KR, Scott RJ, Bailey HD, Attia J, Dalla-Pozza L, de Klerk NH, Armstrong BK. Parental Prenatal Smoking and Risk of Childhood Acute Lymphoblastic Leukemia. American journal of epidemiology. 2011;175:43–53. doi: 10.1093/aje/kwr275. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Zhang L, McHale CM, Hammond SK. Paternal Smoking and Risk of Childhood Acute Lymphoblastic Leukemia: Systematic Review and Meta-Analysis. Journal of Oncology. 2011;2011:1–16. doi: 10.1155/2011/854584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Zhang S, Li Z, Zhu J, Bi Y, Bai Y, Wang H. Maternal benzene exposure during pregnancy and risk of childhood acute lymphoblastic leukemia: a meta-analysis of epidemiologic studies. PLoS One. 2014;9:e110466. doi: 10.1371/journal.pone.0110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey HD, Fritschi L, Metayer C, Infante-Rivard C, Magnani C, Petridou E, Roman E, Spector LG, Kaatsch P, Clavel J, Milne E, Dockerty JD, et al. Parental occupational paint exposure and risk of childhood leukemia in the offspring: findings from the Childhood Leukemia International Consortium. Cancer Causes Control. 2014;25:1351–67. doi: 10.1007/s10552-014-0441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey HD, Metayer C, Milne E, Petridou ET, Infante-Rivard C, Spector LG, Clavel J, Dockerty JD, Zhang L, Armstrong BK, Rudant J, Fritschi L, et al. Home paint exposures and risk of childhood acute lymphoblastic leukemia: findings from the Childhood Leukemia International Consortium. Cancer Causes Control. 2015;26:1257–70. doi: 10.1007/s10552-015-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caughey RW, Michels KB. Birth weight and childhood leukemia: A meta-analysis and review of the current evidence. International Journal of Cancer. 2008;124:2658–70. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 15.Hjalgrim LL. Birth Weight as a Risk Factor for Childhood Leukemia: A Meta-Analysis of 18 Epidemiologic Studies. American Journal of Epidemiology. 2003;158:724–35. doi: 10.1093/aje/kwg210. [DOI] [PubMed] [Google Scholar]
- 16.Linabery AM, Jurek AM, Duval S, Ross JA. The Association Between Atopy and Childhood/Adolescent Leukemia: A Meta-Analysis. American Journal of Epidemiology. 2010;171:749–64. doi: 10.1093/aje/kwq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahl S, Schmidt LS, Vestergaard T, Schüz J, Schmiegelow K. Allergy and the risk of childhood leukemia: a meta-analysis. Leukemia. 2009;23:2300–4. doi: 10.1038/leu.2009.162. [DOI] [PubMed] [Google Scholar]
- 18.Urayama KY, Buffler PA, Gallagher ER, Ayoob JM, Ma X. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. International Journal of Epidemiology. 2010;39:718–32. doi: 10.1093/ije/dyp378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudant J, Lightfoot T, Urayama KY, Petridou E, Dockerty JD, Magnani C, Milne E, Spector LG, Ashton LJ, Dessypris N, Kang AY, Miller M, et al. Childhood acute lymphoblastic leukemia and indicators of early immune stimulation: a Childhood Leukemia International Consortium study. Am J Epidemiol. 2015;181:549–62. doi: 10.1093/aje/kwu298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metayer C, Milne E, Dockerty JD, Clavel J, Pombo-de-Oliveira MS, Wesseling C, Spector LG, Schüz J, Petridou E, Ezzat S, Armstrong BK, Rudant J, et al. Maternal supplementation with folic acid and other vitamins and risk of leukemia in offspring: a Childhood Leukemia International Consortium study. Epidemiology. 2014;25:811–22. doi: 10.1097/EDE.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang JS. Parental Smoking and the Risk of Childhood Leukemia. American journal of epidemiology. 2006;163:1091–100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 22.Metayer C, Zhang L, Wiemels JL, Bartley K, Schiffman J, Ma X, Aldrich MC, Chang JS, Selvin S, Fu CH, Ducore J, Smith MT, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol Biomarkers Prev. 2013;22:1600–11. doi: 10.1158/1055-9965.EPI-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X, Buffler PA, Wiemels JL, Selvin S, Metayer C, Loh M, Does MB, Wiencke JK. Ethnic difference in daycare attendance, early infections, and risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2005;14:1928–34. doi: 10.1158/1055-9965.EPI-05-0115. [DOI] [PubMed] [Google Scholar]
- 24.Ward MH, Colt JS, Metayer C, Gunier RB, Lubin J, Crouse V, Nishioka MG, Reynolds P, Buffler PA. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ Health Perspect. 2009;117:1007–13. doi: 10.1289/ehp.0900583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chokkalingam AP, Buffler PA. Genetic susceptibility to childhood leukaemia. Radiation Protection Dosimetry. 2008;132:119–29. doi: 10.1093/rpd/ncn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North American Association of Central Cancer Registries (NAACCR) Guideline for Enhancing Hispanic-Latino Identification: revised NAACCR Hispanic/Latino Identification Algorithm. Springfield, IL: North American Association of Central Cancer Registries (NAACCR); [Google Scholar]
- 27.SERVICES USDOHAH. A report of the Surgeon General. Atlanta, GA: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking - 50 Years of Progress. [Google Scholar]
- 28.Caetano R, Vaeth PA, Chartier KG, Mills BA. Epidemiology of drinking, alcohol use disorders, and related problems in US ethnic minority groups. Handb Clin Neurol. 2014;125:629–48. doi: 10.1016/B978-0-444-62619-6.00037-9. [DOI] [PubMed] [Google Scholar]
- 29.Klimentopoulou A, Antonopoulos CN, Papadopoulou C, Kanavidis P, Tourvas A-D, Polychronopoulou S, Baka M, Athanasiadou-Piperopoulou F, Kalmanti M, Sidi V, Moschovi M, Petridou ET. Maternal smoking during pregnancy and risk for childhood leukemia: A nationwide case-control study in Greece and meta-analysis. Pediatric Blood & Cancer. 2011;58:344–51. doi: 10.1002/pbc.23347. [DOI] [PubMed] [Google Scholar]
- 30.Trends in Tobacco Use. American Lung AssociationResearch and Program Services Epidemiology and Statistics Unit. 2011. [Google Scholar]
- 31.Metayer C, Colt JS, Buffler PA, Reed HD, Selvin S, Crouse V, Ward MH. Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia. J Expo Sci Environ Epidemiol. 2013;23:363–70. doi: 10.1038/jes.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter-Pokras O, Zambrana RE, Poppell CF, Logie LA, Guerrero-Preston R. The environmental health of Latino children. J Pediatr Health Care. 2007;21:307–14. doi: 10.1016/j.pedhc.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–55. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinlen LJ. An examination, with a meta-analysis, of studies of childhood leukaemia in relation to population mixing. 2012:1–6. doi: 10.1038/bjc.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichter DT, Parisi D, Taquino MC, Grice SM. Residential segregation in new Hispanic destinations: Cities, suburbs, and rural communities compared. Social Science Research. 2009;39:215–30. [Google Scholar]
- 36.Chang JS, Wiemels JL, Buffler PA. Allergies and childhood leukemia. Blood Cells, Molecules, and Diseases. 2009;42:99–104. doi: 10.1016/j.bcmd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Jackson KDHL, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011 NCHS data brief. Vol. 121. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 38.Akinbami LJMJ, Baily C, Zahran HS, King M, Johnson CA, Liu X. Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010. Hyattsville, MD: National Center for Health Statistics; 2012. NCHS data brief, no 94. [Google Scholar]
- 39.Milne E, Greenop KR, Metayer C, Schüz J, Petridou E, Pombo-de-Oliveira MS, Infante-Rivard C, Roman E, Dockerty JD, Spector LG, Koifman S, Orsi L, et al. Fetal growth and childhood acute lymphoblastic leukemia: Findings from the childhood leukemia international consortium. Int J Cancer. 2013;133:2968–79. doi: 10.1002/ijc.28314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overpeck MD, Hediger ML, Zhang J, Trumble AC, Klebanoff MA. Birth weight for gestational age of Mexican American infants born in the United States. Obstet Gynecol. 1999;93:943–7. doi: 10.1016/s0029-7844(98)00553-5. [DOI] [PubMed] [Google Scholar]
- 41.Aldhafiri FK, McColl JH, Reilly JJ. Prevalence of being underweight and overweight and obesity at diagnosis in UK patients with childhood acute lymphoblastic leukaemia 1985–2002. J Hum Nutr Diet. 2013 doi: 10.1111/jhn.12112. [DOI] [PubMed] [Google Scholar]
- 42.Stamatakis E, Wardle J, Cole TJ. Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes (Lond) 2010;34:41–7. doi: 10.1038/ijo.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cynthia O, Margaret C. Health E-Stat Center for Disease Control, National Center for Health Statistics, editor. Prevalence of Obesity Among Children and Adolescents: United States, Trends 1963–1965 Through 2007–2008. 2010. [Google Scholar]
- 44.Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity (Silver Spring) 2006;14:301–8. doi: 10.1038/oby.2006.39. [DOI] [PubMed] [Google Scholar]
- 45.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 46.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–9. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 47.Fernberg P, Odenbro A, Bellocco R, Boffetta P, Pawitan Y, Zendehdel K, Adami J. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: a nationwide cohort study in Sweden. Cancer Res. 2007;67:5983–6. doi: 10.1158/0008-5472.CAN-07-0274. [DOI] [PubMed] [Google Scholar]
- 48.Engeland A, Tretli S, Hansen S, Bjørge T. Height and body mass index and risk of lymphohematopoietic malignancies in two million Norwegian men and women. Am J Epidemiol. 2007;165:44–52. doi: 10.1093/aje/kwj353. [DOI] [PubMed] [Google Scholar]
- 49.Castillo JJ, Reagan JL, Ingham RR, Furman M, Dalia S, Merhi B, Nemr S, Zarrabi A, Mitri J. Obesity but not overweight increases the incidence and mortality of leukemia in adults: a meta-analysis of prospective cohort studies. Leuk Res. 2012;36:868–75. doi: 10.1016/j.leukres.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 50.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122:1418–21. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 51.Yun JP, Behan JW, Heisterkamp N, Butturini A, Klemm L, Ji L, Groffen J, Müschen M, Mittelman SD. Diet-induced obesity accelerates acute lymphoblastic leukemia progression in two murine models. Cancer Prev Res (Phila) 2010;3:1259–64. doi: 10.1158/1940-6207.CAPR-10-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 53.Shimon I, Shpilberg O. The insulin-like growth factor system in regulation of normal and malignant hematopoiesis. Leuk Res. 1995;19:233–40. doi: 10.1016/0145-2126(94)00133-u. [DOI] [PubMed] [Google Scholar]
- 54.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 55.Metayer C, Milne E, Clavel J, Infante-Rivard C, Petridou E, Taylor M, Schüz J, Spector LG, Dockerty JD, Magnani C, Pombo-de-Oliveira MS, Sinnett D, et al. The Childhood Leukemia International Consortium. Cancer Epidemiol. 2013;37:336–47. doi: 10.1016/j.canep.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.