Abstract
Excessive daytime sleepiness (EDS) and fatigue increases with age. The aim of this study was to investigate the association between EDS and fatigue with cortical thickness and hippocampal volume in cognitively normal, late middle-aged and older adults. We performed a cross-sectional observational study of 1374 cognitively-normal subjects aged 50 years and older who had a structural MRI. Regional cortical thickness and hippocampal volume were measured. Multiple linear regression models were fit to explore associations between EDS and fatigue and structural MRI measures in different brain regions, adjusting for multiple covariates. EDS was defined as Epworth Sleepiness Scale ≥10. Fatigue severity was assessed with the Beck Depression Inventory-2. 208 participants had EDS, 27 had significant fatigue, and 11 had both. Participants with EDS or fatigue had significantly lower cognitive scores, more disturbed sleep, and medical comorbidities. The presence of EDS was associated with both global and regional atrophy, whereas fatigue was more associated with frontal and temporal changes. Cortical thinning predicted by EDS and fatigue was maximal in the temporal region with average reduction of 34.2 µm (95% CI, −54.1, −14.3; P=.001) and 90.2 µm (95% CI, −142.1, −38.2; P=.001), respectively. Fatigue was also associated with hippocampal volume reduction of −374.2 mm3 (95%CI, −670.8, −77.7; P=.013). Temporal cortical thinning predicted by presence of EDS and fatigue was equivalent to more than 3.5 and nine additional years of aging, respectively. EDS and fatigue were associated with cortical thickness reduction primarily in regions with increased age-susceptibility, which may indicate accelerated brain aging.
Keywords: sleepiness, fatigue, structural MRI, cortical thickness, hippocampal volume, aging
1. Introduction
Sleep disturbances increase with aging (Coleman, et al., 1981,Foley, et al., 1995,Phillips and Ancoli-Israel, 2001) and are often associated with excessive daytime sleepiness (EDS) and fatigue (Endeshaw, 2015,Hossain, et al., 2005,Young, 2004), which significantly impact patient’s daily functioning (Gooneratne, et al., 2003). EDS is defined as difficulty in maintaining desired wakefulness, or a complaint of an excessive amount of sleep (American Academy of Sleep Medicine, 2005). Large longitudinal studies suggest that EDS has become more prevalent (Ford, et al., 2015). It is estimated to affect up to 20% of adults overall and increases with age, especially when the propensity of falling asleep in the daytime is considered (Hara, et al., 2004,Hayley, et al., 2014,Young, 2004). Elderly subjects with EDS are at higher risk of developing cognitive decline and dementia (Elwood, et al., 2011,Foley, et al., 2001,Jaussent, et al., 2012,Keage, et al., 2012,Merlino, et al., 2010,Tsapanou, et al., 2015).
EDS and fatigue often overlap, but are also separate entities (Shen, et al., 2006). Fatigue has been defined as an overwhelming sense of tiredness, lack of energy, and a feeling of exhaustion, associated with impaired physical and/or cognitive functioning (Shen, et al., 2006). The estimated prevalence of fatigue in the general US population is approximately 14.3% in men and 20.4% in women (Chen, 1986). Fatigue-related symptoms in the general German population were shown to increase with advancing age (Schwarz, et al., 2003). Fatigue is independent and complementary to EDS in the assessment of sleep disorders. In insomnia, fatigue is especially prominent while EDS is less common (Hossain, et al., 2005,Pigeon, et al., 2003,Shen, et al., 2006). Unfortunately, most studies have overlooked fatigue symptoms or failed to evaluate both EDS and fatigue within the same study. Although much less explored than EDS, fatigue has also been associated with more rapid cognitive decline and with dementia in the elderly (Lin, et al., 2013,Sterniczuk, et al., 2013).
If EDS and fatigue are associated with cognitive decline, then one would also expect these symptoms to be associated with structural brain abnormalities, akin to the specific cortical thinning signature related to brain pathology in preclinical Alzheimer’s disease (AD) (Dickerson, et al., 2009,Vemuri, et al., 2008). In light of recent findings suggesting that normal sleep physiology serves a vital role in the clearance of neurotoxic metabolic byproducts, (ie, beta-amyloid (Xie, et al., 2013)), disturbed sleep manifested by EDS and fatigue may also be a harbinger of an ongoing neurodegenerative process. It remains unknown whether EDS or fatigue are associated with global or regional cortical abnormalities in individuals who are clinically determined to be cognitively normal. Understanding how novel (and especially prevalent and modifiable) risk factors for cognitive impairment affect the brain is fundamental to allowing appropriate interventions. The aim of this study was to quantify the independent association between EDS and fatigue, with cortical thickness and hippocampal volume in cognitively-normal late middle-aged and older adults.
2. Methods
2.1. Participant Selection
Study participants were a population-based sample of Olmsted County (Minnesota) residents aged 50 years and older enrolled in the Mayo Clinic Study of Aging (MCSA) from November 2004 through November 2014. This study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards and informed consent was obtained from all participants or their surrogates. Details of the MCSA design have been published elsewhere (Roberts, et al., 2008). For the present study, we included all 1374 cognitively normal participants who completed all core questions of the sleep and fatigue assessment and also had a usable 3T structural MRI scan available. There were no subsequent exclusion criteria.
2.2. Cognitive Assessments
Participants underwent a neuropsychological battery as previously described (Roberts, et al., 2008). Four cognitive domains (executive, language, memory, and visual spatial) were assessed. Individual test scores were converted to z-scores using the mean and standard deviation of this sample.
2.3 Sleep and Fatigue Assessments
Participants responded to core questions of the Mayo Sleep Questionnaire (Boeve, et al., 2013), assessing for general sleep disorder symptoms. When individuals had a bed partner, collateral information was obtained. The questionnaire assessed whether subjects had been experiencing 1) changes in their sleep duration,2) dream enactment behavior (acting out of dreams), 3) snoring or choking during sleep, 4) stopping breathing during sleep (witnessed apneas), 5) bedtime restlessness, 6) bedtime leg cramps, and 7) sleepwalking. Subjective daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS) (Johns, 1991). Subjects with ESS score ≥ 10 were considered to have excessive daytime sleepiness (EDS). The ESS has been found to be a valid and reliable tool to separate normal adult subjects from patients with sleep disorders (Johns, 1991,Pallesen, et al., 2007). Studies have shown that ESS scores correlate at least moderately with objective measures of sleepiness (Cai, et al., 2013,Johns, 1991,Leng, et al., 2003). Scores range from 0–24. Although there are no universally adapted cut-off scores for EDS, studies with similar population have utilized ESS score ≥ 10 (Hayley, et al., 2014). Fatigue was assessed using question #20 of the Beck Depression Inventory-II (BDI-II) (Beck, et al., 1996). Subjects that endorsed being too tired or fatigued “to do a lot” (Score=2) or “most of things they used to do” (Score=3) were considered to have clinically significant fatigue. This method of assessing fatigue is exploratory and has not been previously validated. We chose a cut-off score (≥ 2) as a proxy f or clinically significant fatigue given that scores of 2 or more are higher than the average scores seen in chronic fatigue syndrome (mean 1.48 ± 1.06) (Brown, et al., 2012) and in primary care settings (mean 0.65 ± 0.72) (Arnau, et al., 2001). For individuals with reported and witnessed apneas, CPAP use was inquired. However, due to the epidemiological nature of the present study, polysomnographic confirmation of obstructive sleep apnea (OSA) diagnosis or compliance data were not obtained.
2.4. Medical and Psychiatric Comorbidities Assessment
History of medical conditions (diabetes, hypertension, atrial fibrillation, dyslipidemia) was abstracted by trained nurses using the Rochester Epidemiology Project (REP) medical records-linkage system. Information about obesity (body mass index [BMI]>30), history of tobacco use, and depression (defined by BDI-II score ≥13) (Beck, et al., 1996) were acquired from the structured interview conducted and direct measurement of height and weight by the nurses.
2.5. Structural MRI assessment
Cortical thickness from 3-Tesla magnetization-prepared rapid acquisition gradient echo (MP RAGE) image sequences were estimated using FreeSurfer version 5.3 (Fischl, et al., 2002). Thicknesses were computed from a total of 34 regions of interest (ROIs) with automated cortical parcellation using the Desikan-Killiany Atlas (Desikan, et al., 2006). They were further averaged over left and right hemispheres and grouped into thickness in four lobes: frontal, parietal, temporal, and occipital. The average thickness for each lobe and the global thickness (overall average of all lobes) were used as the primary outcome measures. Additionally, we also used hippocampal volume estimation using FreeSurfer as an outcome measure and computed total intracranial volume using SPM12 software.
2.6. Statistical Analysis
Participants were categorized into 4 groups: 1) without EDS or fatigue (No EDS/fatigue), 2) with EDS only (EDS), 3) with fatigue only (fatigue) and 4) with both EDS and fatigue (EDS and fatigue). Due to the low number of individuals in the EDS and fatigue group (n=11), they were described separately in the results section only. Parametric numerical variables were compared by means of ANOVA followed by Tukey's HSD or Games-Howell post-hoc tests, as appropriate. Non-parametric data were compared by means of the Kruskal-Wallis test followed by Dunn’s post-hoc test. Chi-Square test or Fisher’s exact test followed by post-hoc pairwise comparisons with Bonferroni correction were applied to compare categorical variables, as applicable.
In order to test the association between EDS and fatigue with structural MRI measures, multiple linear regression models were generated using averaged cortical thickness and hippocampal volume as the dependent variable. Multiple linear regression models were created for regional and global cortical thickness. Using the simultaneous entry method, whereby all variables are included at the same time (as opposed to stepwise) (Mundry and Nunn, 2009), EDS and fatigue were included as independent factors. Age, gender, years of education, respiratory symptoms (snore, choke or witnessed apneas), dream enactment, hypertension, diabetes, obesity, depression, and total intracranial volume were also analyzed as independent variables for appropriate control of potential confounders. A secondary analysis checked for a possible interaction between EDS and Fatigue. P-value was set at 0.05 for two-tailed significance levels. Statistical Analyses were performed with IBM SPSS Statistics for Windows Version 20 (Armonk, NY: IBM Corp).
3. Results
3.1. Demographic characteristics
Of the 1374 cognitively normal participants included in this study, 208 (15%) subjects had EDS only, 27 (2%) had fatigue only, and 11 (0.8%) had both EDS and fatigue symptoms. 1128 participants (82%) had neither EDS nor Fatigue. As shown in Table 1, EDS and fatigue individuals were older than those with No EDS/fatigue. There were no age differences between subjects with EDS and fatigue. There were more men in the EDS group, when compared to the No EDS/fatigue and fatigue groups. No EDS/fatigue subjects had significantly more years of education when compared to fatigue individuals. Subjects with both EDS and fatigue were similarly aged to individuals with either alone and level of education was comparable to the individuals with fatigue only.
Table 1.
Subject demographic and clinical characteristics
| P-values | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variables | No EDS/Fatigue (N=1128) |
EDS (N=208) |
Fatigue (N=27) |
No EDS/Fatigue vs. EDS |
No EDS/Fatigue vs. Fatigue |
EDS vs. Fatigue |
| Age (Years) | 71.6 ± 8.7 | 74.5 ± 8.6 | 77.2 ± 7.8 | <.001 | .002 | .266 |
| Gender (Male) (%) | 699 (62) | 167 (80.3) | 15 (55.6) | <.001 | 1 | .011 |
| Education (Years) * | 15 (12 – 16) | 14 (12 – 16) | 12 (12 – 15) | .079 | .007 | .116 |
| Cognitive Evaluation | ||||||
| Executive (z-score) | 0.78 ± 0.87 | 0.44 ± 0.86 | −0.05 ± 0.99 | <.001 | <.001 | .018 |
| Memory (z-score) | 0.82 ± 0.96 | 0.47 ± 0.94 | 0.33 ± 1.05 | <.001 | .023 | .753 |
| Language (z-score) | 0.63 ± 0.85 | 0.41 ± 0.88 | 0.18 ± 1.16 | .003 | .021 | .398 |
| Visual spatial (z-score) | 0.71 ± 0.94 | 0.51 ± 0.95 | 0.12 ± 1.12 | .013 | .005 | .121 |
| Global (z-score) | 0.91 ± 0.89 | 0.55 ± 0.91 | 0.19 ± 1.08 | <.001 | <.001 | .124 |
| Sleep Screening | ||||||
| ESS Score* | 4 (3 – 6) | 11 (10 – 13) | 4 (2 –7) | <.001 | 1 | <.001 |
| Bedpartner (yes) | 988 (87.6) | 175 (84.1) | 20 (74.1) | .518 | .210 | .553 |
| Reduced sleep (yes) | 240 (21.3) | 32 (15.8) | 11 (47.8) | .157 | .046 | .009 |
| Snore or choke (yes) | 266 (23.6) | 87 (41.8) | 4 (14.8) | <.001 | .862 | .020 |
| Witnessed apneas (yes) | 180 (16) | 71 (34.1) | 6 (22.2) | <.001 | 1 | .644 |
| Dream enactment (yes) | 78 (6.9) | 35 (16.8) | 3 (11.1) | <.001 | 1 | 1 |
| Bedtime restlessness (yes) | 81 (7.2) | 34 (16.3) | 5 (18.5) | <.001 | .134 | 1 |
| Leg cramps (yes) | 332 (29.4) | 82 (39.4) | 9 (33.3) | .013 | 1 | 1 |
| Sleepwalking (yes) | 7 (0.6) | 3 (1.4) | 0 (0) | .619 | 1 | 1 |
| Comorbidities | ||||||
| Hypertension (yes) | 704 (62.4) | 148 (71.2) | 21 (77.8) | .048 | .308 | 1 |
| Diabetes (yes) | 161 (14.3) | 45 (21.6) | 11 (40.7) | .021 | .003 | .085 |
| Obesity (yes) | 319 (28.3) | 63 (30.3) | 11 (40.7) | 1 | .470 | .814 |
| Dyslipidemia (yes) | 898 (79.6) | 174 (83.7) | 23 (85.2) | .535 | 1 | 1 |
| Atrial Fibrillation (yes) | 96 (8.5) | 37 (17.8) | 7 (25.9) | <.001 | .022 | .924 |
| Smoking history (yes) | 518 (45.9) | 104 (50) | 18 (66.7) | .836 | .098 | .309 |
| Depression (yes) | 37 (3.3) | 16 (7.7) | 12 (44.4) | .008 | <.001 | <.001 |
Data are shown as median (25 – 75% percentiles). ESS: Epworth Sleepiness Scale. P-values for all post-hoc pairwise comparisons (Chi-square or Fisher’s exact test) between categorical variables underwent Bonferroni Correction. Numerical variables were compared with ANOVA followed by Tukey's HSD post-hoc tests (age, cognitive evaluation) or Kruskal-Wallis followed by Dunn’s post-hoc test (education and ESS).
3.2. Cognitive assessment
EDS and fatigue subjects had significantly lower scores in all cognitive domains tested, when compared to No EDS/fatigue individuals. Average z-scores demonstrated lower performance in the fatigue group when compared with EDS for all cognitive domains, but this was only significant for the executive domain. Individuals with both EDS and fatigue had even lower scores than fatigue subjects in most domains (global zscore 0.06 ± 0.82).
3.3. Sleep disturbance
The EDS subjects had significantly higher frequency of sleep disturbances compared with the No EDS/fatigue group, including symptoms of snoring or choking, witnessed apneas, dream enactment, bedtime restlessness, and leg cramps. Fatigue subjects reported more frequent reduced sleep and less frequent snore or choke symptoms than all groups. Individuals with both EDS and fatigue had the highest symptom frequency of snoring or choking (54.5%), witnessed apnea (54.5%), and leg cramps (54.5%). Approximately 20% of all subjects were reported to have witnessed apneas by their bed partners. The use of CPAP on this subsample was similar between all groups: 45.5% (No EDS/fatigue), 42.3% (EDS), 33.3% (fatigue) and 33.3% (EDS and fatigue).
3.4. Medical Comorbidities
EDS and fatigue groups had more frequent comorbidities of diabetes, atrial fibrillation, and depression compared to No EDS/fatigue individuals. Hypertension was also more frequent in the EDS and fatigue groups, but only reached statistical significance in the comparison between EDS and No EDS/Fatigue groups. EDS and fatigue groups were similar in their comorbidity profile, except for depression, which was significantly more frequent in the fatigue group. Subjects with both EDS and fatigue had the highest frequency of hypertension (90.9%), obesity (45.5%), dyslipidemia (100%) and depression (63.6%).
3.5. Cortical Thickness and Hippocampal Volume
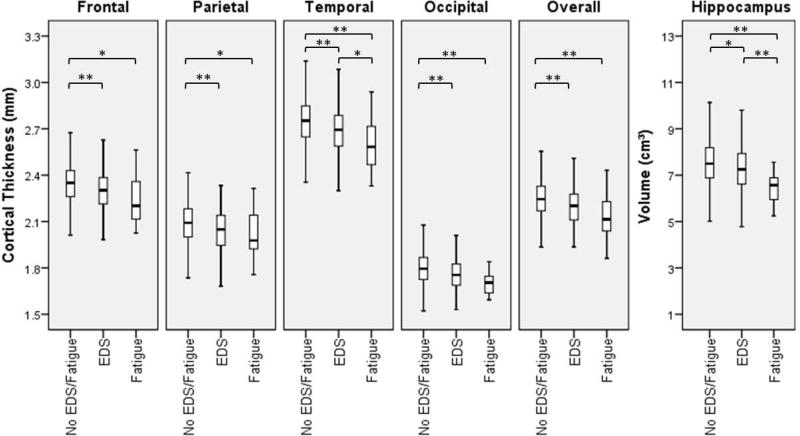
Cortical thickness was reduced in both the EDS and fatigue groups, when compared to No EDS/fatigue subjects in all regions. The same effect was also seen for hippocampal volume (Figure 1). EDS and fatigue were independently associated with cortical thickness after controlling for multiple confounders (Table 2).
Figure 1.
Boxplot representation shows decrease in cortical thickness in different brain regions and reduced hippocampal size in subjects with EDS and fatigue when compared to individuals without these symptoms. EDS: excessive daytime sleepiness; *: p<0.05; **:p<0.01.
Table 2.
Multiple linear regression models estimates for predicted cortical thickness and hippocampal volume.
| Frontal Thickness (µm) |
Parietal Thickness (µm) |
Temporal Thickness (µm) |
Occipital Thickness (µm) |
Overall Thickness (µm) |
Hippocampal Volume (mm3) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Predictors | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | B (95% CI) | p |
| Age (years) | −6.3(−7;−5.5) | <.001 | −7.1(−7.9; −6.4) | <.001 | −9.6(−10.5; −8.7) | <.001 | −5.5(−6.1; −4.9) | <.001 | −7.1(−7.8; −6.5) | <.001 | −65.7(−70.7; −60.7) | <.001 |
| Gender (male) | −5.9(−22.9;11.1) | .494 | −31.7(−48.4; −14.9) | <.001 | −12.1(−31.3;7.1) | .216 | −24.7(−38.2;−11.1) | <.001 | −18.6(−33; −4.2) | .011 | −52(−161.5;57.5) | .352 |
| Education (years) | 2.6(0.3;4.9) | .030 | 2.1(−0.2;4.4) | .078 | 2(−0.6;4.6) | .132 | 1.8(0;3.7) | .053 | 2.1(0.2;4.1) | .035 | −9.1(−24.1;5.9) | .235 |
| EDS | −26.5(−44.1; −8.9) | .003 | −23.1(−40.4; −5.7) | .009 | −34.2(−54.1; −14.3) | .001 | −20.9(−34.9; −6.8) | .004 | −26.1(−41.1; −11.2) | .001 | −52.1(−165.7;61.4) | .368 |
| Fatigue | −48.7(−94.7; −2.7) | .038 | −14.2(−59.6;31.1) | .539 | −90.2(−142.1; −38.2) | .001 | −27.4(−64.1;9.2) | .142 | −45.1(−84.1; −6.2) | .023 | −374.2(−670.8; −77.7) | .013 |
| Dream Enactment | −1.5(−23.7;20.6) | .892 | 2.4(−19.4;24.2) | .830 | −1.2(−26.2;23.8) | .925 | 4.2(−13.5;21.8) | .643 | 1(−17.8;19.7) | .920 | 26.3(−116.5;169.1) | .718 |
| Respiratory Symp. | −6.8(−20.4;6.7) | .323 | −1(−14.3;12.4) | .889 | −1.5(−16.9;13.8) | .846 | −1.2(−12;9.7) | .835 | −2.6(−14.1;8.9) | .655 | 17.8(−69.7;105.3) | .690 |
| Hypertension | −4.2(−18.1;9.7) | .553 | −0.6(−14.3;13.1) | .930 | −11.6(−27.3;4.2) | .150 | −5.2(−16.3;5.9) | .359 | −5.4(−17.2;6.4) | .369 | −63.2(−152.9;26.5) | .167 |
| Diabetes | −27.2(−44.7; −9.6) | .002 | −21.9(−39.2; −4.6) | .013 | −21.5(−41.3; −1.7) | .034 | −14.4(−28.4; −0.4) | .044 | −21.2(−36.1; −6.4) | .005 | −83.1(−196.1;30) | .150 |
| Obesity | −13.9(−28.1;0.3) | .055 | −5.7(−19.7;8.3) | .424 | −20.7(−36.7; −4.6) | .012 | −0.3(−11.6;11) | .960 | −10.1(−22.1;1.9) | .098 | −20.7(−112.2;70.7) | .656 |
| Depression | −18(−47.8;11.9) | .237 | −13.1(−42.5;16.3) | .383 | −21.2(−54.9;12.6) | .219 | −14.6(−38.4;9.2) | .228 | −16.7(−42;8.6) | .195 | −177.8(−370.2;14.6) | .070 |
|
| ||||||||||||
| Model performance | R | 0.49 | R | 0.53 | R | 0.59 | R | 0.52 | R | 0.59 | R | 0.69 |
| models p< 0.001 | Adj. R2 | 0.24 | Adj. R2 | 0.28 | Adj. R2 | 0.35 | Adj. R2 | 0.27 | Adj. R2 | 0.35 | Adj. R2 | 0.47 |
EDS: excessive daytime sleepiness; Respiratory symp. (snore, choke or witnessed apneas).
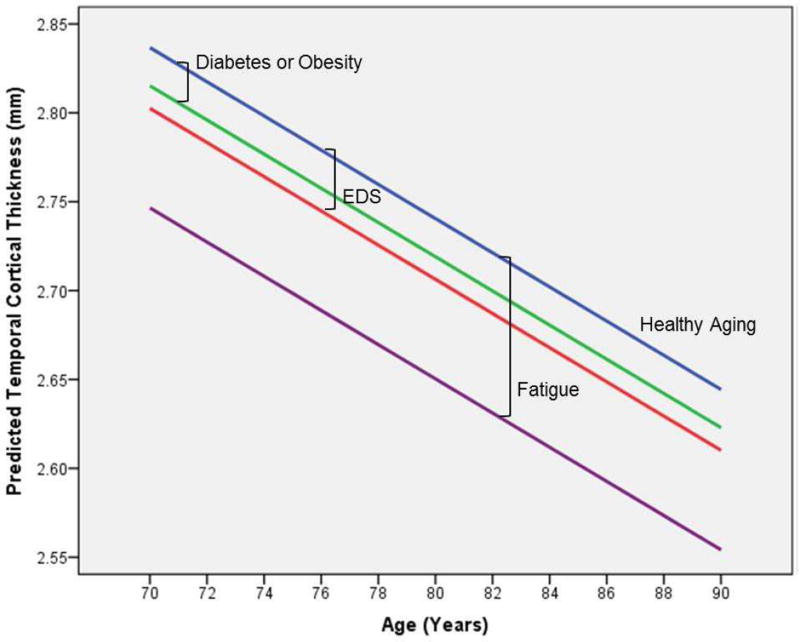
EDS was significantly associated with reduction of cortical thickness in all studied regions, which was maximal in the temporal region. The estimated change was equivalent to more than 3.5 years of aging (95% CI, 1.4–6.2) when compared to the average cortical thinning of 9.6 µm reduction/year predicted by our model. Although fatigue was also associated with overall averaged cortical thickness reduction, this was likely attributed to associations in the frontal and temporal regions. Fatigue- associated cortical thickness reduction was also maximal in the temporal region, and was equivalent to approximately nine additional years (95% CI, 3.6–16) of aging. As opposed to EDS, presence of fatigue also predicted hippocampal volume reduction, which was equivalent to 5.7 years of aging (95% CI, 1–11.1), estimated in 65.7 mm3 reduction/year by our model. In the hippocampus, only age and fatigue were significantly associated with volume reduction. Cortical thickness was significantly associated with age, gender, years of education, diabetes and obesity, in addition to EDS and fatigue. However, presence of EDS and fatigue were associated with the most prominent reductions in cortical thickness. A secondary analysis failed to reveal a significant interaction between EDS and fatigue, or the association between cortical thicknesses and reduced sleep. Figure 2 illustrates the reduction in temporal cortical thickness predicted by EDS and fatigue over time in comparison to diabetes or obesity.
Figure 2.
Illustration reveals the predicted temporal cortical thickness by age according to presence of EDS, fatigue or either diabetes or obesity (similar predicted reduction). EDS: excessive daytime sleepiness. Healthy Aging: individuals without the predictors associated with temporal cortical thickness by our model.
A sensitivity analysis excluding subjects with witnessed apneas on CPAP from the regression analysis had less power to identify associations between fatigue and cortical thickness other than in the temporal region. The overall results were very similar to the ones herein described (Table A.1 - Appendix).
4. Discussion
To our knowledge, this study is the first to assess the association between EDS and fatigue with regional brain structure in cognitively normal late middle-aged and older adults. EDS was associated with global cortical thinning, whereas fatigue was more associated with regional changes in the frontal and temporal region, including the hippocampus. Cortical thickness reduction predicted by presence of EDS or fatigue was larger than the reduction predicted by presence of obesity and diabetes. Subjects with EDS or fatigue had more frequent medical comorbidities, sleep symptoms, and depression.
4.1. Complex Interplay with Comorbid Disorders
Our results corroborate previous findings indicating the presence of multiple comorbidities in patients with EDS or fatigue (Chen, 1986, Lin, et al., 2015, Ohayon, 2012), and suggest a multifactorial cause for these symptoms. EDS and fatigue symptoms may signal a more profound level of sleep disturbance, which has been associated with increased dementia risk (Benedict, et al., 2015,Sterniczuk, et al., 2013,Yaffe, et al., 2015). Snoring or choking, reduced sleep duration, and depression were the most important discriminating factors between subjects with EDS or fatigue. More frequent symptoms of snoring, choking, and witnessed apneas in EDS subjects are likely associated with underlying obstructive sleep apnea (OSA). OSA patients appear to be at higher risk of developing mild cognitive impairment (MCI) and AD-dementia (Chang, et al., 2013,Osorio, et al., 2015,Yaffe, et al., 2011). They were found to have similar brain structural abnormalities as the ones attributed to EDS and fatigue in our study, with reduced grey matter volume (GMV) or cortical thickness in frontal, parietal and temporal regions (Joo, et al., 2013,Macey, et al., 2002,Torelli, et al., 2011), including the hippocampus (Dusak, et al., 2013,Macey, et al., 2002,Morrell, et al., 2003,Torelli, et al., 2011). In one study, hippocampal volume reduction was associated with ESS scores (Dusak, et al., 2013). Interestingly, in our regression analysis, symptoms of snoring, choking, or witnessed apneas did not predict cortical thinning, possibly because EDS is a more sensitive marker of severe OSA (Seneviratne and Puvanendran, 2004). However, EDS is not simply a surrogate of severe OSA (Ohayon, 2012,Whitney, et al., 1998) and may be influenced by other factors such as sleep duration, depression, sedating medications and obesity (Sforza, et al., 2015). EDS may also represent a higher degree of sleep fragmentation, which is associated with a reduction in the total cortical GMV (Lim, et al., 2015).
On the other hand, reduced sleep duration was more frequent in the fatigue group, suggesting inadequate sleep duration or more frequent insomnia. Our results may corroborate previous findings of bilateral hippocampal atrophy and frontal cortical thinning in insomniacs (Joo, et al., 2014,Suh, et al., 2016) as well as greater age-related brain atrophy with reduced sleep in healthy older adults (Lo, et al., 2014). However, there is likely a complicated relationship between reduced sleep, fatigue, and depression, which often coexist. Reduced sleep duration increases dementia risk, but the association disappeared after controlling for depression (Hahn, et al., 2014). Moreover, there is a significant body of evidence suggesting that depression increases the risk for AD-dementia (Ownby, et al., 2006). Similar to our findings in subjects with fatigue, depression is associated with reduced hippocampal and frontal GMV, especially in more severe disease (Lorenzetti, et al., 2009). However, fatigue cannot be considered a surrogate of depression alone, as subjects in this group also had high frequencies of medical comorbidities.
Our study focused primarily on cardiovascular and psychiatric comorbidities, but sleepiness and fatigue in the elderly have also been associated with other important comorbid disorders, especially chronic pain and chronic pulmonary diseases (Chen, 1986). Pain and sleep appear to have an important reciprocal relationship. Poor sleep seems to reduce pain threshold and predispose to chronic pain, which may worsen sleep quality (Finan, et al., 2013). Akin to our findings in fatigue patients, hippocampal volume reduction has been reported in healthy elderly adults with increased pain duration and intensity (Zimmerman, et al., 2009). Primarily frontal cortical thickness (Chen, et al., 2016) and fronto-temporal GMV (Zhang, et al., 2013) reduction has also been described in chronic obstructive pulmonary disease.
4.2. EDS and Fatigue may Indicate Brain Atrophy
Very little is known about EDS-related brain structural abnormalities in the elderly. One historical community-based study failed to find an association between daytime sleepiness and brain atrophy in an elderly population using less sensitive imaging measures of global atrophy (ventricle size, central sulcus, and bifrontal distance) (Whitney, et al., 1998). The only study that specifically evaluated the relationship between daytime sleepiness and GMV in healthy individuals was a small sample of nonelderly adults, where left frontal GMV reduction (ventromedial pre-frontal cortex) was associated with higher ESS scores (Killgore, et al., 2012). Nothing is known about fatigue-related brain structural abnormalities in the normal elderly population, only in neurological diseases, which are likely different than normal aging and beyond the scope of the present work.
It is possible that EDS and fatigue may indicate accelerated brain aging. Normal aging is associated with global cortical thinning in longitudinal studies, but the identification of the most susceptible areas remains controversial (Shaw, et al., 2016,Storsve, et al., 2014,Thambisetty, et al., 2010). The cortical thickness reduction predicted by age, EDS and fatigue was maximal in the temporal region, which is consistent with studies showing more age-related susceptibility in this region (Shaw, et al., 2016,Storsve, et al., 2014,Yang, et al., 2016).
However, changes in the temporal lobe, particularly in the hippocampus, are involved with pre-clinical Alzheimer’s disease (Andrews, et al., 2016,Dickerson, et al., 2009,Vemuri, et al., 2008) as well as time to progression from MCI to dementia (Jack, et al., 2010), suggesting that EDS and fatigue could be associated with underlying neurodegeneration. This association may occur through the interaction between sleep disorders, cardiovascular risk factors, and depression. Although not fully elucidated, there is a growing body of evidence suggesting that sleep is crucial for the removal of neurotoxic byproducts (Berezuk, et al., 2015,Lucey and Bateman, 2014,Sanchez-Espinosa, et al., 2014,Spira, et al., 2013,Sprecher, et al., 2015,Xie, et al., 2013). However, poor sleep (reduced, fragmented, with apneas) leads to metabolic and cardiovascular dysfunction (Mesas, et al., 2014,Mullington, et al., 2009,Roux, et al., 2000,Strand, et al., 2015), which are also associated with imaging (Goldstein, et al., 2002,Kharabian Masouleh, et al., 2016,Kumar, et al., 2015,Langbaum, et al., 2012,Moran, et al., 2015,Peng, et al., 2015,Soininen, et al., 1992,Villeneuve, et al., 2014) and neuropathological (Gelber, et al., 2015) findings of neurodegeneration, to which depression may also contribute (Elcombe, et al., 2015). Therefore, EDS and fatigue can possibly be an epiphenomenon of impaired clearance/accumulation of metabolic neurotoxic byproducts during sleep or neurodegeneration of areas related to maintenance of wakefulness.
4.3. Limitations
The cross-sectional design did not allow determination of the temporal relationship between EDS and fatigue with changes in cortical thickness. Due to the epidemiological nature of this study, EDS and fatigue were based solely on self-reported subjective variables. Although the Epworth Sleepiness Scale has been extensively used in the literature, it has not been validated in the elderly. A sleep quality assessment such as the Pittsburgh Sleep Quality Index might have been more sensitive to detect self-reported sleep disturbance (Niu, et al., 2016,Waller, et al., 2016). Lack of a detailed assessment of sleep duration might have limited its potential association with cortical thickness (Spira, et al., 2016). Although fatigue assessment was not optimal, through more comprehensive scales (Shen, et al., 2006), our methods were similar to previously reported methodology in epidemiological studies (Chen, 1986,Sterniczuk, et al., 2013). The low sample size in the fatigue group may have obscured potential group differences and the interaction with EDS. Subjects with both EDS and fatigue were also rare in this sample, not allowing for appropriate comparison. We accounted for only the most common medical comorbidities associated with cognitive decline.
5. Conclusion
EDS and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Our work corroborates the literature suggesting that EDS and fatigue symptoms may also be risk factors for cognitive decline or dementia, especially given their association with significantly altered brain structure primarily in the temporal region, and potential relationship with comorbid sleep disorders, cardiovascular disease and depression. Future large scale longitudinal prospective studies are necessary to clarify the association between incident EDS and fatigue, comorbid disorders, and neurodegeneration.
Supplementary Material
Highlights.
-
*
Sleepiness and fatigue are associated with significantly altered brain structure
-
*
Sleepiness is associated with global cortical thinning in cognitively-normal elderly
-
*
Fatigue is associated with fronto-temporal cortical thinning in normal elderly
-
*
Fatigue is associated with hippocampal volume reduction in normal elderly
-
*
Sleepiness and fatigue may suggest accelerated brain aging
Acknowledgments
This work was supported by NIH grants R00 AG37573, P50 AG16574/P1, R01 AG11378, R01-AG041851, R01AG034676, U01 AG06786; and Opus building NIH grant C06 RR018898. The funding institutions did not participate in study design, data collection, data analysis, manuscript writing, or submission process. Carvalho and Vemuri contributed to study concept and design. All authors participated in data collection. Carvalho contributed to the statistical analysis and drafting of the first manuscript. All co-authors participated in the critical revision of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Academy of Sleep Medicine. The international classification of sleep disorders : diagnostic and coding manual. 2. American Academy of Sleep Medicine; Westchester, IL: 2005. [Google Scholar]
- Andrews KA, Frost C, Modat M, Cardoso MJ, Rowe CC, Villemagne V, Fox NC, Ourselin S, Schott JM. Acceleration of hippocampal atrophy rates in asymptomatic amyloidosis. Neurobiol Aging. 2016;39:99–107. doi: 10.1016/j.neurobiolaging.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2001;20(2):112–9. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment. 1996;67(3):588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Benedict C, Byberg L, Cedernaes J, Hogenkamp PS, Giedratis V, Kilander L, Lind L, Lannfelt L, Schioth HB. Self-reported sleep disturbance is associated with Alzheimer's disease risk in men. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11(9):1090–7. doi: 10.1016/j.jalz.2014.08.104. [DOI] [PubMed] [Google Scholar]
- Berezuk C, Ramirez J, Gao F, Scott CJ, Huroy M, Swartz RH, Murray BJ, Black SE, Boulos MI. Virchow-Robin Spaces: Correlations with Polysomnography-Derived Sleep Parameters. Sleep. 2015;38(6):853–8. doi: 10.5665/sleep.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Molano JR, Ferman TJ, Lin SC, Bieniek K, Tippmann-Peikert M, Boot B, St Louis EK, Knopman DS, Petersen RC, Silber MH. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2013;9(5):475–80. doi: 10.5664/jcsm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Kaplan C, Jason L. Factor analysis of the Beck Depression Inventory-II with patients with chronic fatigue syndrome. Journal of health psychology. 2012;17(6):799–808. doi: 10.1177/1359105311424470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai SJ, Chen R, Zhang YL, Xiong KP, Lian YX, Li J, Shen JC, Liu CF. Correlation of Epworth Sleepiness Scale with multiple sleep latency test and its diagnostic accuracy in assessing excessive daytime sleepiness in patients with obstructive sleep apnea hypopnea syndrome. Chinese medical journal. 2013;126(17):3245–50. [PubMed] [Google Scholar]
- Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, Huang HL, Tsai SJ. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PloS one. 2013;8(10):e78655. doi: 10.1371/journal.pone.0078655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lin IT, Zhang H, Lin J, Zheng S, Fan M, Zhang J. Reduced cortical thickness, surface area in patients with chronic obstructive pulmonary disease: a surface-based morphometry and neuropsychological study. Brain imaging and behavior. 2016;10(2):464–76. doi: 10.1007/s11682--015-9403-7. [DOI] [PubMed] [Google Scholar]
- Chen MK. The epidemiology of self-perceived fatigue among adults. Preventive medicine. 1986;15(1):74–81. doi: 10.1016/0091-7435(86)90037-x. [DOI] [PubMed] [Google Scholar]
- Coleman RM, Miles LE, Guilleminault CC, Zarcone VP, Jr, van den Hoed J, Dement WC. Sleep-wake disorders in the elderly: polysomnographic analysis. Journal of the American Geriatrics Society. 1981;29(7):289–96. doi: 10.1111/j.1532-5415.1981.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusak A, Ursavas A, Hakyemez B, Gokalp G, Taskapilioglu O, Parlak M. Correlation between hippocampal volume and excessive daytime sleepiness in obstructive sleep apnea syndrome. European review for medical and pharmacological sciences. 2013;17(9):1198–204. [PubMed] [Google Scholar]
- Elcombe EL, Lagopoulos J, Duffy SL, Lewis SJ, Norrie L, Hickie IB, Naismith SL. Hippocampal volume in older adults at risk of cognitive decline: the role of sleep, vascular risk, and depression. Journal of Alzheimer's disease : JAD. 2015;44(4):1279–90. doi: 10.3233/JAD-142016. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Bayer AJ, Fish M, Pickering J, Mitchell C, Gallacher JE. Sleep disturbance and daytime sleepiness predict vascular dementia. Journal of epidemiology and community health. 2011;65(9):820–4. doi: 10.1136/jech.2009.100503. [DOI] [PubMed] [Google Scholar]
- Endeshaw YW. Do sleep complaints predict persistent fatigue in older adults? Journal of the American Geriatrics Society. 2015;63(4):716–21. doi: 10.1111/jgs.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. The journal of pain : official journal of the American Pain Society. 2013;14(12):1539–52. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, Launer L. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. Journal of the American Geriatrics Society. 2001;49(12):1628–32. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among U.S.. adults from 2002 to 2012. Sleep medicine. 2015;16(3):372–8. doi: 10.1016/j.sleep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber RP, Redline S, Ross GW, Petrovitch H, Sonnen JA, Zarow C, Uyehara-Lock JH, Masaki KH, Launer LJ, White LR. Associations of brain lesions at autopsy with polysomnography features before death. Neurology. 2015;84(3):296–303. doi: 10.1212/WNL.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology. 2002;59(5):713–9. doi: 10.1212/wnl.59.5.713. [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Weaver TE, Cater JR, Pack FM, Arner HM, Greenberg AS, Pack AI. Functional outcomes of excessive daytime sleepiness in older adults. Journal of the American Geriatrics Society. 2003;51(5):642–9. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2014;22(11):1262–71. doi: 10.1016/j.jagp.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Hara C, Lopes Rocha F, Lima-Costa MF. Prevalence of excessive daytime sleepiness and associated factors in a Brazilian community: the Bambui study. Sleep medicine. 2004;5(1):31–6. doi: 10.1016/j.sleep.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Hayley AC, Williams LJ, Kennedy GA, Berk M, Brennan SL, Pasco JA. Prevalence of excessive daytime sleepiness in a sample of the Australian adult population. Sleep medicine. 2014;15(3):348–54. doi: 10.1016/j.sleep.2013.11.783. [DOI] [PubMed] [Google Scholar]
- Hossain JL, Ahmad P, Reinish LW, Kayumov L, Hossain NK, Shapiro CM. Subjective fatigue and subjective sleepiness: two independent consequences of sleep disorders? Journal of sleep research. 2005;14(3):245–53. doi: 10.1111/j.1365-2869.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, Bernstein MA, Gunter JL, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, Knopman DS Alzheimer's Disease Neuroimaging, I. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain : a journal of neurology. 2010;133(11):3336–48. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaussent I, Bouyer J, Ancelin ML, Berr C, Foubert-Samier A, Ritchie K, Ohayon MM, Besset A, Dauvilliers Y. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35(9):1201–7. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Joo EY, Jeon S, Kim ST, Lee JM, Hong SB. Localized cortical thinning in patients with obstructive sleep apnea syndrome. Sleep. 2013;36(8):1153–62. doi: 10.5665/sleep.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37(7):1189–98. doi: 10.5665/sleep.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep medicine. 2012;13(7):886–92. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Kharabian Masouleh S, Arelin K, Horstmann A, Lampe L, Kipping JA, Luck T, Riedel-Heller SG, Schroeter ML, Stumvoll M, Villringer A, Witte AV. Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol Aging. 2016;40:1–10. doi: 10.1016/j.neurobiolaging.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Schwab ZJ, Kipman M, DelDonno SR, Weber M. Voxel-based morphometric gray matter correlates of daytime sleepiness. Neuroscience letters. 2012;518(1):10–3. doi: 10.1016/j.neulet.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Kumar R, Yadav SK, Palomares JA, Park B, Joshi SH, Ogren JA, Macey PM, Fonarow GC, Harper RM, Woo MA. Reduced regional brain cortical thickness in patients with heart failure. PloS one. 2015;10(5):e0126595. doi: 10.1371/journal.pone.0126595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbaum JB, Chen K, Launer LJ, Fleisher AS, Lee W, Liu X, Protas HD, Reeder SA, Bandy D, Yu M, Caselli RJ, Reiman EM. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol Aging. 2012;33(4):827, e11–9. doi: 10.1016/j.neurobiolaging.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng PH, Low SY, Hsu A, Chong SF. The clinical predictors of sleepiness correlated with the multiple sleep latency test in an Asian Singapore population. Sleep. 2003;26(7):878–81. doi: 10.1093/sleep/26.7.878. [DOI] [PubMed] [Google Scholar]
- Lim AS, Fleischman DA, Dawe RJ, Yu L, Arfanakis K, Buchman AS, Bennett DA. Regional Neocortical Gray Matter Structure and Sleep Fragmentation in Older Adults. Sleep. 2015 doi: 10.5665/sleep.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Chen DG, Vance DE, Ball KK, Mapstone M. Longitudinal relationships between subjective fatigue, cognitive function, and everyday functioning in old age. International psychogeriatrics / IPA. 2013;25(2):275–85. doi: 10.1017/S1041610212001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WQ, Jing MJ, Tang J, Wang JJ, Zhang HS, Yuan LX, Wang PX. Factors Associated with Fatigue among Men Aged 45 and Older: A Cross-Sectional Study. International journal of environmental research and public health. 2015;12(9):10897–909. doi: 10.3390/ijerph120910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Loh KK, Zheng H, Sim SK, Chee MW. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171–8. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. Journal of affective disorders. 2009;117(1–2):1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer's disease pathogenesis. Neurobiol Aging. 2014;35(Suppl 2):S29–34. doi: 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. American journal of respiratory and critical care medicine. 2002;166(10):1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- Merlino G, Piani A, Gigli GL, Cancelli I, Rinaldi A, Baroselli A, Serafini A, Zanchettin B, Valente M. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep medicine. 2010;11(4):372–7. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Mesas AE, Guallar-Castillon P, Lopez-Garcia E, Leon-Munoz LM, Graciani A, Banegas JR, Rodriguez-Artalejo F. Sleep quality and the metabolic syndrome: the role of sleep duration and lifestyle. Diabetes/metabolism research and reviews. 2014;30(3):222–31. doi: 10.1002/dmrr.2480. [DOI] [PubMed] [Google Scholar]
- Moran C, Beare R, Phan TG, Bruce DG, Callisaya ML, Srikanth V Alzheimer's Disease Neuroimaging, I. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85(13):1123–30. doi: 10.1212/WNL.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep medicine. 2003;4(5):451–4. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Progress in cardiovascular diseases. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundry R, Nunn CL. Stepwise model fitting and statistical inference: turning noise into signal pollution. The American naturalist. 2009;173(1):119–23. doi: 10.1086/593303. [DOI] [PubMed] [Google Scholar]
- Niu J, Han H, Wang Y, Wang L, Gao X, Liao S. Sleep quality and cognitive decline in a community of older adults in Daqing City, China. Sleep medicine. 2016;17:69–74. doi: 10.1016/j.sleep.2015.07.033. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Determining the level of sleepiness in the American population and its correlates. Journal of psychiatric research. 2012;46(4):422–7. doi: 10.1016/j.jpsychires.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, Wohlleber ME, Ducca EL, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport DM, de Leon MJ Alzheimer's Disease Neuroimaging, I. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–71. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of general psychiatry. 2006;63(5):530–8. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen S, Nordhus IH, Omvik S, Sivertsen B, Tell GS, Bjorvatn B. Prevalence and risk factors of subjective sleepiness in the general adult population. Sleep. 2007;30(5):619–24. doi: 10.1093/sleep/30.5.619. [DOI] [PubMed] [Google Scholar]
- Peng B, Chen Z, Ma L, Dai Y. Cerebral alterations of type 2 diabetes mellitus on MRI: A pilot study. Neuroscience letters. 2015;606:100–5. doi: 10.1016/j.neulet.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Phillips B, Ancoli-Israel S. Sleep disorders in the elderly. Sleep medicine. 2001;2(2):99–114. doi: 10.1016/s1389-9457(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. Journal of psychosomatic research. 2003;54(1):61–9. doi: 10.1016/s0022-3999(02)00542-1. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, D'Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. The American journal of medicine. 2000;108(5):396–402. doi: 10.1016/s0002-9343(00)00302-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Espinosa MP, Atienza M, Cantero JL. Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-beta and cortical thinning. NeuroImage. 2014;98:395–404. doi: 10.1016/j.neuroimage.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003;26(2):140–4. doi: 10.1159/000069834. doi:69834. [DOI] [PubMed] [Google Scholar]
- Seneviratne U, Puvanendran K. Excessive daytime sleepiness in obstructive sleep apnea: prevalence, severity, and predictors. Sleep medicine. 2004;5(4):339–43. doi: 10.1016/j.sleep.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Sforza E, Pichot V, Martin MS, Barthelemy JC, Roche F. Prevalence and determinants of subjective sleepiness in healthy elderly with unrecognized obstructive sleep apnea. Sleep medicine. 2015;16(8):981–6. doi: 10.1016/j.sleep.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Shaw ME, Sachdev PS, Anstey KJ, Cherbuin N. Age-related cortical thinning in cognitively healthy individuals in their 60s: the PATH Through Life study. Neurobiology of aging. 2016;39:202–9. doi: 10.1016/j.neurobiolaging.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep medicine reviews. 2006;10(1):63–76. doi: 10.1016/j.smrv.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Soininen H, Puranen M, Helkala EL, Laakso M, Riekkinen PJ. Diabetes mellitus and brain atrophy: a computed tomography study in an elderly population. Neurobiol Aging. 1992;13(6):717–21. doi: 10.1016/0197-4580(92)90095-f. [DOI] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA neurology. 2013;70(12):1537–43. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Gonzalez CE, Venkatraman VK, Wu MN, Pacheco J, Simonsick EM, Ferrucci L, Resnick SM. Sleep Duration and Subsequent Cortical Thinning in Cognitively Normal Older Adults. Sleep. 2016;39(5):1121–8. doi: 10.5665/sleep.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36(9):2568–76. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Current Alzheimer research. 2013;10(7):767–75. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(25):8488–98. doi: 10.1523/JNEUROSCI.0391-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand LB, Carnethon M, Biggs ML, Djousse L, Kaplan RC, Siscovick DS, Robbins JA, Redline S, Patel SR, Janszky I, Mukamal KJ. Sleep Disturbances and Glucose Metabolism in Older Adults: The Cardiovascular Health Study. Diabetes care. 2015;38(11):2050–8. doi: 10.2337/dc15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S, Kim H, Dang-Vu TT, Joo E, Shin C. Cortical Thinning and Altered Cortico-Cortical Structural Covariance of the Default Mode Network in Patients with Persistent Insomnia Symptoms. Sleep. 2016;39(1):161–71. doi: 10.5665/sleep.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. NeuroImage. 2010;52(4):1215–23. doi: 10.1016/j.neuroimage.2010.04.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torelli F, Moscufo N, Garreffa G, Placidi F, Romigi A, Zannino S, Bozzali M, Fasano F, Giulietti G, Djonlagic I, Malhotra A, Marciani MG, Guttmann CR. Cognitive profile and brain morphological changes in obstructive sleep apnea. NeuroImage. 2011;54(2):787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapanou A, Gu Y, Manly J, Schupf N, Tang MX, Zimmerman M, Scarmeas N, Stern Y. Daytime Sleepiness and Sleep Inadequacy as Risk Factors for Dementia. Dementia and geriatric cognitive disorders extra. 2015;5(2):286–95. doi: 10.1159/000431311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Whitwell JL, Kantarci K, Josephs KA, Parisi JE, Shiung MS, Knopman DS, Boeve BF, Petersen RC, Dickson DW, Jack CR., Jr Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. NeuroImage. 2008;42(2):559–67. doi: 10.1016/j.neuroimage.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S, Reed BR, Madison CM, Wirth M, Marchant NL, Kriger S, Mack WJ, Sanossian N, DeCarli C, Chui HC, Weiner MW, Jagust WJ. Vascular risk and Abeta interact to reduce cortical thickness in AD vulnerable brain regions. Neurology. 2014;83(1):40–7. doi: 10.1212/WNL.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller KL, Mortensen EL, Avlund K, Osler M, Fagerlund B, Lauritzen M, Jennum P. Subjective sleep quality and daytime sleepiness in late midlife and their association with age-related changes in cognition. Sleep medicine. 2016;17:165–73. doi: 10.1016/j.sleep.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21(1):27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama. 2011;306(6):613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Nettiksimmons J, Yesavage J, Byers A. Sleep Quality and Risk of Dementia Among Older Male Veterans. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2015;23(6):651–4. doi: 10.1016/j.jagp.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wen W, Jiang J, Crawford JD, Reppermund S, Levitan C, Slavin MJ, Kochan NA, Richmond RL, Brodaty H, Trollor JN, Sachdev PS. Age-associated differences on structural brain MRI in nondemented individuals from 71 to 103 years. Neurobiol Aging. 2016;40:86–97. doi: 10.1016/j.neurobiolaging.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Young TB. Epidemiology of daytime sleepiness: definitions, symptomatology, and prevalence. The Journal of clinical psychiatry. 2004;65(Suppl 16):12–6. [PubMed] [Google Scholar]
- Zhang H, Wang X, Lin J, Sun Y, Huang Y, Yang T, Zheng S, Fan M, Zhang J. Reduced regional gray matter volume in patients with chronic obstructive pulmonary disease: a voxel-based morphometry study. AJNR American journal of neuroradiology. 2013;34(2):334–9. doi: 10.3174/ajnr.A3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: a pilot study. Neurology. 2009;73(19):1567–70. doi: 10.1212/WNL.0b013e3181c0d454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.