Abstract
Human metapneumovirus (hMPV) infections pose a serious health risk to young children, particularly in cases of premature birth. No licensed vaccine exists and there is no standard treatment for hMPV infections apart from supportive hospital care. We describe the production of a Sendai virus (SeV) recombinant that carries a gene for a truncated hMPV fusion (F) protein (SeV-MPV-Ft). The vaccine induces binding and neutralizing antibody responses toward hMPV and protection against challenge with hMPV in a cotton rat system. Results encourage advanced development of SeV-MPV-Ft to prevent the morbidity and mortality caused by hMPV infections in young children.
Keywords: Metapneumovirus, Sendai virus, recombinant vaccine, respiratory virus, immunity, neutralizing antibodies
INTRODUCTION
Human metapneumovirus (hMPV) is a respiratory pathogen, first discovered in 2001, that causes significant morbidity and mortality in young children, particularly in children who were born prematurely [1–5]. Unfortunately, there remains no licensed vaccine for hMPV. Several vaccine candidates for hMPV are now being developed [6]. A variety of research strategies are used for this purpose, including attenuation of hMPV by mutation or deletion of genes, construction of DNA plasmids that express hMPV proteins, construction of subunit vaccines, assembly of CD8+ T cell epitopes, and construction of recombinant virus-based replication-competent viruses or virus-like particles (VLPs). Recombinant virus-based vaccines include Venezuelan equine encephalitis virus-based viral replicon particles (VEE-VRP [7]) and recombinant bovine/human parainfluenza virus type 3 (b/h PIV3/hMPV F [8]). Today, reverse genetics technologies provide valuable tools, both for the mutation of hMPV and for the creation of chimeric viral vaccines [6].
SeV is a murine parainfluenza virus that was discovered in 1952, and is closely related to the human parainfluenza virus type 1 (hPIV-1). Although SeV is pathogenic in mice, there has never been a case of confirmed SeV-associated disease in humans. The unmanipulated virus has already been tested in clinical trials in adults and children as a vaccine for hPIV-1, and has been shown to be well-tolerated and immunogenic. A recombinant SeV carrying HIV genes was also tested clinically, and a recombinant SeV vaccine for respiratory syncytial virus (RSV) is advancing toward clinical trials [9–14]. Here, we describe the production and testing of a recombinant SeV, named SeV-MPV-Ft that was produced by reverse genetics and carries a gene for a truncated hMPV fusion (F) protein. The F protein was selected for expression in the recombinant vaccine due to its known immunogenicity and conservation among hMPV isolates (>90% protein conservation)[15].
To test the immunogenicity and efficacy of SeV-MPV-Ft, we have used a cotton rat model. The cotton rat model is particularly attractive for hMPV vaccine testing, because it can be challenged with clinical isolates of hMPV without virus adaptation [16]. A primary infection with hMPV is protective against subsequent infections in cotton rats, demonstrating that a robust anti-hMPV immunological response can be generated [16]. Here, we show that SeV-MPV-Ft can be used as an effective intranasal vaccine against hMPV in cotton rats. The vaccine is immunogenic, induces neutralizing antibodies against a variety of hMPV isolates, and is protective against an hMPV challenge.
MATERIALS AND METHODS
Construct design
A plasmid was ordered from Life Technologies (Gene Art®Gene Synthesis) that incorporated a synthetically-produced, full-length-MPV F gene sequence from a Canadian isolate (CAN00-16, Genbank accession # AY145301.1, A2 lineage [17–19]). The sequence, which was confirmed by sequencing, was shuttled into pSVc, a plasmid containing a modified, full-length sequence from SeV Enders, using reverse genetics technology [10, 12, 20–22]. The position of the hMPV F sequence was between SeV F and HN genes. Cloning utilized a unique Not 1 site in the vector positioned adjacent to a natural transcription initiation site (Figure 1, panel A). Virus was next rescued in 293T cells. Briefly, this involved co-transfection of cells with the recombinant SeV plasmid plus supporting plasmids carrying genes for T7, SeV NP, SeV P, and SeV L. The rescued recombinant SeV was amplified in tissue culture and then in eggs. Sequencing of the virus was performed by extracting RNA from allantoic fluid using an RNeasy Mini Kit (Qiagen). hMPV-F cDNA was then generated with a OneStep RT-PCR Kit (Qiagen) using forward and reverse primers designed to match sequences adjacent to the NotI cloning sites of pSVc. The cDNA was fractionated on a 2% agarose gel and a 1.7kb band of the expected size for the hMPV-F gene insert was excised and purified using a QIAquick Gel Extraction Kit (Qiagen). The cDNA was mixed with primers in molecular grade water and was submitted to the Hartwell Center at St. Jude Children’s Research Hospital for Sanger sequencing.
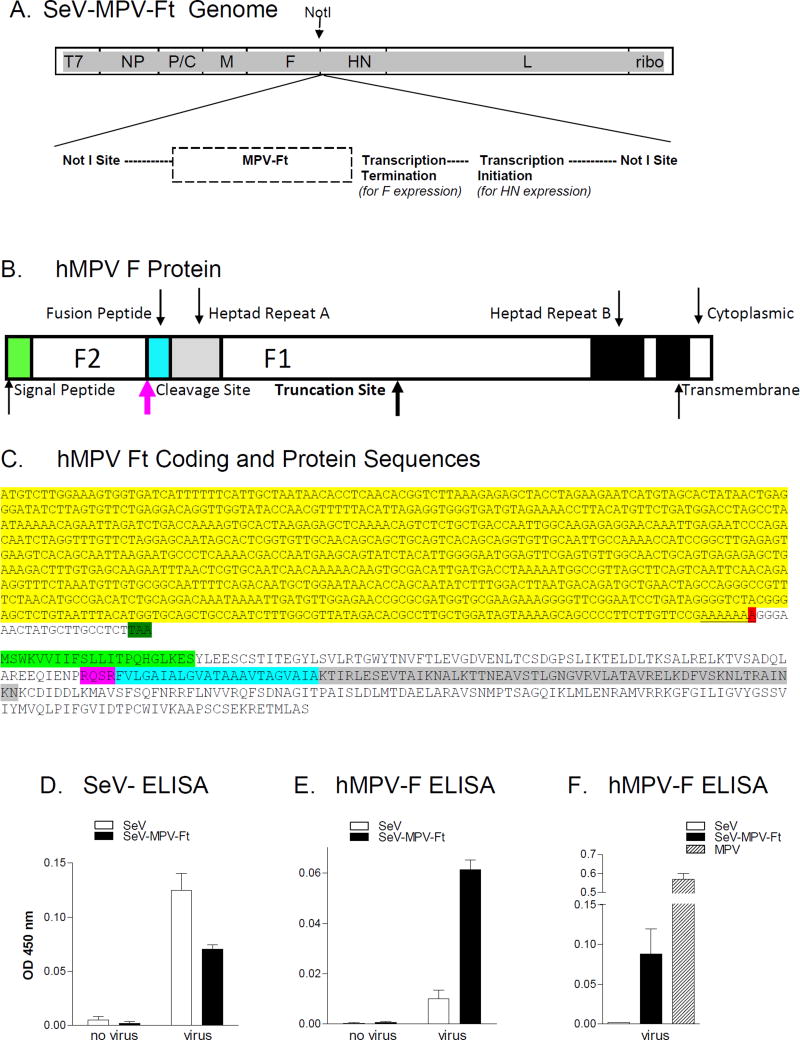
Figure 1. Characterization of recombinant SeV-MPV-Ft.
A. SeV-MPV-Ft rescue: A construct carrying the hMPV F sequence was produced by Invitrogen using GeneArt™Gene Synthesis technology, and confirmed to encompass the correct F sequence. The sequence was then recombined between F and HN genes at a unique NotI site in a vector carrying the complete SeV genome. Virus was rescued and amplified. B. hMPV F Protein: A cartoon of the hMPV F protein is shown indicating functional segments. Shown are positions of the signal peptide and F2 segment. Within the F1 segment are the fusion peptide, heptad repeats A and B, and transmembrane and cytoplasmic domains. The site of predicted F protein truncation is shown. C. hMPV-Ft coding and protein sequences: The N-terminal coding sequence of the hMPV-Ft gene is shown. The bases highlighted in yellow were unaltered from the original clone. The red highlight indicates the position of the A deletion, followed by a stop codon (highlighted in dark green) resulting from a frame-shift. The predicted protein sequence is shown below the gene sequence. The signal peptide, cleavage site, fusion peptide and heptad repeat A are highlighted in light green, pink, blue, and grey, respectively, corresponding to the similar color scheme in panel B. D, E and F. ELISAs with SeV-specific or hMPV-F-specific monoclonal antibodies for the detection of hMPV F expression: Uninfected and infected cell cultures were tested for viral protein expression in assays with SeV-specific (Panel D) or hMPV-specific (Panels E and F) monoclonal antibodies.
Metapneumovirus isolates
Replication-competent hMPV isolates included a clinical isolate, 2008#1, isolated from a pediatric patient at St. Jude Children’s Research Hospital by amplification in hybrid cell line shell vial cultures containing Madin-Darby canine kidney (MDCK) and human lung carcinoma (A549) cells (R-Mix Too™ ReadyCells®, Diagnostic Hybrids, Athens, OH). Identification of hMPV was confirmed by direct fluorescent antibody staining (D3 DFA Metapneumovirus Identification Kit, Diagnostic Hybrids). The clinical sample was completely de-identified and was therefore exempt from institutional review board oversight. Additional hMPV isolates were Cl.A1 (an A1 stock virus, kindly provided by Dr. John V. Williams [23]) and the CAN97-83 (GenBank #AY297749) A2 virus [15, 17, 18]. Viruses were in some cases amplified in LLC-MK2 cells in OptiMEM (Life technologies) supplemented with trypsin.
Immunizations and hMPV challenges in cotton rats
Cotton rats (Sigmodon hispidus; Harlan Sprague Dawley, Indianapolis, IN) were grouped (up to six animals per group) to receive SeV-MPV-Ft or non-recombinant SeV intranasally (i.n., 2 x 106 TCID50, 100 µl per animal) or no vaccine. All experiments were repeated to ensure reproducibility. Animals were anesthetized using isoflurane prior to manipulations including infections and samplings, and animals were euthanized with CO2. St. Jude Children’s Research Hospital (St. Jude) follows the standards established by the Animal Welfare Act and by the Principles for the Use of Animals and Guide for the Care and Use of Laboratory Animals. The animals are housed according to AAALAC guidelines, and St. Jude maintains AAALAC approval.
Sera were sampled before and after immunizations. Four-to-six weeks post-vaccination, animals were challenged i.n. with the hMPV clinical isolate 2008#1 at a dose of 2 × 105 - 3 × 106 TCID50 in 100 µl per cotton rat. Animals were euthanized on day 4 post-challenge and lungs were removed for freezing at -80°C. To titer virus, lungs were each placed in three ml Dulbecco’s PBS (D-PBS) and homogenized with a mechanical Dounce homogenizer (PowerGen125 PCR Tissue Homogenizing kit; Fisher Scientific). Serial dilutions were made of the homogenates (from 10−2 to 10−6 dilutions) in DMEM with 0.2% bovine serum albumin (BSA) and acetylated trypsin (final concentration 5 µg/ml). Serial dilutions of virus were added to LLC-MK2 cell (ATCC CCL-7) monolayers in 96-well plates, using six wells per sample dilution, 200 µl/well. Plates were incubated for four days at 37°C, 5% CO2. Media were then removed and cells were fixed with 80% acetone/20% D-PBS for 10 minutes, after which fixed cells were washed with D-PBS and blocked overnight (ON) with 1% BSA in D-PBS, 200 µl/well. Supernatants were aspirated and plates were next incubated with mouse monoclonal anti-MPV F-specific antibodies (a mixture of two monoclonal antibodies, each diluted 1:1000, Millipore Cat#MAB80122 and Cat#MAB80124), 100 µl/well, room temperature (RT) for three hours. Plates were next washed 3X and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (H+L) antibody (diluted 1:3000), 100 µl/well, RT for 1.5 hours. Plates were washed 3X and developed with TMB Peroxidase, 150 µl/well. Reactions were stopped after ~15 minutes with 50 µl/well 4N H3PO4. Plates were read at 450 nm. Wells scoring above 0.025 were considered positive. TCID50 in the lungs of each animal were calculated using the Reed and Muench method [24].
Enzyme-linked immunosorbent assay (ELISA) for antibody analyses
Sucrose gradient-purified SeV or hMPV (2008#1) from virus cultures were disrupted for 5 minutes at RT in 10x disruption buffer (0.05% TritonX-100, 60 mM KCl, 10 mM Tris pH7.8) and then diluted at least 10X with D-PBS. Disrupted viruses were added to ELISA plates at a concentration of 10 µg/ml (defined by BCA testing) in 50 µl/well, and incubated ON at 4°C. Plates were then washed 3X with 150 µl D-PBS and blocked with 100 µl 3% BSA in D-PBS ON at 4°C. Serially diluted cotton rat serum samples were prepared in 3% BSA and 0.1% Tween in D-PBS and added to wells in replicate in 50 µl/well for a 1hr incubation at RT. Plates were then washed 7X with 0.1% Tween in D-PBS. Wells were incubated with 50 µl/well polyclonal rabbit anti-cotton rat IgG (kindly provided by Virion Systems, Rockville, MD) in 3% BSA, 0.1% Tween in D-PBS for 1 hr RT. Plates were washed 7X followed by incubation with 50 µl goat anti-rabbit IgG-AP conjugate (H+L, SBA) for 1 hour RT. After washes, wells were developed with p- nitrophenyl phosphate (Sigma) in 1 mg/ml diethanolamine buffer (50µl/well) and plates were read after 15 minutes at OD 405 nm on a Versa Max microplate reader. The antibody titer was defined as the inverse serum dilution that scored 0.1 by nonlinear regression analyses (GraphPad Prism).
Microneutralization assays
Microneutralization assays were conducted using LLC-MK2 cells grown in 96-well tissue culture flat-bottom plates in NaHC03-buffered MEM with glutamine, gentamicin and 5% fetal bovine serum at 37°C, 5% CO2. Cells were plated at 2×104 cells/well one day prior to assay. On the day of assay and in a separate plate, serially diluted test and control sera (1:2 dilutions starting at 1:40, 100 µl per well) were mixed with hMPV (100 TCID50 in 50 µl per well). There were 4–6 replicate wells per serum dilution. Wells with no virus or virus with no sera served as controls. After 1 hour incubation at 37°C, 5% CO2, the antibody-virus mixtures were transferred to plates carrying LLC-MK2 cells for ON incubation. Supernatants were then removed and replaced with 200 µl DMEM with 0.1% BSA, glutamine, gentamicin and acetylated trypsin (2–4 µg/ml), for an additional 3 day incubation. Supernatants were then aspirated and cells were fixed with 80% acetone/20% D-PBS. After fixation, wells were washed with D-PBS, blocked with 1% BSA in D- PBS, and rewashed. Then 100 ul of a mixture of anti-MPV monoclonal antibodies were added (1:1,000–1:3,000 dilution of AbCam Cat#94800, Millipore Cat#MAB80124, and Millipore Cat#MAB80135). After 1 hour at RT, plates were washed 6X. Then horseradish peroxidase- conjugated goat anti-mouse IgG (anti H chain, Southern Biolotech Associates, 1:1000) was added, 100 µl/well, for a 1 hour incubation at RT. Plates were washed 6X with D-PBS, followed by addition of 100 µl TMB Peroxidase/well. Upon color development, reactions were stopped with 100 µl 4N H3PO4. Readings were at 450 nm. The titer was defined as the highest dilution of sample that resulted in a 50% reduction of signal compared to the no-antibody control, in at least 50% of test wells.
hMPV-F and SeV Protein Expression by SeV-MPV-Ft-infected cultures
To identify hMPV antigen expression by SeV-MPV-Ft infected cells, Hep2 cells were plated at 2x104 cells/well EMEM with 10% FCS in 96 well flat bottom plates and cultured ON at 37°C, 5% CO2. Medium was removed and 100 µl SeV, SeV-MPV-Ft, or hMPV (2008#1 isolate) were added per well in DMEM in 10% FCS. Input viruses were tested at concentrations between 103–106 TCID50 per ml to optimize ELISA signals. Control cultures received no virus. Cells were incubated for 3–4 days. Cells were then permeabilized and fixed with 80% acetone/20% D-PBS. Monoclonal antibodies were next used to detect SeV or hMPV antigens in wells. For hMPV detection, assays were with anti-hMPV monoclonal antibodies as described above for the scoring of neutralization assays. For SeV detection, anti- SeV antibodies were used in place of anti-hMPV antibodies (S2 and M57 monoclonal anti SeV HN antibodies, 1µg/ml [25]).
RESULTS
Rescue of the SeV-MPV-Ft recombinant virus vaccine
Synthesis of a full-length hMPV F gene (Genbank accession # AY145301.1, [17]) was conducted at Invitrogen using GeneArt™Gene Synthesis technology, followed by incorporation of this gene into a plasmid containing the full-length SeV sequence. A recombinant SeV was rescued by plasmid transfection into 293T cells. When the virus was sequenced, it exhibited a single nucleotide (A) deletion in the hMPV F gene. This deletion shortened the otherwise heptameric poly A sequence central to the F1 coding sequence, forcing out-of-frame translation and truncation of the predicted hMPV F1 protein fragment (see Figure 1, panels B and C). The position of predicted truncation is shown by a bolded arrow in panel 1B. Mutations of this type have been described previously in hMPV, and are a likely consequence of polymerase stuttering [26]. There was also a compensatory, non-coding nucleotide addition immediately upstream of the NotI insertion site, as would be required to accommodate the “rule-of-six” for SeV genome packaging [27, 28].
The predicted, truncated hMPV F protein (Ft) produced by our new recombinant virus (SeV- MPV-Ft) was 303 amino acids in length. It retained the full F2 segment (including the signal peptide), the cleavage site, and the N-terminal F1 segment (including the fusion peptide, heptad repeat A (HR-A), and multiple known antibody binding sites [29]; Figure 1, panels B and C). The protein is predicted to lack C-terminal amino acids including the transmembrane domain. The two base changes in the viral genome were stable in that they were identified in three separate sequencing experiments, one performed after the first viral passage, and two performed after independent sets of seven additional viral passages in vitro.
To confirm expression of the recombinant hMPV Ft protein, SeV-infected and SeV-MPV-Ft- infected cells were tested with cocktails of SeV-specific or hMPV-F-specific monoclonal antibodies. As demonstrated in Figure 1, the SeV-specific antibodies bound cells infected with both non-recombinant and recombinant viruses (panel D), whereas hMPV-F-specific antibodies preferentially bound cells infected with SeV-MPV-Ft (panel E). A student’s T test (GraphPad Prism Software) showed that the binding of hMPV-F-specific monoclonal antibodies was significantly (p<.001) stronger toward cells infected with SeV-MPV-Ft compared to cells infected with non-recombinant SeV (panel E). Panel F shows that the signal in SeV-MPV-Ft- infected cells was weaker than the signal in wildtype MPV-infected cells, suggesting a relatively low amount of protein in SeV-MPV-Ft-infected cells and/or that the protein was of a conformation that was bound poorly by the developing antibodies in this assay.
The monoclonal antibodies used to identify hMPV F expression in tissue culture were not successful at scoring F protein fragments on Western blots. We therefore utilized monoclonal antibodies, polyclonal antibodies from humans, and sera from hMPV-infected cotton rats to support additional testing of virus-infected cultures. Although MPV F expression by cells from wildtype MPV-infected cultures was detected by immunoprecipitation experiments and gel analysis, we could not visualize MPV Ft expression from SeV-MPV-Ft-infected culture products on gels. This was despite analyses of cells, supernatants, and concentrated supernatants (data not shown).
Vaccination with SeV-MPV-Ft elicits hMPV-specific binding and neutralizing antibodies in cotton rats
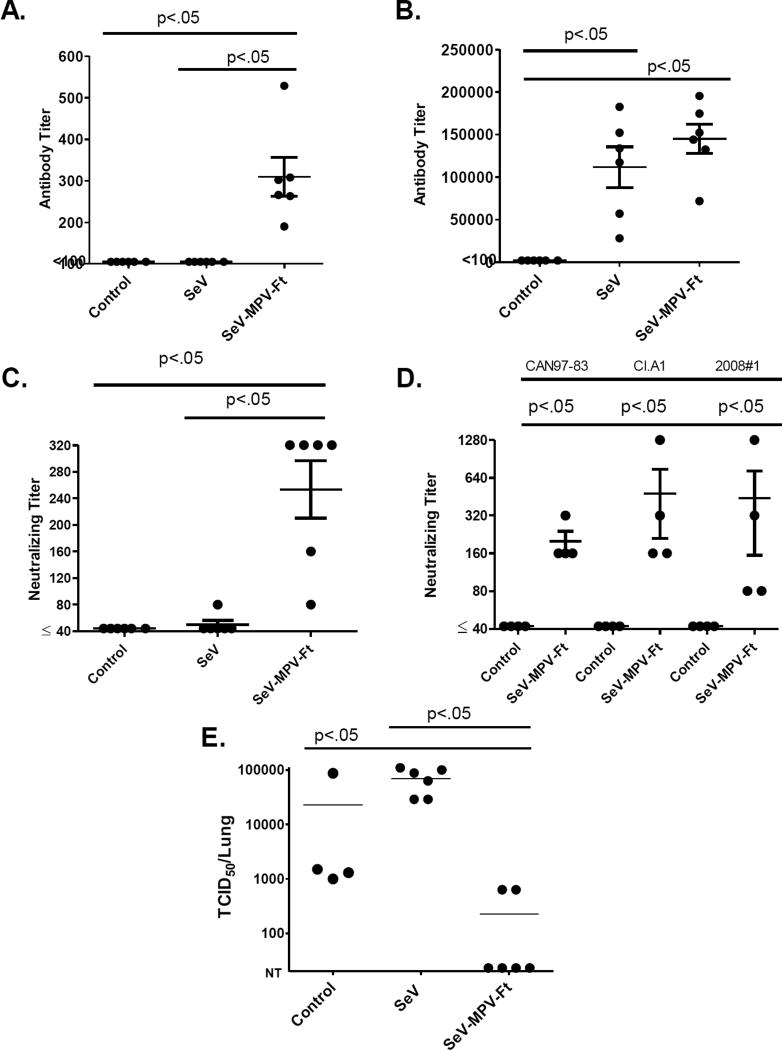
To determine whether the SeV-MPV-Ft vaccine induced an antibody response toward hMPV, we inoculated groups of 4–6 cotton rats by the i.n. route (2×106 TCID50/cotton rat). Control animals received non-recombinant SeV instead of recombinant SeV-MPV-Ft, or no vaccine. Blood samples were collected 4–6 weeks after vaccination for antibody testing. As shown in Figures 2A and B, antibodies from SeV-MPV-Ft-vaccinated animals bound both hMPV 2008#1 (a clinical isolate) and SeV, whereas antibodies from SeV-vaccinated animals bound SeV well and hMPV poorly. Serum samples were also tested for neutralization of hMPV 2008#1. Sera from SeV-MPV-Ft-infected cotton rats neutralized hMPV 2008#1, whereas sera from SeV-infected animals exhibited non-significant neutralization (Figure 2C). In separate experiments, sera from SeV-MPV-Ft infected cotton rats were tested for neutralization across a panel of hMPV isolates. These included an A2 isolate CAN97-83 and an A1 isolate (Cl.A1) along with the uncharacterized clinical isolate 2008#1. As expected, based on the conserved nature of the hMPV-F protein, all tested hMPV isolates were neutralized [15, 17–19]. Cotton rats primed with non-recombinant SeV did not generate significant neutralizing antibodies toward any of the three viruses (data not shown).
Figure 2. Cotton rats primed with SeV-MPV-Ft generate hMPV-specific binding and neutralizing antibodies, and are protected from hMPV challenge.
Groups of 4–6 cotton rats were inoculated with 2 × 106 TCID50 SeV-MPV-Ft or non-recombinant SeV. Sera were collected after 4–6 weeks. Sera were serially diluted for testing in an ELISA against hMPV or SeV (panels A and B). Serum samples were also tested for neutralization activity with 2008#1 (panel C), and with a panel of viral isolates (panel D). All ELISAs and neutralization assays revealed statistically significant differences between vaccinated and control samples using Mann Whitney Tests (p<.05, GraphPad Prism software, San Diego, CA). After resting for 4–6 weeks, vaccinated animals were challenged with hMPV, isolate 2008#1. Virus replication in the lungs was then measured (panel E). Each symbol represents virus from the lungs of an individual cotton rat. Two animals died in the naïve group (original n=6) before analysis. Mann-Whitney tests with GraphPad Prism Software demonstrated that differences in virus loads between vaccinated and control animals were statistically significant (p<.05). Challenge experiments have been conducted three times using the cotton rat model, and each experiment demonstrated significant protection induced by SeV-MPV-Ft.
SeV-MPV-Ft protects cotton rats from hMPV challenge
SeV-MPV-Ft vaccination was next tested for protection of animals from heterologous hMPV challenge. 4–6 weeks after vaccination, animals were challenged i.n. with hMPV 2008#1 and sacrificed 4 days later. As shown in Figure 2E, animals vaccinated with SeV-MPV-Ft, but not control animals, were significantly protected from hMPV challenge. hMPV challenge experiments were conducted three times, twice comparing SeV-MPV-Ft-vaccinated mice with SeV-vaccinated and naïve mice, and once comparing SeV-MPV-Ft-vaccinated mice with naïve mice. Altogether, among 20 SeV-MPV-Ft-vaccinated animals, only three exhibited virus in lungs post-hMPV challenge, whereas 30 of 30 tested control animals were positive for virus in lungs post-hMPV challenge. Reproducible, statistically significant differences in protection were clearly observed between SeV-MPV-Ft- vaccinated animals and controls.
DISCUSSION
We describe the preparation and testing of an SeV recombinant, SeV-MPV-Ft, carrying a gene for truncated hMPV F. We immunized cotton rats with SeV-MPV-Ft, and then measured robust antibody responses and protection from challenge with hMPV. Upon initiation of this project, a construct was designed to express full-length hMPV F, but following virus rescue, a point deletion (A) central to the hMPV F1 gene fragment was discovered. This type of mutation has been described previously in hMPV, and is a likely consequence of polymerase stuttering [26]. Polymerase stuttering is a mechanism associated with polyadenylation, and a cis-acting sequence immediately upstream of the edited site has been shown to modulate the frequency and quality of nucleotide changes [26].
The predicted protein expressed by hMPV-Ft retained the F2 sequence and a significant portion of F1, but lacked the transmembrane region. The retained N-terminal sequence included known antibody binding sites such as a conserved site comparable to that in the respiratory syncytial virus F protein bound by palivizumab, a monoclonal antibody used routinely to protect vulnerable infants from RSV [29, 30]. Known antibody binding sites dependent on C-terminal sequences were predicted to be absent, and it was expected that the conformation of expressed hMPV F fragments could differ from the conformation of matched sequences expressed in the context of full-length F [3, 31]. Nonetheless, we found that antibodies elicited by SeV-MPV-Ft were capable of neutralizing several hMPV isolates, and vaccinated animals were protected from hMPV infection in vivo [15].
We were able to confirm hMPV-Ft expression by cells infected with SeV-MPV-Ft using hMPV-specific monoclonal antibodies. However, our efforts to characterize hMPV-Ft from cultures infected with SeV-MPV-Ft by Western blot or immunoprecipitation were unfortunately unsuccessful. Perhaps difficulties arose in part due to relatively low expression of Ft by SeV-MPV-Ft-infected cells in tissue culture, and/or conformational differences between Ft and wildtype F proteins (against which developing reagents were produced). Continued efforts to characterize the SeV-MPV-Ft protein expression using sensitive methodologies and reagents [32–35] may assist further characterization of Ft to support advanced vaccine development.
Also important for advanced vaccine development will be the testing of SeV-MPV-Ft for induction of immunopathological responses. Of note, vaccination with a formalin- inactivated (FI) hMPV vaccine can cause immunopathology in small and large animal models [6]. Immunopathologies can occur even when vaccines induce neutralizing antibodies and decrease virus replication [6]. Our previous studies showed that a SeV expressing RSV F did not cause enhanced immunopathology upon subsequent RSV challenge [12], but parallel experiments with SeV-MPV-Ft using the hMPV challenge system have not yet been done.
The success of our SeV-MPV-Ft vaccine in protecting animals from hMPV challenge was clear. These new data support further efforts to develop SeV-based vaccines. We have previously shown that SeV induces rapid and durable B and T cell responses systemically and in upper respiratory tract (URT) and lower respiratory tract (LRT) tissues [36, 37]. Non-recombinant SeV is currently in clinical trials and has been shown to be well tolerated in adults and 3–6 year old children [9, 11]. Tests in 1–2 year old children are ongoing. A recombinant SeV that expresses the full length RSV F has been tested in cotton rats, and has been shown to induce protective responses against both RSV A and B isolates without immunopathology [12]. The vaccine further protects against infection in the LRT after RSV challenge in African green monkeys [38], and is expected to enter clinical trials shortly. SeV is an attractive vaccine backbone, in part because of its safety profile, in part because SeV can be amplified in hens’ eggs and in mammalian cell cultures, and in part because SeV recombinants can be combined in cocktails to target multiple pediatric pathogens at once [39, 40]. Possibly a SeV-MPV-Ft vaccine will ultimately serve to protect young children from the morbidity and mortality caused by hMPV infections.
In conclusion, we demonstrated that SeV-MPV-Ft induced hMPV-specific neutralizing antibodies and conferred protection against hMPV challenge in a cotton rat model. Results encourage continued study of this and other recombinant, SeV-based vaccines to protect children from the disease consequences of hMPV and other respiratory viral infections.
HIGHLIGHTS.
A Sendai virus-based human metapneumovirus (hMPV) vaccine is being developed
SeV-MPV-Ft carries a gene for a truncated metapneumovirus fusion protein
The intranasal vaccine induces hMPV-specific binding antibodies in cotton rats
The vaccine induces hMPV-specific neutralizing antibodies in cotton rats
A single immunization protects against hMPV challenge in vivo
Acknowledgments
We thank Virion Systems and Dr. Jorge Blanco (Sigmovir) for providing cotton rat antibody reagents. We thank Dr. John V. Williams (University of Pittsburgh) for the Cl.A1 MPV isolate. We thank Dr. Allen Portner for SeV-specific monoclonal antibodies generated at St. Jude. This work was supported by grants NIH NIAID P01 AI054955, NIH NIAID R01 AI088729, NIHI NIAID R01 AI083370, NIH NCI P30-CA21765, and ALSAC.
Abbreviations
- hMPV
human metapneumovirus
- hPIV-1
human parainfluenza virus type 1
- SeV
Sendai virus
- F
fusion protein
- Ft
truncated fusion protein
- RT
room temperature
- ON
overnight
- MEM
Modified Eagle’s Medium
- D-MEM
Dulbecco’s MEM
- HN
hemagglutinin-neuraminidase
- NP
nucleoprotein
- P
phosphoprotein
- L
large protein
- HR
heptad repeat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, et al. Burden of human metapneumovirus infection in young children. The New England journal of medicine. 2013;368:633–43. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schildgen V, van den Hoogen B, Fouchier R, Tripp RA, Alvarez R, Manoha C, et al. Human Metapneumovirus: lessons learned over the first decade. Clinical microbiology reviews. 2011;24:734–54. doi: 10.1128/CMR.00015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster JE, Williams JV. Human metapneumovirus. Pediatrics in review. 2013;34:558–65. doi: 10.1542/pir.34-12-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams JV. Human Metapneumovirus: An Important Cause of Respiratory Disease in Children and Adults. Current infectious disease reports. 2005;7:204–10. doi: 10.1007/s11908-005-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pancham K, Sami I, Perez GF, Huseni S, Kurdi B, Rose MC, et al. Human Metapneumovirus Infection is Associated with Severe Respiratory Disease in Preschool Children with History of Prematurity. Pediatrics and neonatology. 2016;57:27–34. doi: 10.1016/j.pedneo.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen SC, Williams JV. New Approaches for Immunization and Therapy against Human Metapneumovirus. Clin Vaccine Immunol. 2015;22:858–66. doi: 10.1128/CVI.00230-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok H, Tollefson SJ, Podsiad AB, Shepherd BE, Polosukhin VV, Johnston RE, et al. An alphavirus replicon-based human metapneumovirus vaccine is immunogenic and protective in mice and cotton rats. J Virol. 2008;82:11410–8. doi: 10.1128/JVI.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang RS, Schickli JH, MacPhail M, Fernandes F, Bicha L, Spaete J, et al. Effects of human metapneumovirus and respiratory syncytial virus antigen insertion in two 3' proximal genome positions of bovine/human parainfluenza virus type 3 on virus replication and immunogenicity. J Virol. 2003;77:10819–28. doi: 10.1128/JVI.77.20.10819-10828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adderson E, Branum K, Sealy RE, Jones BG, Surman SL, Penkert R, et al. Safety and immunogenicity of an intranasal Sendai virus-based parainfluenza virus type 1 vaccine in 3–6 year old children. ClinVaccine Immunol. 2014 doi: 10.1128/CVI.00618-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell CJ, Hurwitz JL. Sendai virus as a backbone for vaccines against RSV and other human paramyxoviruses. Expert Rev Vaccines. 2015:1–12. doi: 10.1586/14760584.2016.1114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slobod KS, Shenep JL, Lujan-Zilbermann J, Allison K, Brown B, Scroggs RA, et al. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004;22:3182–6. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 12.Zhan X, Hurwitz JL, Krishnamurthy S, Takimoto T, Boyd K, Scroggs RA, et al. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25:8782–93. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu S, Feng X, Shu T, Matano T, Hasegawa M, Wang X, et al. Potent specific immune responses induced by prime-boost-boost strategies based on DNA, adenovirus, and Sendai virus vectors expressing gag gene of Chinese HIV-1 subtype B. Vaccine. 2008;26:6124–31. doi: 10.1016/j.vaccine.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Ramanathan VD, Kumar M, Mahalingam J, Sathyamoorthy P, Narayanan PR, Solomon S, et al. A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res Hum Retroviruses. 2009;25:1107–16. doi: 10.1089/aid.2009.0096. [DOI] [PubMed] [Google Scholar]
- 15.Boivin G, Mackay I, Sloots TP, Madhi S, Freymuth F, Wolf D, et al. Global genetic diversity of human metapneumovirus fusion gene. Emerging infectious diseases. 2004;10:1154–7. doi: 10.3201/eid1006.031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JV, Tollefson SJ, Johnson JE, Crowe JE., Jr The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J Virol. 2005;79:10944–51. doi: 10.1128/JVI.79.17.10944-10951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastien N, Normand S, Taylor T, Ward D, Peret TC, Boivin G, et al. Sequence analysis of the N, P, M and F genes of Canadian human metapneumovirus strains. Virus research. 2003;93:51–62. doi: 10.1016/S0168-1702(03)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peret TC, Boivin G, Li Y, Couillard M, Humphrey C, Osterhaus AD, et al. Characterization of human metapneumoviruses isolated from patients in North America. J Infect Dis. 2002;185:1660–3. doi: 10.1086/340518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biacchesi S, Skiadopoulos MH, Boivin G, Hanson CT, Murphy BR, Collins PL, et al. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315:1–9. doi: 10.1016/s0042-6822(03)00528-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhan X, Slobod KS, Jones BG, Sealy RE, Takimoto T, Boyd K, et al. Sendai virus recombinant vaccine expressing a secreted, unconstrained respiratory syncytial virus fusion protein protects against RSV in cotton rats. Int Immunol. 2015;27:229–36. doi: 10.1093/intimm/dxu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai Y. Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev Med Virol. 1999;9:83–99. doi: 10.1002/(sici)1099-1654(199904/06)9:2<83::aid-rmv244>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Nagai Y, Kato A. Paramyxovirus reverse genetics is coming of age. Microbiol Immunol. 1999;43:613–24. doi: 10.1111/j.1348-0421.1999.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 23.Piyaratna R, Tollefson SJ, Williams JV. Genomic analysis of four human metapneumovirus prototypes. Virus research. 2011;160:200–5. doi: 10.1016/j.virusres.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. American Journal of Hygeine. 1938;27:493–7. [Google Scholar]
- 25.Thompson SD, Portner A. Localization of functional sites on the hemagglutinin-neuraminidase glycoprotein of Sendai virus by sequence analysis of antigenic and temperature-sensitive mutants. Virology. 1987;160:1–8. doi: 10.1016/0042-6822(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 26.Hausmann S, Garcin D, Delenda C, Kolakofsky D. The versatility of paramyxovirus RNA polymerase stuttering. J Virol. 1999;73:5568–76. doi: 10.1128/jvi.73.7.5568-5576.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–9. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vulliemoz D, Roux L. "Rule of six": how does the Sendai virus RNA polymerase keep count? J Virol. 2001;75:4506–18. doi: 10.1128/JVI.75.10.4506-4518.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulbrandt ND, Ji H, Patel NK, Barnes AS, Wilson S, Kiener PA, et al. Identification of antibody neutralization epitopes on the fusion protein of human metapneumovirus. The Journal of general virology. 2008;89:3113–8. doi: 10.1099/vir.0.2008/005199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. JPediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Schuster JE, Cox RG, Hastings AK, Boyd KL, Wadia J, Chen Z, et al. A broadly neutralizing human monoclonal antibody exhibits in vivo efficacy against both human metapneumovirus and respiratory syncytial virus. J Infect Dis. 2015;211:216–25. doi: 10.1093/infdis/jiu307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schickli JH, Kaur J, Ulbrandt N, Spaete RR, Tang RS. An S101P substitution in the putative cleavage motif of the human metapneumovirus fusion protein is a major determinant for trypsin-independent growth in vero cells and does not alter tissue tropism in hamsters. J Virol. 2005;79:10678–89. doi: 10.1128/JVI.79.16.10678-10689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schowalter RM, Smith SE, Dutch RE. Characterization of human metapneumovirus F protein-promoted membrane fusion: critical roles for proteolytic processing and low pH. J Virol. 2006;80:10931–41. doi: 10.1128/JVI.01287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biacchesi S, Pham QN, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. Modification of the trypsin-dependent cleavage activation site of the human metapneumovirus fusion protein to be trypsin independent does not increase replication or spread in rodents or nonhuman primates. J Virol. 2006;80:5798–806. doi: 10.1128/JVI.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cseke G, Wright DW, Tollefson SJ, Johnson JE, Crowe JE, Jr, Williams JV. Human metapneumovirus fusion protein vaccines that are immunogenic and protective in cotton rats. J Virol. 2007;81:698–707. doi: 10.1128/JVI.00844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology. 2011;410:429–36. doi: 10.1016/j.virol.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sealy R, Jones BG, Surman SL, Hurwitz JL. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine. 2010;28:6749–56. doi: 10.1016/j.vaccine.2010.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones BG, Sealy RE, Rudraraju R, Traina-Dorge VL, Finneyfrock B, Cook A, et al. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine. 2012;30:959–68. doi: 10.1016/j.vaccine.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones B, Zhan X, Mishin V, Slobod KS, Surman S, Russell CJ, et al. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine. 2009;27:1848–57. doi: 10.1016/j.vaccine.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan X, Slobod KS, Krishnamurthy S, Luque LE, Takimoto T, Jones B, et al. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine. 2008;26:3480–8. doi: 10.1016/j.vaccine.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]