Abstract
Background
BRAF-mutant metastatic colorectal cancers (mCRCs) share many clinicopathologic features with right-sided colon tumors, including frequent peritoneal involvement. Because of the poorer outcomes associated with BRAF mutations, early enrollment in clinical trials has been encouraged. However, the use of standard eligibility and assessment criteria, such as measurable disease, has anecdotally impeded patient accrual and restricted appraisal of treatment response. We investigated whether the presence of a BRAF V600E mutation is differentially associated with sites and appearance of metastatic disease in patients matched by primary tumor location.
Methods
A total of 40 patients with BRAF-mutant mCRC were matched to 80 patients with BRAF wild-type mCRC by location of primary tumor (right or left colon; rectum), sex, and age. Associations between BRAF mutation status and clinicopathologic characteristics and metastatic sites were analyzed using proportion tests. Survival was summarized with Kaplan-Meier and Cox regression methods.
Results
The distribution of primary tumor locations was: 60% right colon, 30% left colon, and 10% rectum. Compared with BRAF wild-type tumors, BRAF-mutant tumors more commonly associated with peritoneal metastases (50% vs 31%; P=.045) and ascites (50% vs 24%; P=.0038). In patients with left colon primaries, BRAF mutations were associated with more frequent ascites (58% vs 12%; P=.0038) and less frequent liver metastases (42% vs 79%; P=.024). Among patients with right colon primaries, no significant difference in sites of disease by BRAF mutation status was observed. Disease was not measurable by RECIST 1.1 in 24% of patients with right-sided primary tumors, irrespective of BRAF mutation status. In the BRAF-mutated cohort, ascites correlated unfavorably with survival (hazard ratio, 2.35; 95% CI, 1.14, 4.83; P=.02).
Conclusions
Greater frequency of ascites and peritoneal metastases, which pose challenges for RECIST 1.1 interpretation of therapeutic outcomes, are seen with BRAF-mutant mCRC, even when patients are matched for primary tumor location.
Background
Missense mutation of BRAF occurs in 5% to 10% of metastatic colorectal cancers (mCRCs).1,2 BRAF encodes a protein kinase in the mitogen-activated protein kinase (MAPK) pathway that may be constitutively activated by substitution at valine 600 to glutamic acid (V600E). This mutation defines a unique molecular subtype of mCRC, commonly originating from a serrated adenoma, more often in the right colon, with associated microsatellite instability (MSI), hypermutation, and a high degree of CpG island methylation (CIMP-H).3–10 BRAF mutations are more frequent in women, older patients, and in whites compared with Asians or blacks with CRC.2,11,12 Importantly, BRAF V600E–mutated mCRC is associated with a poor prognosis in patients treated with conventional chemotherapy.13–22 In the TRIBE and FIRE-3 studies, patients with BRAF wild-type tumors treated with FOLFIRI plus bevacizumab had a median overall survival (mOS) of 2 or more years, whereas mOS of patients with BRAF-mutated tumors was approximately 1 year.21,22 The explanation for the poor survival of patients with BRAF-mutated mCRC is incompletely understood but may relate to inherent chemoresistance, aggressive disease biology and kinetics, and/or a distinct pattern of metastatic spread.19,23
At diagnosis of mCRC, Yaeger et al23 found that BRAF-mutated tumors were associated with more frequent peritoneal metastases (26% vs 14%; P<.01) and less frequent liver-limited metastases (41% vs 63%; P<.01). Over the course of disease, Tran et al19 observed significantly higher rates of peritoneal metastases (46% vs 24%; P=.001) and distant lymph node metastases (53% vs 38%; P=.044) and lower rates of lung metastases (35% vs 49%; P=.049) in BRAF-mutant tumors.19 Of note, two-thirds of BRAF-mutated tumors originated in the right versus the left colon (68% vs 32%); this ratio was reversed for BRAF wild-type tumors (35% vs 65%; P<.001).19
“Sidedness,” differences between cancers originating in the left versus right colon, is an area of active research. Consensus molecular subtypes, mutations, and other genetic and epigenetic features vary in prevalence from the proximal to distal colon,3,5,24–31 possibly relating to the distinct embryologic origins of the right (from the midgut) and left (from the hindgut) colon. Patients with left-sided tumors experience superior OS compared with those with right-sided primaries.30–37 Right-sided tumors are associated with female sex, older age, and peritoneal metastases, whereas left-sided tumors are associated with more frequent liver and lung metastases.32 Although these differences cannot be explained by BRAF mutation alone, they may relate to enrichment of a “BRAF mutant–like” poor prognosis gene expression signature in right-sided BRAF wild-type tumors.38 It remains undetermined whether BRAF-mutated tumors really have a distinct pattern of metastatic spread, or whether the BRAF “phenotype” is representative of right-sided tumors, independent of mutation status.
Meanwhile, several clinical trials for BRAF-mutated mCRC are underway,39–46 and radiologic assessment of disease appearance is critical, both for patient management and for evaluating efficacy. Quantitative tumor burden assessments, such as RECIST 1.1, depend on finding “measurable disease.” Several findings suggestive of metastases that are known to be difficult to quantify in the RECIST 1.1 system are commonly observed with BRAF-mutated mCRC, including ascites, which is nonspecific and may be disproportionate to disease burden, and peritoneal metastases, which are often diffuse, mobile, band-like, or interdigitated with fat, and therefore difficult to quantify accurately with scan-to-scan comparisons.
As we work to translate our expanding knowledge of mCRC heterogeneity and the role of primary tumor location to improved clinical outcomes, we remain reliant on a handful of biomarkers (KRAS, NRAS, BRAF, MSI, and carcinoembryonic antigen)47 and serial radiographic assessments. We propose that careful radiologic review may identify novel radiogenomic “biomarkers” for prediction and prognosis. By matching patients with BRAF V600E–mutant mCRC to their BRAF wild-type counterparts, we queried whether there is a differential radiographic appearance of BRAF-mutant mCRC and whether radiographic appearance has implications for treatment or survival.
Methods
Patients
Institutional Review Board approval was obtained for retrospective review of imaging and clinical data from patients with mCRC seen in an academic gastrointestinal oncology practice. All known cases of BRAF-mutant mCRC seen from January 2013 to September 2015 with available contrast-enhanced CT images were included in this analysis. Each patient with BRAF-mutant mCRC identified was matched to 2 patients with BRAF wild-type mCRC, according to location of primary tumor (right colon, left colon, rectum), sex, and age (<50 years, ≥50 years at diagnosis). If multiple matches were identified, patients were selected based on availability of expanded RAS mutation testing results and age proximity.
Image Analysis
All available cross-sectional imaging studies from date of diagnosis to December 2015 were reviewed by a radiologist with expertise in abdominal imaging who was blinded to patients’ clinical histories. Images were evaluated for presence of liver, lung, lymph node, and osseous metastases, and for peritoneal implants and ascites. Liver and lung metastases were defined as lesions measuring greater than 1 cm or lesions that were new or increasing in size from prior studies. Lymph node metastases were defined as nodes measuring greater than 1.5 cm in short axis. Both sclerotic and lytic osseous metastases were noted. Peritoneal or omental disease was considered present when soft tissue densities or nodular infiltration soft tissue were present that could not be attributed to a vessel, lymph nodes, or prior surgeries. All studies were assessed for whether the bulk of disease was measurable according to RECIST 1.1. Followup was extended to August 2016 to evaluate time to ascites (details in supplementary eAppendix 1, available with this article at JNCCN.org).
Statistical Analysis
Univariate analyses of BRAF mutation status, clinicopathologic characteristics, sites of metastases, and measurable disease were examined using frequencies and proportions for categorical data and t tests for continuous variables. OS, stratified by BRAF mutation status and ascites (within BRAF mutation status), was summarized with Kaplan-Meier curves and Cox proportional hazards models after ensuring that the hazard assumption was met. For multivariate analysis, backward deletion was applied to capture negatively confounded predictors. Cumulative incidence function was used to estimate the probability of developing ascites from the date of diagnosis with mCRC in patients who did not have ascites at initial presentation. Death without ascites was treated as a competing event.
Results
Clinicopathologic Characteristics
A cohort of 40 patients with BRAF-mutant mCRC was identified. Of these 40 patients, 24 (60%) were women, 9 (23%) were younger than 50 years at diagnosis, 24 (60%) had right-sided colon primaries, 12 (30%) had left-sided colon primaries, and 4 (10%) had rectal primaries (Table 1 and supplemental eAppendix 2). Female sex was associated with right-sided tumors (P=.013); there was no statistically significant association between age and primary tumor location. A total of 60% of patients with BRAF-mutated tumors had received treatment including a BRAF inhibitor on a clinical trial.40,41,43
Table 1.
Clinicopathologic Features by BRAF Mutation Status
| Variable |
BRAF V600E (N=40) |
BRAF Wild-Type (N=80) |
|---|---|---|
| Sex (female) | 24 (60%) | 48 (60%) |
| Median age at diagnosis (range), y | 56 (28–88) | 58 (20–77) |
| Race, n (%) | ||
| Asiana | 7 (18%) | 14 (20%) |
| White | 31 (82%) | 49 (71%) |
| Black | 0 (0%) | 6 (9%) |
| Hispanic, n (%) | 2 (5%) | 5 (7%) |
| Primary tumor location, n (%)b | ||
| Right colon | 24 (60%) | 48 (60%) |
| Left colon | 12 (30%) | 24 (30%) |
| Rectum | 4 (10%) | 8 (10%) |
| Primary resected, n (%) | 30 (74%) | 71 (89%) |
| Metastases resected, n (%) | 7 (17.5%) | 32 (40%)c |
| Stage at diagnosis | ||
| II | 3 (7.5%) | 8 (10%) |
| III | 10 (25%) | 22 (27.5%) |
| IV | 27 (67.5%) | 50 (62.5%) |
| Median time to metastasis (range), mo | ||
| Stage II | 12 (9–13) | 38 (2–93) |
| Stage III | 9 (2–92) | 14 (2–100) |
| Stage II/III combined | 9 (2–92) | 16 (2–100) |
| Tumor grade (high), n (%)d | 11 (32%) | 16 (22%) |
| Mucinous features (present), n (%)e | 6 (15%) | 5 (6%) |
| MSI/MMR, n (%) | ||
| Present | 6 (15%) | 4 (5%) |
| Absent | 15 (37.5%) | 53 (66%) |
| Unknown | 19 (47.5%) | 23 (29%) |
| RAS mutated, n (%)f | 0 (0%) | 47 (59%) |
Abbreviations: MMR, mismatch repair (present=deficient); MSI, microsatellite instability (present=high).
Native Hawaiian or other Pacific Islander were grouped with Asian (2 BRAF-wild-type).
Right colon: cecum, ascending colon, hepatic flexure, and proximal transverse colon. Left colon: distal transverse colon, splenic flexure, descending colon, and sigmoid colon. Unspecified location in transverse colon was coded as left colon.
P=.013; all other P values insignificant.
Intermediate-high grade was included with high-grade.
Signet-ring adenocarcinoma was grouped with mucinous features present.
Of patients without a known RAS or BRAF mutation, 15/33 (45%) lacked full expanded RAS testing (KRAS and NRAS codons 12, 13, 61, 117 and 146). The following variables contain missing values: Race (2 BRAF-mutant; 11 wild-type); Hispanic (3 BRAF-mutant; 9 wild-type); grade (6 BRAF-mutant; 8 wild-type).
Compared with matched patients with BRAF wild-type tumors, a significantly lower proportion of patients with BRAF-mutant tumors underwent surgical resection of metastases (Table 1; P=.013), and when metastasectomy was performed, it was typically for palliation. Among patients originally diagnosed with stage II or III CRC, the time to radiographic detection of metastatic disease was shorter with BRAF-mutant as opposed to BRAF wild-type tumors (median, 9 vs 16 months). All other clinico-pathologic characteristics were balanced.
Sites of Metastatic Disease
We observed no significant differences in distribution of sites of metastases by sex or age. MSI-high/mismatch repair–deficient tumors exhibited less frequent lung metastases and more frequent lymph node metastases (supplemental eAppendix 3 and 4). In the cohort as a whole, a significantly higher frequency of ascites (P=.0038) occurred in patients with BRAF-mutant tumors (eAppendix 3). The mean number of sites of metastatic disease was 2.7 ± 1.4 with BRAF-mutant and 2.3 ± 1.2 with BRAF wild-type tumors (P=.13). Comparing BRAF- and KRAS-mutated tumors, BRAF mutation was again associated with a higher proportion of patients with ascites (P=.01). Among BRAF wild-type tumors, RAS mutations were overrepresented in right- versus left-sided tumors: 72% versus 37.5% (P=.0045).
In patients with right colon primaries, we observed no significant difference in metastatic disease sites by BRAF mutation status. In patients with left colon primaries, BRAF mutations were associated with less frequent liver metastases (42% vs 79%; P=.024) and more frequent ascites (58% vs 12%; P=.0038) (Table 2). If rectal tumors were grouped with left-sided primaries, BRAF mutations remained associated with ascites (56% vs 9%; P=.0004).
Table 2.
Patterns of Metastasis by BRAF Mutation Status and Location of CRC Primary
| Metastatic Sites | Entire Cohort | Right Colon Primaries | Left Colon Primaries | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mutant (N=40) |
Wild-Type (N=80) |
P Value | Mutant (N=24) |
Wild-Type (N=48) |
P Value | Mutant (N=12) |
Wild-Type (N=24) |
P Value | |
| Liver | 21 (53%) | 54 (68%) | .11 | 14 (58%) | 31 (65%) | .61 | 5 (42%) | 19 (79%) | .024 |
| Lung | 12 (30%) | 33 (41%) | .23 | 7 (29%) | 16 (33%) | .72 | 4 (33%) | 11 (46%) | .47 |
| Bone | 4 (10%) | 7 (9%) | .82 | 3 (12%) | 2 (4%) | .19 | 1 (8%) | 4 (17%) | .49 |
| Lymph node | 21 (54%) | 37 (46%) | .44 | 11 (50%) | 23 (48%) | .87 | 8 (73%) | 12 (50%) | .21 |
| Peritoneal | 20 (50%) | 25 (31%) | .045 | 14 (58%) | 20 (42%) | .18 | 5 (42%) | 4 (17%) | .10 |
| Ascites | 20 (50%) | 19 (24%) | .0038 | 11 (46%) | 16 (33%) | .30 | 7 (58%) | 3 (12%) | .0038 |
| RECIST measurable | 32 (80%) | 66 (83%) | .74 | 18 (75%) | 37 (77%) | .84 | 10 (83%) | 22 (92%) | .45 |
Using the false discovery method of Benjamini and Hochberg (1995)48 as implemented by Glickman et al (2014),49 the P values related to frequency of ascites in BRAF mutant versus wild-type CRC remain statistically significant, whereas the P value of .045 for peritoneal metastases in the entire cohort no longer meets the criteria for statistical significance.
Abbreviation: CRC, colorectal cancer.
In our initial analysis, tumors in the proximal transverse colon (1 BRAF-mutated; 3 wild-type) were classified as right-sided, and tumors in the distal transverse colon (3 BRAF-mutated) or unspecified location in transverse colon (2 BRAF-mutated) were classified as left-sided. Our observations were similar after exclusion of patients with primary tumors in the transverse colon (supplemental eAppendix 5).
Measurable Disease
The presence of peritoneal disease and ascites were each associated with nonmeasurable disease by RECIST 1.1 (P=.001 and P=.0032, respectively) (Figure 1). Conversely, liver, lung, and lymph node metastases were associated with measurable disease by RECIST 1.1 (P<.0001, P=.0023, and P=.0003, respectively). The association between nonmeasurable disease and right-sided tumors had a P value of 0.12. No association was observed between BRAF mutation status and measurable disease, even on further stratification by location of primary tumor. Overall, 20% of patients with BRAF-mutated tumors and 17.5% of BRAF wild-type tumors had nonmeasurable disease by RECIST 1.1 (eAppendix 3).
Figure 1.
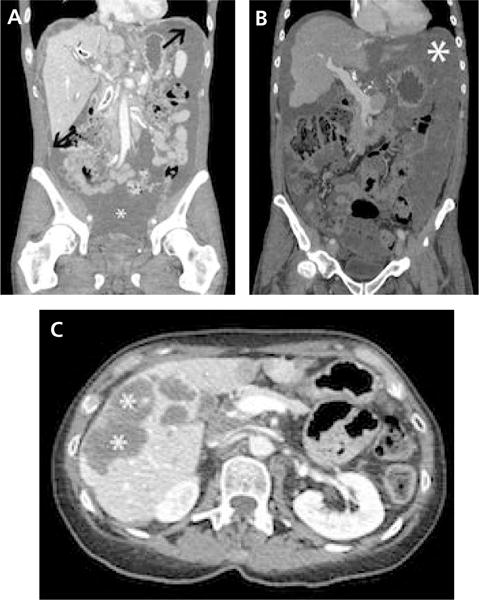
Representative CT images from patients with BRAF-mutated tumors. (A) Coronal CT image showing extensive ascites (*) with multiple nodular soft tissue implants (arrows) in a patient with BRAF-mutated metastatic colorectal cancer (mCRC). No other measurable disease was found. (B) Coronal CT image showing extensive mixed low attenuating peritoneal implants and ascites (*) in a patient with BRAF-mutated mCRC. No other measurable disease was found. (C) Axial CT image showing predominantly low attenuating liver metastases (*) due to the high level of mucin, another radiographic feature of BRAF-mutated mCRC.
Survival
The mOS of patients with BRAF-mutant mCRC was 24 months compared with 45 months with BRAF wild-type mCRC (P<.001) (Figure 2A). In patients with BRAF-mutated tumors, the presence of ascites was negatively correlated with survival (hazard ratio [HR], 2.35; 95% CI, 1.14, 4.83; P=.02) (Figure 2B). Variables positively correlated with survival were female sex (HR, 0.37; 95% CI, 0.18, 0.76; P=.009) and having the primary tumor resected (HR, 0.30; 95% CI, 0.13, 0.70; P=.009). There was a trend toward improved survival among clinical trial participants, with an mOS of 18 versus 27 months (HR, 0.5; 95% CI, 0.23, 1.05; P=.07) (supplemental eAppendix 6). A trend toward inferior survival with left-sided colon primaries was observed, with an mOS of 18 months for left versus 25 months for right-sided primaries (HR, 0.47; 95% CI, 0.21, 1.04; P=.07). No statistically significant differences in mOS by age or stage at diagnosis, tumor grade, other sites of metastases, metastasectomy, or presence of RECIST-measurable disease were detected (eAppendix 6).
Figure 2.
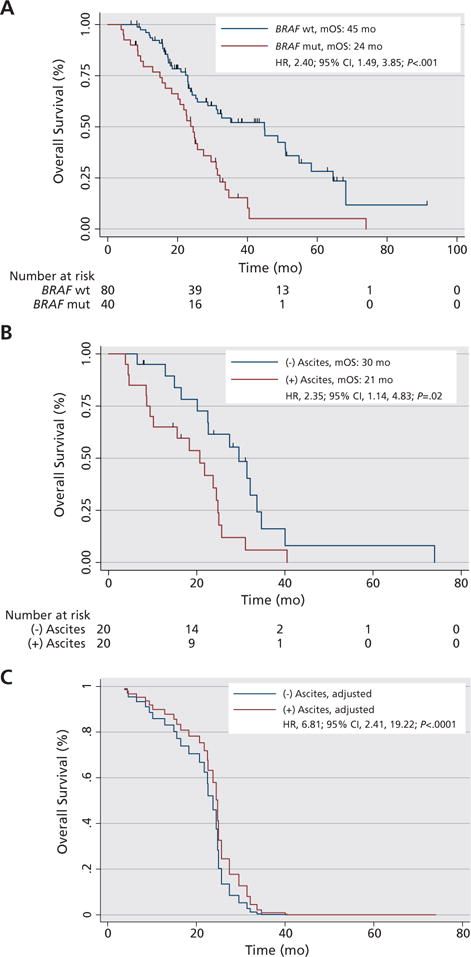
Survival by BRAF mutation status and presence of ascites. (A) Kaplan-Meier curves for median overall survival (mOS) from diagnosis with metastatic colorectal cancer by BRAF mutation status. Median follow-up among 40 censored patients with BRAF wild-type (wt) colorectal cancer was 29 months (range, 6–91 months). Median follow-up among 7 censored patients with BRAF-mutated (mut) colorectal cancer was 25 months (range, 8–37 months). (B) Kaplan-Meier curves for mOS of patients with BRAF-mutated tumors by absence or presence of ascites on CT imaging. (C) Cox regression for radiographic detection of ascites after adjusting for sex, primary tumor location (left or right colon), age, and peritoneal metastases patients with BRAF-mutated tumors.
Abbreviation: HR, hazard ratio.
Multivariate Analysis
The best model fit for the association of radiographic detection of ascites and survival in patients with BRAF-mutated tumors followed adjustment for sex, age at diagnosis, primary tumor location, and peritoneal metastases. Adjustment for these confounders greatly strengthened the negative association between ascites and survival (HR, 6.81; 95% CI, 2.41, 19.22; likelihood ratio, P<.0001) (Figure 2C, eAppendix 6). The interaction product term for ascites and peritoneal metastases had a P value of 0.98.
Time to Ascites
With extended follow-up, no additional patients with BRAF-mutant mCRC developed ascites; 3 patients with BRAF wild-type mCRC developed ascites. The proportion of patients who developed ascites within 3 months of mCRC diagnosis versus within 3 months of death did not differ by BRAF-mutation status (P=.56) (supplemental eAppendix 7). The cumulative incidence of ascites was higher among patients with BRAF-mutant disease (2-year cumulative incidence of 35% vs 15%; P=.018) (Figure 3).
Figure 3.
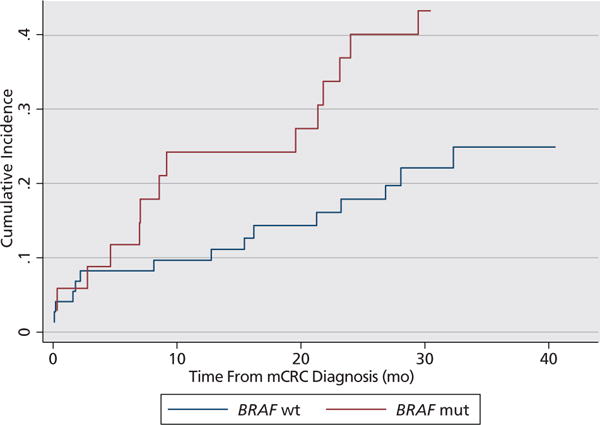
Time to ascites. Cumulative incidence of ascites in patients who did not have radiographic evidence of ascites at the time of initial presentation with metastatic colorectal cancer (mCRC). BRAF-mutated (mut): 14 patients developed ascites after diagnosis out of 34 patients total (6 patients with ascites at diagnosis excluded). BRAF wild-type (wt): 15 patients developed ascites after diagnosis out of 73 patients total (7 patients with ascites at diagnosis excluded).
Discussion
A cohort of patients with BRAF-mutated mCRC was matched 1:2 to patients with BRAF wild-type mCRC to determine whether BRAF-mutated tumors have a differential radiographic appearance after controlling for sex, age, and primary tumor location. In the matched cohort, ascites was the radiographic feature most strongly associated with the presence of a BRAF mutation.
Among patients with right-sided tumors, no statistically significant differences in metastatic sites were observed, although ascites and peritoneal disease were more common in patients whose tumors harbored a BRAF mutation. Among patients with left-sided colon primaries, ascites was associated with the presence of a BRAF mutation and peritoneal disease, which is often seen in conjunction with ascites, was also more frequent. Conversely, liver metastases were significantly more common in BRAF wild-type left-sided colon primaries.
We hypothesized that the association between ascites and BRAF mutation, significant in the cohort as a whole and the subset of patients with left- but not right-sided primaries, might be attributable to tumors in the transverse colon classified as originating from the left colon (5 BRAF-mutated, no BRAF wild-type). However, even after exclusion of patients with transverse colon primaries, the association between ascites and BRAF-mutant tumors persisted. Furthermore, with longer-follow up we did not find evidence that the difference in the frequency of ascites between BRAF-mutant and wild-type mCRC cases was due to the compressed course of disease for BRAF-mutant mCRC.
Compared with the study by Tran et al,19 in which the pattern of metastatic spread was analyzed in an unmatched cohort of BRAF-mutant and wild-type mCRC (68% vs 35% right-sided primaries, respectively), the association of BRAF mutations with peritoneal metastases was consistent; however, we did not recapitulate the weaker correlations between BRAF-mutant tumors and lymph node metastases or BRAF wild-type tumors and lung metastases that they observed. We did, however, consistently find more frequent lymph node metastases and less frequent lung metastases in BRAF-mutated tumors, so it is possible that these associations could become significant with enhanced sample size.
Because ascites and peritoneal disease are more common with BRAF-mutant tumors, we hypothesized that more of these patients would have disease that is nonmeasurable by RECIST 1.1. Instead, in our matched cohort, we found similar, and quite high, frequencies of RECIST nonmeasurable disease with or without a BRAF mutation, particularly in mCRC originating from a right-sided primary tumor (24%). This has implications for clinical trial design, wherein response and progression-free survival end points rely on RECIST assessments, and also for standard clinical care, wherein contrast-enhanced CT imaging is the gold standard for therapeutic decision-making.
Survival of patients with BRAF-mutated tumors was longer than that reported previously with standard therapies, even in clinical trial populations.13–22 Here, 60% of patients with BRAF-mutated mCRC were treated on a clinical trial including a BRAF targeted inhibitor40,41,43; the mOS of these patients was 27 months, comparable to the survival of patients with BRAF wild-type mCRC, and double that of patients with BRAF-mutated mCRC who received first-line FOLFIRI plus bevacizumab on 2 recent studies.21,22 In the BRAF-mutated cohort, ascites was associated with shorter survival, and this correlation strengthened in an exploratory multivariate model. Unexpectedly, among patients with BRAF-mutated mCRC, female sex associated favorably with mOS, and there was also a trend toward longer survival with right-sided colon primaries. If reiterated in other data sets, this could represent a real biological difference from mCRC overall, wherein right-sided tumors are associated with inferior survival30,32–35; however, it could also be that typical presentation of BRAF-mutated mCRC (female sex with a right colon primary)12 resulted in earlier mutation testing and referral for clinical trial participation.
Limitations of this study include the relative rarity of BRAF-mutated mCRC, which limits the ability to establish a large cohort for research purposes at any single institution. Thus, our study may have been underpowered for detection of clinicopathologic characteristics associated with BRAF mutation. In addition, we were unable to report on the frequency of brain metastases because a minority of patients underwent neuroimaging as part of routine care (eAppendix 3). Finally, due to the retrospective nature of this analysis, treatments administered for management of mCRC varied among patients in both the BRAF-mutant and BRAF-wild type cohorts; therefore, the survival analysis does not account for different treatments delivered.
With this analysis, we demonstrate for the first time the importance of using a matched cohort to characterize the effects of a mutation, BRAF V600E, which is differentially distributed in tumors arising in the proximal to distal colon and rectum. We conclude that ascites is the radiographic feature most robustly correlated with BRAF-mutant mCRC. BRAF mutation testing should be strongly considered with a left-sided colon primary if ascites is present; however, BRAF testing should not be limited to patients with this clinical presentation. Ascites correlated with inferior survival among patients with BRAF-mutated tumors. Moreover, a quarter of patients with mCRC with right colon primaries, with or without a BRAF mutation, had disease that was not measurable by RECIST 1.1.
Our future research directions will include the development of an alternative to RECIST 1.1 to characterize the burden of peritoneal-predominant disease and biomarkers that may be used in conjunction with CTs when disease is difficult to follow radiographically (eg, cell-free DNA). Given the limitations of single-institution analyses in this rare subset of mCRC, future multicenter clinical trials in which treatments are standardized should include correlative studies to validate these findings. Finally, we are encouraged by the survival of patients with BRAF-mutated tumors in our cohort, suggesting that our research efforts may be positively impacting individuals with this especially aggressive form of cancer.
Supplementary Material
Acknowledgments
The authors acknowledge editorial assistance on this manuscript from Amy J. Markowitz, JD.
Dr. Atreya has disclosed that she receives grant/research funding from Novartis. Dr. Yeh has disclosed that he is a consultant for and received a grant from GE Healthcare. The remaining authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors. Dr. Atreya received support from the NIH and NCI under Award Number K08CA175143.
Footnotes
Author Contributions: Conception and design: Atreya, Greene, McWhirter, and Behr. Collection and assembly of data: Atreya, Greene, McWhirter, Ikram, and Behr. Data analysis and interpretation: Atreya, Greene, McWhirter, Ikram, Allen, Van Loon, Venook, Yeh, and Behr. Manuscript writing: Atreya, Greene, McWhirter, Ikram, Allen, Van Loon, Venook, Yeh, and Behr. Final approval of manuscript: Atreya, Greene, McWhirter, Ikram, Allen, Van Loon, Venook, Yeh, and Behr.
References
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Tie J, Gibbs P, Lipton L, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. 2011;128:2075–2084. doi: 10.1002/ijc.25555. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151–1156. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Sousa EM, Wang X, Jansen M, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–618. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 7.Li WQ, Kawakami K, Ruszkiewicz A, et al. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer. 2006;5:2. doi: 10.1186/1476-4598-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann Oncol. 2014;25:1032–1038. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettington M, Walker N, Clouston A, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 11.Yoon HH, Shi Q, Alberts SR, et al. Racial differences in BRAF/KRAS mutation rates and survival in stage III colon cancer patients. J Natl Cancer Inst. 2015;107:pii:djv186. doi: 10.1093/jnci/djv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loupakis F, Moretto R, Aprile G, et al. Clinico-pathological nomogram for predicting BRAF mutational status of metastatic colorectal cancer. Br J Cancer. 2016;114:30–36. doi: 10.1038/bjc.2015.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 14.Yokota T, Ura T, Shibata N, et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104:856–862. doi: 10.1038/bjc.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price TJ, Hardingham JE, Lee CK, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol. 2011;29:2675–2682. doi: 10.1200/JCO.2010.34.5520. [DOI] [PubMed] [Google Scholar]
- 16.Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 17.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 18.Morris V, Overman MJ, Jiang ZQ, et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer. 2014;13:164–171. doi: 10.1016/j.clcc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 21.Stintzing S, Jung A, Rossius L, et al. Mutations within the EGFR signaling pathway: Influence on efficacy in FIRE-3—a randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients [abstract] J Clin Oncol. 2014;32(Suppl 3) Abstract 445. [Google Scholar]
- 22.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 23.Yaeger R, Cercek A, Chou JF, et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer. 2014;120:2316–2324. doi: 10.1002/cncr.28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Int Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 25.Maus MK, Hanna DL, Stephens CL, et al. Distinct gene expression profiles of proximal and distal colorectal cancer: implications for cytotoxic and targeted therapy. Pharmacogenomics J. 2015;15:354–362. doi: 10.1038/tpj.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 27.Glebov OK, Rodriguez LM, Nakahara K, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12:755–762. [PubMed] [Google Scholar]
- 28.Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, et al. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54:374–384. doi: 10.1136/gut.2003.036848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 31.Lee M, Advani S, Morris J, et al. Association of primary (1°) site and molecular features with progression-free survival (PFS) and overall survival (OS) of metastatic colorectal cancer (mCRC) after anti-epidermal growth factor receptor (αEGFR) therapy [abstract] J Clin Oncol. 2016;34(Suppl) Abstract 3506. [Google Scholar]
- 32.Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right-and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 33.Meguid RA, Slidell MB, Wolfgang CL, et al. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–2394. doi: 10.1245/s10434-008-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results—Medicare data. J Clin Oncol. 2011;29:4401–4409. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venook A, Niedzwiecki D, Innocenti F, et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance) [abstract] J Clin Oncol. 2016;34(Suppl) Abstract 3504. [Google Scholar]
- 37.Schrag D, Weng S, Brooks G, Meyerhardt J, Venook A. The relationship between primary tumor sidedness and prognosis in colorectal cancer [abstract] J Clin Oncol. 2016;34(Suppl) Abstract 3505. [Google Scholar]
- 38.Popovici V, Budinska E, Tejpar S, et al. Identification of a poor-prognosis BRAF-mutant-like population of patients with colon cancer. J Clin Oncol. 2012;30:1288–1295. doi: 10.1200/JCO.2011.39.5814. [DOI] [PubMed] [Google Scholar]
- 39.Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33:4032–4038. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol. 2015;33:4023–4031. doi: 10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atreya C, Van Cutsem E, Bendell J, et al. Updated efficacy of the MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E mutated (BRAFm) metastatic colorectal cancer (mCRC) [abstract] J Clin Oncol. 2015;33(Suppl) Abstract 103. [Google Scholar]
- 42.Hong D, Morris V, El Osta B, et al. Phase Ib study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated metastatic colorectal cancer and advanced cancers [abstract] J Clin Oncol. 2015;33(Suppl) Abstract 3511. [Google Scholar]
- 43.Kopetz S, McDonough S, Morris VK, et al. S1406: Randomized phase II study of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (mCRC) [abstract] J Clin Oncol. 2015;33(Suppl) doi: 10.1200/JCO.20.01994. Abstract TPS790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21:1313–1320. doi: 10.1158/1078-0432.CCR-14-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Geel R, Elez E, Bendell J, et al. Phase I study of the selective BRAFV600 inhibitor encorafenib (LGX818) combined with cetuximab and with or without the α-specific PI3K inhibitor BYL719 in patients with advanced BRAF-mutant colorectal cancer [abstract] J Clin Oncol. 2014;32(Suppl) Abstract 3514. [Google Scholar]
- 46.Tabernero J, Chan E, Baselga J, et al. VE-BASKET, a Simon 2-stage adaptive design, phase II, histology-independent study in nonmelanoma solid tumors harboring BRAF V600 mutations (V600m): activity of vemurafenib (VEM) with or without cetuximab (CTX) in colorectal cancer (CRC) [abstract] J Clin Oncol. 2014;32(Suppl) Abstract 3518. [Google Scholar]
- 47.Atreya CE, Corcoran RB, Kopetz S. Expanded RAS: refining the patient population. J Clin Oncol. 2015;33:682–685. doi: 10.1200/JCO.2014.58.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 49.Glickman M, Sowmya RR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67:850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


