Abstract
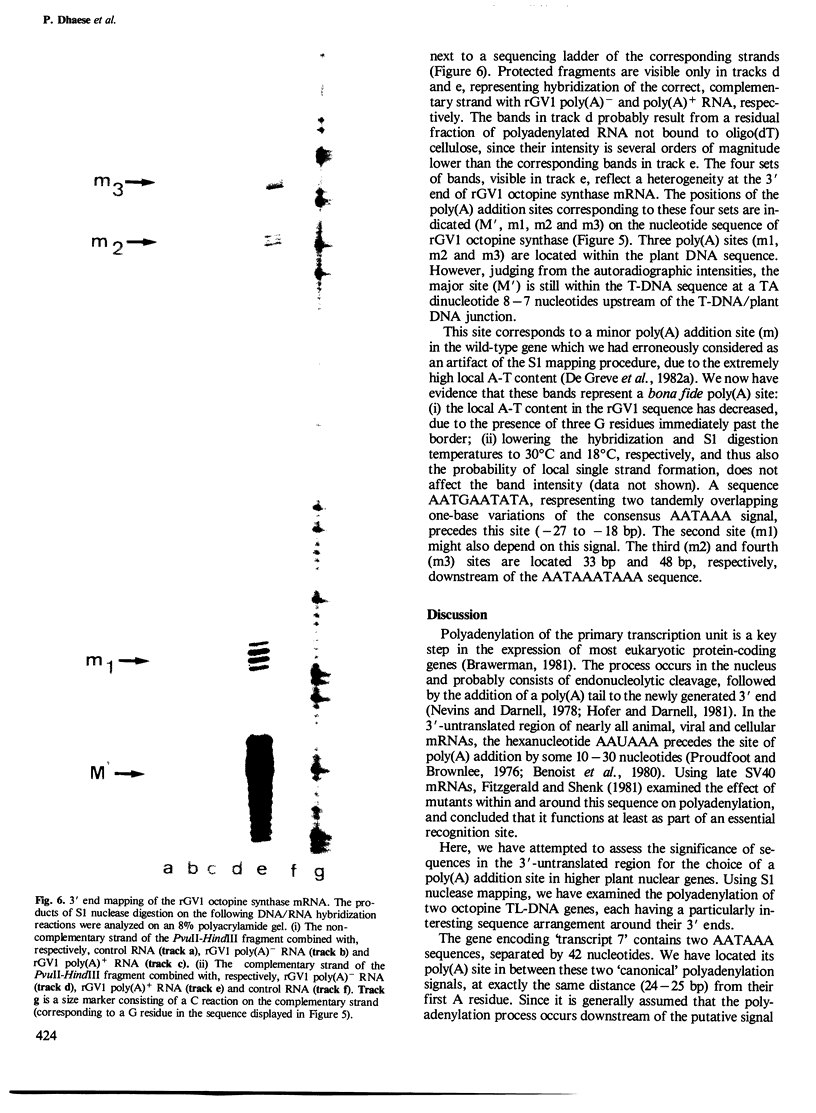
Sequences in the 3'-untranslated region of two different octopine T-DNA genes were analyzed with regard to their significance in polyadenylation. Poly(A) addition sites were localized precisely by S1 nuclease mapping with T-DNA-derived mRNAs isolated from tobacco. The gene encoding transcript 7' contains two AATAAA hexanucleotides, respectively 119 bp and 170 bp downstream of the TAA stop codon. A single poly(A) site was mapped 24-25 bp downstream of the first AATAAA. Further, we show that a mutant octopine synthase gene, which has lost part of its 3'-untranslated region by deletion, is still active. This mutant gene terminates 19 bp upstream from the major wild-type polyadenylation site. The deletion also removes the AATAAT signal preceding this site. The mutant octopine synthase gene contains a minimum of four different poly(A) sites. The most prominent of these sites is identical to the minor poly(A) site of the wild-type gene, and is preceded by a sequence AATGAATATA. Three other sites are located within the adjacent plant DNA, giving rise to hybrid T-DNA/plant DNA transcripts. The two most distal sites are probably dependent on a motif AATAAATAAA, found 29 bp away from the T-DNA/plant DNA junction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Braun A. C., Wood H. N. Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci U S A. 1976 Feb;73(2):496–500. doi: 10.1073/pnas.73.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. The Role of the poly(A) sequence in mammalian messenger RNA. CRC Crit Rev Biochem. 1981;10(1):1–38. doi: 10.3109/10409238109114634. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Wolgemuth D. J., Hsu M. T., Darnell J. E., Jr Transcription and accurate polyadenylation in vitro of RNA from the major late adenovirus 2 transcription unit. Cell. 1982 Mar;28(3):575–584. doi: 10.1016/0092-8674(82)90212-4. [DOI] [PubMed] [Google Scholar]
- De Beuckeleer M., Lemmers M., De Vos G., Willmitzer L., Van Montagu M., Schell J. Further insight on the transferred-DNA of octopine crown gall. Mol Gen Genet. 1981;183(2):283–288. doi: 10.1007/BF00270630. [DOI] [PubMed] [Google Scholar]
- De Greve H., Dhaese P., Seurinck J., Lemmers M., Van Montagu M., Schell J. Nucleotide sequence and transcript map of the Agrobacterium tumefaciens Ti plasmid-encoded octopine synthase gene. J Mol Appl Genet. 1982;1(6):499–511. [PubMed] [Google Scholar]
- De Vos G., De Beuckeleer M., Van Montagu M., Schell J. Restriction endonuclease mapping of the octopine tumor-inducing plasmid pTiAch5 of Agrobacterium tumefaciens. Plasmid. 1981 Sep;6(2):249–253. doi: 10.1016/0147-619x(81)90070-6. [DOI] [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Engler G., Depicker A., Maenhaut R., Villarroel R., Van Montagu M., Schell J. Physical mapping of DNA base sequence homologies between an octopine and a nopaline Ti plasmid of Agrobacterium tumefaciens. J Mol Biol. 1981 Oct 25;152(2):183–208. doi: 10.1016/0022-2836(81)90239-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B., Thomashow M. F., McPherson J. C., Gordon M. P., Nester E. W. Sizes and map positions of several plasmid-DNA-encoded transcripts in octopine-type crown gall tumors. Proc Natl Acad Sci U S A. 1982 Jan;79(1):76–80. doi: 10.1073/pnas.79.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty D., Peifer M. A., Rubenstein I., Messing J. The primary structure of a plant storage protein: zein. Nucleic Acids Res. 1981 Oct 10;9(19):5163–5174. doi: 10.1093/nar/9.19.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Wiborg O., Garrett R., Jørgensen P., Marcker K. A. The primary structures of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jan 22;10(2):689–701. doi: 10.1093/nar/10.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans J., Deblaere R., Willmitzer L., De Greve H., Hernalsteens J. P., Van Montagu M., Schell J. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J. 1982;1(1):147–152. doi: 10.1002/j.1460-2075.1982.tb01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. D., Larkins B. A. Analysis of sequence microheterogeneity among zein messenger RNAs. J Biol Chem. 1982 Sep 10;257(17):9976–9983. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Otten L., De Greve H., Hernalsteens J. P., Van Montagu M., Schieder O., Straub J., Schell J. Mendelian transmission of genes introduced into plants by the Ti plasmids of Agrobacterium tumefaciens. Mol Gen Genet. 1981;183(2):209–213. doi: 10.1007/BF00270619. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Tosi M., Young R. A., Hagenbüchle O., Schibler U. Multiple polyadenylation sites in a mouse alpha-amylase gene. Nucleic Acids Res. 1981 May 25;9(10):2313–2323. doi: 10.1093/nar/9.10.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Marcker K. A. The nucleotide sequences of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jun 11;10(11):3487–3494. doi: 10.1093/nar/10.11.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Schmalenbach W., Schell J. Transcription of T-DNA in octopine and nopaline crown gall tumours is inhibited by low concentrations of alpha-amanitin. Nucleic Acids Res. 1981 Oct 10;9(19):4801–4812. doi: 10.1093/nar/9.19.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]