Abstract
Autoimmune congenital heart block (CHB) is an immune-mediated acquired disease that is associated with the placental transference of maternal antibodies specific for Ro and La autoantigens. The disease develops in a fetal heart without anatomical abnormalities that could otherwise explain the block, and which is usually diagnosed in utero, but also at birth or within the neonatal period. Autoantibody-mediated damage of fetal conduction tissues causes inflammation and fibrosis and leads to blockage of signal conduction at the atrioventricular (AV) node. Irreversible complete AV block is the principal cardiac manifestation of CHB, although some babies might develop other severe cardiac complications, such as endocardial fibroelastosis or valvular insufficiency, even in the absence of cardiac block. In this Review, we discuss the epidemiology, classification and management of women whose pregnancies are affected by autoimmune CHB, with a particular focus on the autoantibodies associated with autoimmune CHB and how we should test for these antibodies and diagnose this disease. Without confirmed effective preventive or therapeutic strategies and further research on the aetiopathogenic mechanisms, autoimmune CHB will remain a severe life-threatening disorder.
Introduction
Autoimmune congenital heart block (CHB) is a passive immune-mediated acquired disease included among the manifestations collectively referred to as neonatal lupus. The disease is associated with the placental transference of maternal antibodies that are specific for Ro (also called SSA [Sjögren-syndrome-related antigen A]) and La (also called SSB [Sjögren-syndrome-related antigen B]) autoantigens and involves not only the development of cardiac abnormalities, but also cutaneous rash, liver damage and cytopenias in the newborn.1 With respect to cardiac damage, anti-Ro and anti-La antibodies cross the placenta as early as week 11 of gestation and might affect cardiac fetal development by damaging fetal conduction tissues and resulting in inflammation, calcification and fibrosis, which can block signal conduction at the atrioventricular (AV) node in an otherwise structurally-normal heart.2 The majority of autoimmune-CHB-affected babies present with type III (complete) AV block (Box 1). These babies have a severe reduction in their fetal ventricular heart rate (often 50–70 bpm; a normal range is 120–160 bpm).
Box 1. AV block classification62.
Type I
Prolonged PR interval >150 ms between atrial and ventricular depolarization; however, P waves still conduct to the ventricles
Type II
A block of one or more, but not all, P waves travelling from the atria to the ventricles
Mobitz type I (Wenckebach phenomenon)
Progressive prolongation of PR interval until one P wave fails to conduct to the ventricles
Mobitz type II
Intermittent nonconducted P waves without prolongation of the PR interval
Advanced second degree
Two or more consecutive P waves fail to conduct to the ventricles
Type III
The absence of AV conduction; no atrial impulses reach the ventricle
Abbreviation: AV, atrioventricular.
Isolated CHB was first reported in 1901 by Morquio;3 27 years later Aylward4 reported the first case associated with a maternal autoimmune disease (Mikulicz disease). Studies in the 1960s and 1970s reported that CHB-affected mothers had underlying systemic lupus erythematosus (SLE),5–7 linking this congenital cardiac disease with autoimmunity. In the 1980s, the advent of fetal echocardiography and the discovery of anti-Ro antibodies in the mothers of affected babies paved the way for a more specific definition of the disease currently known as autoimmune CHB. This Review summarizes current knowledge of the clinical spectrum of autoimmune CHB, focusing on characterizing the main maternal and fetal features and the risk of the development of this autoantibody-mediated cardiac disease. We also provide a systematic review of 39 retrospective or case–control studies to assess the clinical management of autoimmune CHB (Box 2).
Box 2. Systematic review criteria.
We searched MEDLINE (31/07/14) using the MeSH term “congenital heart block” with no restrictions of language and date. Studies were eligible when the majority of the study population fulfilled the following criteria: mothers with autoimmune diseases and/or testing positive for autoantibodies; diagnosis of CHB confirmed by fetal echocardiography; AV block diagnosed in utero, at birth or within the neonatal period (0–27 days after birth);8 absence of anatomical cardiac abnormalities which might be causal of AV block; and studies with sufficient clear information on the characteristics of CHB. Studies including duplicated or mixed data (congenital and postnatal cases, cardiac and noncardiac features) were excluded when data corresponding to autoimmune CHB were not detailed separately. Studies from the same research group that included patients recruited during the same study period were excluded; however, some multicenter studies, in which the degree of overlap with previously-reported single-centre studies could not be evaluated, were included due to the relevance of the data.
Abbreviations: AV, atrioventricular; CHB, congenital heart block.
Classification and definition
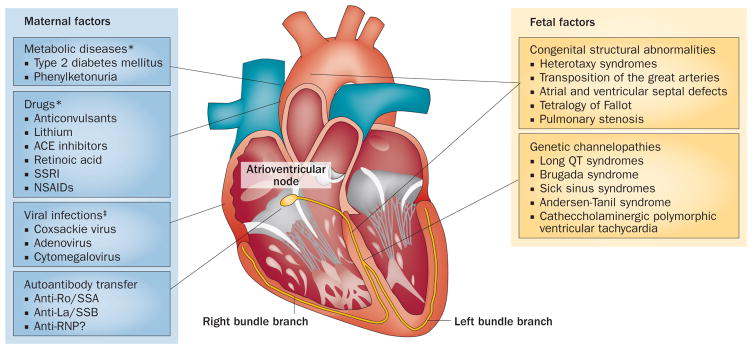
Fetal heart block is associated with two major aetiologies. Anatomical heart abnormalities are reported in 14–42% of cases8 and include AV septal defects, left atrial isomerism and abnormalities of the great arteries (Figure 1), which disturb the electrophysiological continuity between the atria and ventricles. In the remaining cases, heart block might be caused by viral infections, drugs or myocardial ischaemic and infiltrative diseases, although most cases of babies who have structurally normal hearts are associated with the transference of maternal autoantibodies,1 in which case the disease is known as autoantibody-mediated, Ro/La-related, or autoimmune CHB.
Figure 1.
Maternal and fetal factors associated with the development of CHB. Fetal heart block can be associated with maternal metabolic diseases, drugs, viral myocarditis or transference of maternal autoantibodies, or with fetal anatomical heart abnormalities and genetic channelopathies. *Factors associated with an increased risk of heart structural abnormalities. ‡Factors associated with a higher risk of development of myocarditis. Abbreviations: ACE, angiotensin-converting enzyme; RNP, ribonucleoprotein; SSA, Sjögren-syndrome-related antigen A; SSB, Sjögren-syndrome-related antigen B; SSRI, selective serotonin reuptake inhibitor.
An important problem in studying autoimmune CHB has been the absence of a common definition used by specialists involved in the management of affected babies and mothers, with a lack of consensus on the principal characteristics of the disease (Box 3). In this Review, we follow the definition used by the most experienced international groups,8 that autoimmune CHB is an AV block in the baby of a mother with autoimmune disease and/or positive for autoantibodies, with no anatomical abnormalities of the fetal heart that would otherwise explain the block, and which is diagnosed in utero, at birth or within the neonatal period (0–27 days after birth).
Box 3. Recommendations for homogeneous reporting of autoimmune CHB.
Autoimmune CHB should be diagnosed in mothers positive for anti-Ro or anti-La antibodies
CHB should be diagnosed in utero, at birth or within the neonatal period (0–27 days after birth)8
For each case of CHB, the mother’s underlying disease, the autoantibody profile and the type of previous (if relevant) and current CHB should be detailed on a case-by-case basis
Technical methods for autoantibody detection should be reported carefully
Mothers should be tested for anti-Ro52, anti-Ro60 and anti-La antibodies
Native Ro60 should be used for the detection of anti-Ro60 antibodies
Not only should type III (complete) CHB be included, but also types I and II; also, a specific definition for type I is required
Mothers who are positive for anti-Ro or anti-La antibodies and who have a baby with EFE or valvular disease, even in the absence of concomitant CHB, should also be included
Maternal autoimmune diseases should be clearly defined, including the fulfillment of the criteria corresponding to each disease
In asymptomatic mothers or in those classified as having undefined autoimmune disease, details of the specific investigations for an incipient disease (principally pSS) should be included
For incidence studies, the lack of a maternal history of previous CHB or cutaneous neonatal lupus in the pregnant mothers prospectively followed should be clearly specified; mothers with a previous history should be excluded or analysed separately in recurrence studies
Abbreviations: CHB, congenital heart block; EFE, endocardial fibroelastosis; pSS, primary Sjögren syndrome.
Incidence
No epidemiological studies have evaluated the incidence of autoimmune CHB. Two studies, from 1964, estimated the incidence of isolated complete CHB in Finland and the USA to be ~1:20,000 live births (range1:19,000–22,000).9,10 A third study, published in 1998,11 calculated the mean incidence of isolated CHB during 1970–1994 to be 1:17,000, with a wide annual variation of 1 in 6,500–64,000, and a progressive linear increase in the incidence. Unfortunately, no information on the maternal immunological status was reported by these three studies and, therefore, no specific information with respect to the incidence of autoimmune CHB is available. However, studies of CHB (due to all aetiologies) found that 14–42% of cases were associated with anatomical abnormalities;2 therefore, assuming that the remaining 56–86% of cases are autoimmune, the global incidence of autoimmune CHB is probably 1 in 20,000–30,000.
Maternal features
Maternal autoantibodies
The evidence that maternal anti-Ro and anti-La antibodies are important in the aetiopathogenesis of autoimmune CHB is supported not only by many epidemiological and clinical studies, but also by various in vitro and in vivo experimental studies.12,13 In a systematic review for this article (methodology explained in Box 2) of 39 retrospective or case–control studies, we found that anti-Ro and/or anti-La antibodies were detected in 1,230 (87%) out of 1,416 CHB-affected mothers;14–52 although the remaining cases were classified as immunonegative, they overwhelmingly correspond to CHB diagnosed in children aged ≥15 years or were cases in which mothers were not tested for the complete panel of CHB-associated autoantibodies (specific for 52 kDa Ro, 60 kDa Ro, or La).
Anti-Ro autoantibodies
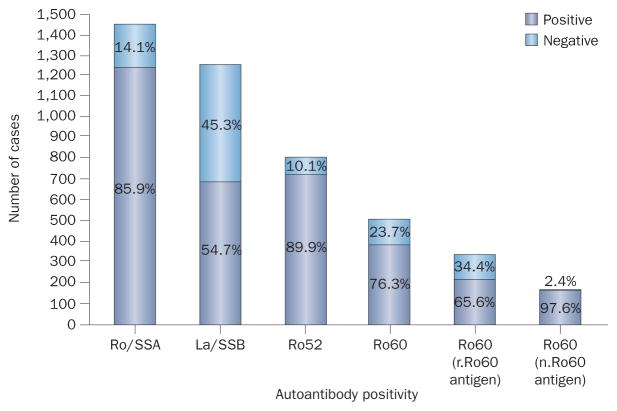
Of the 1,416 affected mothers in our systematic review, 1,216 (86%) were positive for anti-Ro antibodies, showing that this autoantibody is, by far, the auto-antibody most-closely associated with autoimmune CHB (Figure 2). Most antibodies specific for the Ro antigen recognize one, or both, of the 52 kDa and 60 kDa proteins (which are encoded by different genes), and some studies have suggested that anti-Ro52 antibodies have a predominant role in the development of autoimmune CHB.53,54 Anti-Ro52 antibodies were more frequent than anti-Ro60 antibodies (90% versus 76%, respectively) in mothers of autoimmune CHB-affected babies (Figure 2); however, anti-Ro60 antibodies are more easily detected by immunoassay if a native 60 kDa Ro protein is used as the antigen.27 The frequency of anti-Ro60 antibodies in autoimmune-CHB-affected mothers was 98% in studies using native antigen21,27,52 and 66% in those using recombinant antigen (Figure 2).16,18,20,23,27,28,35,37,44,47
Figure 2.
Ratios of autoantibody positivity and negativity amongst CHB-affected mothers.14–52 Data taken from our systematic review (Box 2). Most affected mothers were positive for anti-Ro/SSA antibodies, but only around half were positive for anti-La/SSB antibodies. Two-thirds of affected mothers were positive for anti-Ro60 antibodies when tested using r.Ro60 antigen, whereas almost all were positive when tested using n.Ro60 antigen. Abbreviations: n, native; r, recombinant SSA, Sjögren-syndrome-related antigen A; SSB, Sjögren-syndrome-related antigen B.
As not all mothers positive for anti-Ro antibodies deliver babies with CHB, studies have investigated whether maternal reactivity to a specific epitope of Ro52 might confer a high susceptibility to the induction of CHB. In 2002, Salomonsson et al.43 identified a subset of anti-Ro52 antibodies, specific for amino acids 200–239 of the Ro52 protein, in all of nine (tested) anti-Ro52-antibody-positive mothers with CHB-affected children, indicating a positive association of these autoantibodies (anti-p200 antibodies) with CHB. However, two of three subsequent case–control studies did not find statistically significant differences in the prevalence of anti-p200 antibodies between affected and nonaffected mothers.52,55,56
Most studies have reported that mothers of babies with autoimmune CHB have higher serum levels of anti-Ro and anti-La antibodies than mothers with unaffected babies (Supplementary Table 1 online). Four out of five case–control studies detected higher levels of anti-Ro52 antibodies in affected than in nonaffected pregnancies,14,33,46,48 as did two of three studies that tested levels of anti-Ro60 antibodies.14,48,52 Interestingly, only one study used native antigens for detection, and in this study anti-Ro60 antibodies were significantly higher in mothers either with an affected baby (P = 0.0002) or with a previous history of affected babies (P = 0.0001), compared with women with unaffected babies, whereas there was no statistical difference in anti-Ro52 antibody titres.52
Anti-La antibodies
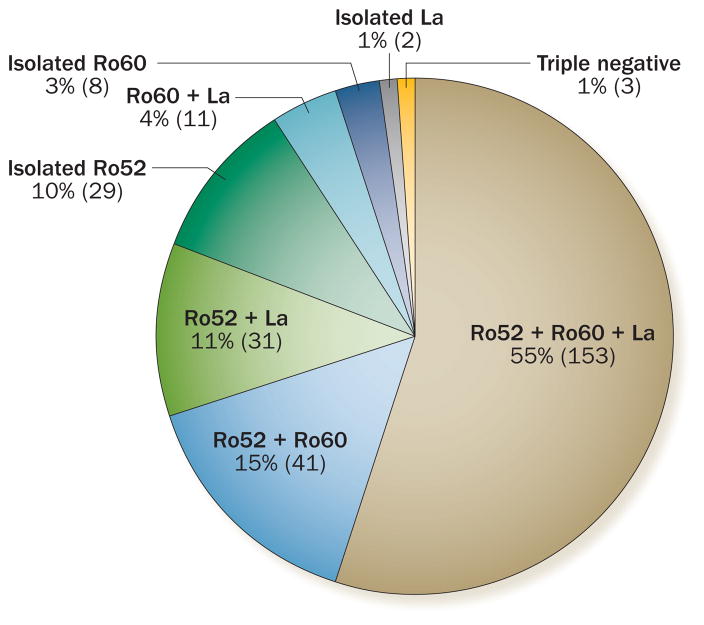
In our systematic review, we calculate that anti-La antibodies were detected in 672 (55%) of 1,229 mothers of babies with CHB, mostly in association with anti-Ro antibodies (Figure 3). The prevalence of these antibodies is similar to their prevalence in patients with primary Sjögren syndrome (pSS), which is considered the systemic autoimmune disease with the highest percentage of anti-La-antibody-positive patients.57 However, the presence of maternal anti-La antibodies in the absence of anti-Ro antibodies is uncommon, and we identified (in the published literature) only 14 cases of CHB that were associated with isolated anti-La antibodies (<1% of reported cases of autoimmune CHB; Figure 3).15,18,31,44,58 With respect to serum levels of anti-La antibodies, four out of five case–control studies have shown higher levels of anti-La antibodies in affected than in nonaffected mothers (Supplementary Table 1 online), similar to reports of anti-Ro antibodies.
Figure 3.
Proportions of isolated and multiple autoantibody positivity. 288 CHB-affected mothers were tested for anti-Ro52, anti-Ro60 and anti-La autoantibodies.16,18,27,28,44,99 Approximately half the mothers were triple positive. Much lower percentages were reported for double positivity of Ro52+Ro50+, Ro52+La+ and Ro60+La+, and less again for isolated Ro52, Ro60 or La positivity. Only three (1%) mothers tested negative for all three autoantibodies.
Other autoantibodies
Detection of concomitant maternal autoantibodies other than anti-Ro and anti-La antibodies is often dependent on the underlying maternal autoimmune disease. These antibodies include anti-dsDNA and anti-Smith (Sm) antibodies in mothers with SLE, rheumatoid factor in mothers with rheumatoid arthritis or pSS, and anti-ribonucleoprotein (RNP) antibodies in mothers with mixed connective tissue disease. A specific role of these concomitant autoantibodies in the development of CHB has not been studied, although studies have suggested a possible independent role for anti-RNP antibodies. However, all reported cases of auto-immune CHB associated with anti-RNP autoantibodies have been mothers who were also positive for anti-Ro or anti-La autoantibodies, and we identified only one case of a transient type I CHB in an anti-RNP-antibody-positive mother who was negative both for anti-Ro and anti-La antibodies.59
Some studies have identified other antigens (other than Ro and La proteins) associated with CHB, including calreticulin, the muscarinic acetylcholine receptor M1, α-fodrin (also known as spectrin α chain, non-erythrocytic 1), α-enolase, and the serotoninergic 5-hydroxytryptamine (5-HT4) receptor. 12,60,61 Antibodies specific for these proteins have not been tested in large international cohorts, and their aetiopathogenic and clinical relevance is uncertain.
Maternal autoimmune disease
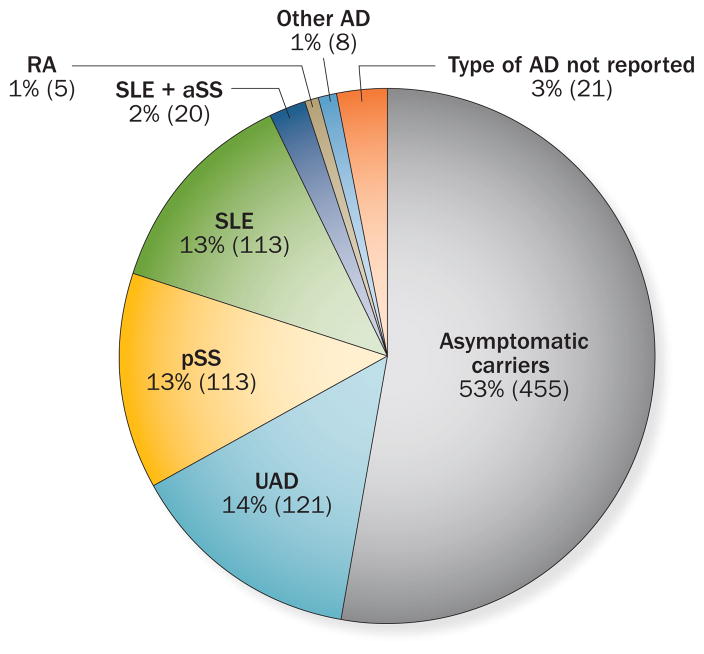
Not all mothers with CHB-affected pregnancies are diagnosed (at the same time) with a specific autoimmune disease. In our systematic review of underlying maternal autoimmune diseases, of 856 affected mothers more than half were classified as asymptomatic carriers of anti-Ro and anti-La antibodies, and ~14% were classified as incomplete or undifferentiated autoimmune disease (Figure 4).16,19,28,48,62–64 The remaining cases were mothers diagnosed with a specific autoimmune disease, almost all with pSS or SLE, or both; only 13 cases were diagnosed with other autoimmune diseases, including five with rheumatoid arthritis. More than half the affected mothers might have been asymptomatic, as anti-Ro and anti-La antibodies can be detected several years before SLE65 or pSS66 are diagnosed. In fact, autoimmune CHB could be one of the first ‘indirect’ signs of pSS in women of childbearing age.67
Figure 4.
Maternal autoimmune diseases associated with CHB. Of 856 CHB-affected mothers who were autoantibody positive, approximately half were asymptomatic Ro/La carriers. 121 (14%) had UAD (lacking fulfillment of the current criteria for systemic autoimmune diseases), 113 (13%) had pSS, 113 (13%) had SLE, 20 (2%) had Sjögren syndrome associated with SLE, and 13 (1.5%) had other ADs (including RA in 5, mixed connective tissue disease in 3, SSc in 2, autoimmune thyroiditis in 1, dermatomyositis in 1 and haemolytic anaemia in 1 case). Data from our systematic review.14–19,21–24,27–29,31–33,36,38,40–43,45–51,100,101 Abbreviations: AD, autoimmune disease; aSS, Sjögren syndrome associated with other systemic autoimmune diseases; CHB, congenital heart block; pSS, primary Sjögren syndrome; RA, rheumatoid arthritis; SSc, systemic sclerosis; SLE, systemic lupus erythematosus; UAD, undifferentiated autoimmune disease.
Fetal features of CHB
Cardiac involvement
Atrioventricular block
AV block is the most common fetal cardiac manifestation of CHB-affected mothers who test positive for anti-Ro or anti-La antibodies. Two specific characteristics of auto-immune CHB should be highlighted. Firstly, AV block is diagnosed predominantly during a specific time-frame. Secondly, infants with autoimmune CHB are more likely than those with nonautoimmune CHB to have complete AV block, which is the most severe form.
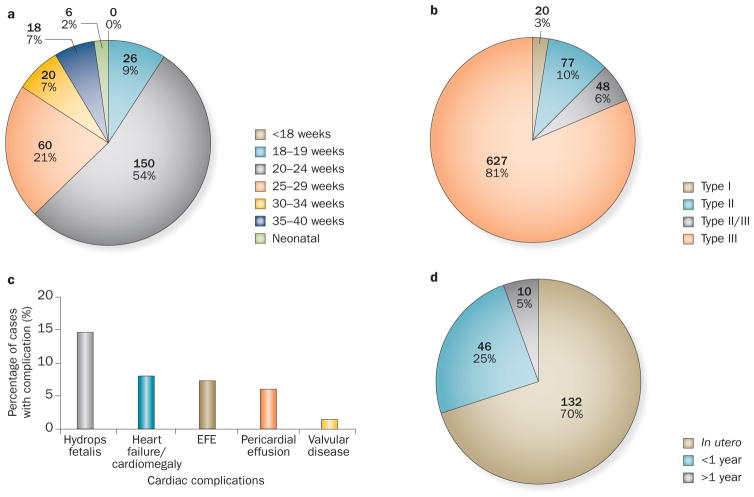
All cases in which the time of CHB diagnosis has been published have recorded the diagnosis at ≥18 weeks of gestation (Figure 5a).15,17,22,24,25,28,32,33,40,42,48,62,68,69 However, isolated cases have been reported earlier than 18 weeks; one case was at 16 weeks,70 one at 17 weeks,70 and two cases during the third month of pregnancy.71 In more than half the cases, AV block was diagnosed at 20–24 weeks of gestation, and in 75% was during weeks 20–29. Only 2% of reported cases were diagnosed at birth or within the neonatal period (<27 days after birth);15,68 however, whether serial echocardiographic surveillance was performed from week 16 in all cases is unknown. The absence of fetal monitoring could explain a late diagnosis of autoimmune CHB because anti-Ro and anti-La antibody detection is not part of routine prenatal testing. With respect to the type of CHB, >80% of the cases analysed were classified as complete AV block (type III), with only 3% corresponding to the less-advanced type I block (Figure 5b).
Figure 5.
Demographics of autoimmune CHB. Data taken from our systematic review show that negative pregnancy outcomes included prematurity (<37 weeks; n = 138 of 360, 38%)14,22,25,30,48, IUGR (n = 5 of 62, 8%),14,22,25,102 and Caesareans (n = 55 of 73, 75%).22,24,25,37,64 The gender of live births was 54% female (n = 362) and 46% male (n = 307).17,22,30,40,68,86 The mean weight of live births was 2,409.56 g (1,260–3,950 g; n =9),22,32,73 and a pacemaker was required in 519 of 809 cases (64%).15,17,21,22,24,25,28,32,40,43,48,49,51,68,69,102–104 Graphs depict a | age at diagnosis of CHB (n =280),15,17,22,24,25,28,32,33,40,42,48,62,68,69 b | CHB type (n =772),2,17,21,23–26,29,33,35,37,44,46,49,51,102 c | other cardiac complications (n =875),17,22,24,25,29,30,32,33,40,48,49,51,69,73,102,103 and d | mortality (n =188).15,17,21,22,24,25,28,30,32,33,38,40,43,48,49,51,68,69,102,103 Abbreviations: CHB, congenital heart block; EFE, endocardial fibroelastosis; IUGR, intrauterine growth restriction.
Other electrophysiological abnormalities
Although AV block is, by far, the major manifestation of autoimmune CHB, other rare electrophysiological abnormalities have been reported, including transient and persistent sinus node dysfunction, long QT interval (≥440 ms), ventricular and junctional tachycardia, and atrial flutter; however, consistent association of these abnormalities and the presence of maternal anti-Ro and anti-La antibodies has not been reported.1
Endocardial fibroelastosis
Endocardial fibroelastosis (EFE) is a form of myocardial fibrosis that can progress to end-stage heart failure and death.72 Prenatal echocardiographic signs of EFE include areas of patchy echogenicity (fibrosis) on the endocardial surfaces of the fetal heart. EFE has been reported in 7% of babies affected by CHB (comprising 19% of non-CHB cardiac abnormalities; Figure 5c), but a clear association with CHB has not been demonstrated. Table 1 summarizes the main characteristics of 116 cases of autoimmune EFE included in 14 studies,30,32,33,40,50,51,73–80 of which almost 20% were diagnosed with EFE in the absence of CHB. The outcome of babies with EFE was detailed by a study of 103 cases in which there were 53 (51%) fatalities; the mortality rate of babies with concomitant cardiomyopathy was 100%.30
Table 1.
Main characteristics of autoimmune EFE
| Features | Feature details | Cases (n) |
|---|---|---|
| CHB (n = 116) | No AV block | 22 (19%) |
| AV block | 94 (81%) | |
| Type I | 4 of 38 (11%)* | |
| Type II | 2 of 38 (5%)* | |
| Type III | 32 of 38 (84%)* | |
|
| ||
| Outcome (n = 103) | Survival | 50 (49%) |
| Death | 53 (51%) | |
| Intrauterine death | 27 of 39 (69%)‡ | |
| Neonatal death | 12 of 39 (31%)‡ | |
|
| ||
| Maternal disease (n = 31) | Asymptomatic Ro/La carriers | 17 (55%) |
| SLE | 9 (29%) | |
| Hypothyroidism | 2 (6%) | |
| Mixed connective tissue disease | 1 (3%) | |
| Raynaud phenomenon | 1 (3%) | |
| Arthritis | 1 (3%) | |
|
| ||
| Pacemaker required (n = 17) | Yes | 13 (76%) |
| No | 4 (24%) | |
Valvular disease
Valvular disease due to dysfunction of the tensor apparatus is a severe complication of autoimmune CHB, and has been reported in 1.6% of cases (comprising 4% of non-CHB cardiac complications; Figure 5c). One study detailed the diagnostic approach and outcomes of six affected babies.81 Areas of patchy echogenicity in the papillary muscle were detected at weeks 19–22, involving mainly the tricuspid and mitral valves. Severe valve insufficiency developed prenatally or postnatally, ranging from as early as 34 weeks of gestation to as late as 26 weeks after birth; all babies required urgent valve surgery, except one who died before surgery.
Pregnancy outcomes
Autoimmune CHB is associated with a mortality rate of 19% (Figure 5d); the majority of deaths (70%) occurred in utero. Risk factors associated with death were analysed in the US Research Registry for Neonatal Lupus,30 which includes 325 babies with autoimmune CHB, of whom 57 (17%) died. In two-thirds of the cases, the cause of death was severe cardiomyopathy. The mortality rate was >50% in babies with either EFE or dilated cardiomyopathy and 100% in those with both complications. Multivariate analysis showed that death in utero is associated with the development of hydrops fetalis and myocarditis, and late gestational age, with a statistically significant trend for a higher risk of death in mothers with an established diagnosis of pSS or SLE, or testing positive for anti-La antibodies. In another study, Eliasson et al.49 correlated mortality with the development of hydrops fetalis, impaired left ventricular function, a low ventricular rate (<50 bpm) and an earlier diagnosis of autoimmune CHB (<20 weeks).
Delivery outcomes
In our systematic review, we found that 81% of autoimmune-CHB-affected pregnancies result in live births, with a mean delivery of 34–37 weeks, a prematurity rate (birth <37 weeks) of 38%, a Caesarean rate of 75%, and a female:male live-birth ratio of 1.07 (Figure 5 legend). The variability in the clinical presentation of CHB in live births is a challenge for paediatric cardiologists, especially with respect to which patients need pacemakers and when they should be implanted.1 We also found that two-thirds of babies with autoimmune CHB required pacemakers, almost all during the first year of life, and in nearly two-thirds of cases during the first 10 days after birth (Supplementary Table 2 online). Babies born with autoimmune CHB require a close follow-up, as a quarter of total deaths related to auto-immune CHB are reported during the first year of life (Figure 5d).
Risk of autoimmune CHB
Global risk
Due to the severity of autoimmune CHB, knowing the risk of its development in anti-Ro and anti-La antibody-positive women of childbearing age is essential. This risk should be calculated from studies prospectively designed to evaluate pregnancy outcomes in these mothers. The first such prospective study was carried out in 2001 by Brucato et al.;82 they studied 100 anti-Ro antibody-positive pregnant mothers and found that two (2%) had babies with autoimmune CHB. Table 2 summarizes the results of eight prospective studies, including 705 anti-Ro antibody-positive mothers, of whom 12 (1.7%) had babies with autoimmune CHB.19,22,26,28,34,62,82,83 The incidence of autoimmune CHB was 0.28% for type I, 0.42% for type II and 1.00% for type III AV block. Different expression patterns of HLA genes have been suggested as an explanation as to why a minority of mothers who are positive for anti-Ro and anti-La antibodies have babies with CHB.84 Maternal factors that might exacerbate the risk of CHB deserve specific analysis. The first genome-wide association study to solely evaluate children with CHB, rather than the mothers, suggested that variation near genes related to inflammatory and apoptotic responses might promote cardiac injury initiated by passively acquired autoantibodies.85
Table 2.
Incidence of CHB in Ro+ mothers without previous history of CHB
| Study (year) | Ro+ mothers | Babies | Cases of CHB | Type and frequency of CHB |
|---|---|---|---|---|
| Brucato et al.82 (2001) | 100 | 126 | 2 | Type III (n =2, 2%) |
| Gladman et al.83 (2002) | 90 | 102 | 0 | None |
| Cimaz et al.19 (2003) | 95 | 95 | 0 | None |
| Costedoat et al.22 (2004) | 99 | 150 | 1 | Type III (n =1, 1.01%) |
| Grava et al.28 (2005) | 41 | 46 | 2 | Type II (n =1, 2.44%) Type III (n =1, 2.44%) |
| Gerosa et al.26 (2007) | 59 | 60 | 1 | Type II (n =1, 1.69%) |
| Friedman et al.62 (2008) | 79 | 79 | 3 | Type I (n =1, 1.27%) Type III (n =2, 2.53%) |
| Jaeggi et al.34 (2011) | 142 | 165 | 3 | Type I (n =1, 0.7%) Type II (n =1, 0.7%) Type III (n =1, 0.7%) |
| Total | 705 | 823 | 12 (1.7%) | Type I (n =2, 0.28%) Type II (n =3, 0.42%) Type III (n =7, 1%) |
Abbreviation: CHB, congenital heart block.
Influence of maternal autoantibody profile
Whether the risk of delivering a child with autoimmune CHB is affected by the maternal anti-Ro and anti-La autoantibody pattern is unknown. Two prospective studies of these autoantibodies, in both affected and nonaffected pregnancies, show that analysis of the risk according to the maternal autoantibody profile is feasible.16,28 The incidence of CHB-affected pregnancies was 3.31% for anti-Ro60-antibody-positive mothers, 3.92% for anti-La-antibody-positive mothers and 4.92% for anti-Ro52-antibody-positive mothers (Supplementary Table 3 online).16,28 The incidence of CHB in mothers who tested positive for isolated anti-Ro antibodies (2.25%) was lower than in those positive for both anti-Ro and anti-La autoantibodies (3.92%);16,28 the only mother who tested positive for isolated anti-La auto-antibodies delivered a baby without autoimmune CHB (Supplementary Table 3 online).28 Two earlier studies also indicated that the more maternal autoantibodies specific for the three antigens (Ro60, Ro52, La) that are detected, the higher the risk of developing CHB.13,20
Other maternal factors
Maternal age,86 the season of birth86 or autoimmune thyroiditis,87,88 can be factors in the risk of CHB. No study has specifically evaluated whether this risk is affected by an underlying systemic autoimmune disease of the mother at the time of pregnancy. However, in our pooled analysis of six prospective studies (Table 3),19,22,28,62,82,83 in which maternal disease was studied in both affected and nonaffected pregnancies, the highest incidence of CHB-affected pregnancy was in asymptomatic mothers (8%), although the total number of asymptomatic mothers was <30. Mothers with pSS had the second-highest incidence (2.9%), followed by undifferentiated autoimmune disease (2.3%) and SLE (1%); mothers with other systemic autoimmune diseases were not CHB-affected. However, these frequencies should be interpreted with caution owing to the small number of cases and the heterogeneity in the diagnostic approaches to evaluate the underlying autoimmune diseases in the mothers. Furthermore, some of the main autoimmune CHB studies were not included in the analysis because maternal underlying autoimmune diseases were not assessed.
Table 3.
Affect of maternal autoimmune disease on risk of CHB—pooled analysis of prospective studies
| Maternal diagnosis | Incidence of CHB19,22,28,62,82,83 | Recurrence of CHB19,36,40,63,83 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Ro+ mothers (n) | Total CHB (n) | Type of CHB among Ro+ mothers | Ro+ mothers with previous CHB (n)‡ | Recurrence of CHB (n) | Type of CHB specified among Ro+ mothers | |
| SLE | 303* | 3 (1%) | NS | 13* | 3 (23.1%) | Type III (n =2) NS (n =1) |
|
| ||||||
| pSS | 104* | 3 (2.9%) | Type II (n =1) Type III (n =2) |
31* | 7 (22.6%) | Type III (n =6) NS (n =1) |
|
| ||||||
| Undifferentiated autoimmune diseases | 87 | 2 (2.3%) | Type III (n =2) | 13 | 1 (7.7%) | Type III (n =1) |
|
| ||||||
| Asymptomatic Ro/La+ | 25 | 2 (8%) | NS | 24 | 3 (12.5%) | Type III (n =2) NS (n =1) |
|
| ||||||
| Other autoimmune diseases | 11 | 0 (0%) | None | 3 | 1 (33%) | Type III (n =1) |
5 mothers had both pSS and SLE, so were included in both groups.
17 Ro+ mothers (6 with SLE, 3 with pSS, 1 with MCTD, 6 asymptomatic and 1 with Raynaud phenomenon) had a previous history of NLE.
Abbreviations: CHB, congenital heart block; NS, not specified; pSS, primary Sjögren syndrome; SLE, systemic lupus erythematosus.
Obstetric history
Another highly specific feature of autoimmune CHB is that the risk is exacerbated in mothers with a previous child with CHB. Table 4 summarizes the data included in 11 prospective studies19,22,36,40,62,63,68,83,86,89,90 that evaluated the pregnancy outcomes of 317 anti-Ro-antibody-positive mothers with a previous child diagnosed with CHB (n = 50; 15.8%). The recurrence of CHB according to the type of AV block was 3.33% for type I, 0.71% for type II and 12.86% for type III. Our pooled analysis of five prospective studies (Table 3)19,36,40,63,83 that detailed maternal disease in both affected and nonaffected pregnancies showed that the rate of recurrence of CHB was twofold higher (23%) in mothers with pSS or SLE, or both, than in mothers who had undifferentiated diseases or no diagnosed disease (8–12%).
Table 4.
Recurrence of CHB in Ro+ mothers with a previous history of CHB
| Study (year) | Inclusion criteria (type of CHB) | Ro+ mothers (n=317) | Babies (n=326) | CHB (n=50) | Types of CHB |
|---|---|---|---|---|---|
| Gladman et al.83 (2002) | Previous type III CHB | 11 | 12 | 1 (9%) | Type III (n =1, 9%) |
| Cimaz et al.19 (2003) | Previous NLE (13 type III CHB, 4 CNLE) | 17* | 17 | 2 (11.76%) | Type III (n =2, 11.76%) |
| Costedoat et al.22 (2004) | Previous CHB (ND) | 7 | 13 | 0 | None |
| Friedman et al.62 (2008) | Previous CHB (ND) | 16 | 16 | 3 (18.75%) | Type I (n =2, 12.5%) Type III (n =1, 6.25%) |
| Buyon et al.68 (1998) | Previous CHB (ND) | 49 | 49 | 8 (16.32%) | ND |
| Julkunen et al.89 (2001) | Previous CHB (ND) | 47 | 47 | 8 (17.02%) | Type I (ND)‡ Type II (n =1, 2.12%) Type III (n =7, 14.89%) |
| Kaaja et al.36 (2003) | Previous CHB (ND) | 8 | 8 | 1 (12.50%) | ND |
| Solomon et al.90 (2003) | Previous type I, II or III CHB | 87 | 87 | 16 (18.39%) | Type I (n =4, 4.59%) Type II or III (n =12, 13.79%) |
| Friedman et al.63 (2010) | Previous type I, II or III CHB | 20* | 20 | 3 (15.00%) | Type III (n =3, 15.00%) |
| Pisoni et al.40 (2010) | Previous type III CHB | 22 | 24 | 4 (18.18%) | Type III (n =4, 18.18%) |
| Ambrosi et al.86 (2012) | Previous CHB (ND) | 33 | 33 | 4 (12.12%) | ND |
| Totals | NA | 317 | 326 | 50 (15.8%) | Type I (n = 6 of 180, 2.54%) Types II or III (n = 12 of 87, 13.79%) Type II (n = 1 of 140, 0.71%) Type III (n = 18 of 140, 12.86%) |
Previous history of NLE (6 cases with isolated cutaneous involvement were also included).
Type I CHB was not included in this study.
Abbreviations: CHB, congenital heart block; CNLE, cutaneous neonatal lupus erythematosus; NA, not applicable; ND, not detailed; NLE, neonatal lupus erythematosus.
Management recommendations
Maternal screening
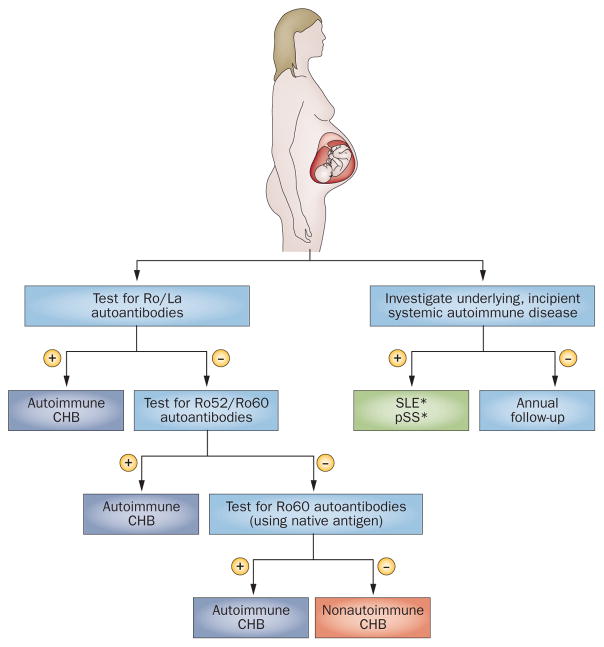
Women who have babies with CHB of any type, EFE or congenital valvular disease should be tested (with commercial tests) for the complete panel of anti-Ro and anti-La antibodies. For women testing immunonegative, we recommend extending immunological studies to include anti-Ro52 and anti-Ro60 antibodies; and when immunonegative for anti-Ro60 antibodies using recombinant proteins, we recommend retesting with native Ro60 antigens (Figure 6). Measurement of serum anti-Ro and anti-La antibody concentrations or testing for anti-p200 antibodies is not recommended for daily practice. Immunopositive mothers should be evaluated extensively for underlying incipient systemic auto-immune diseases (principally for pSS and SLE), and prospectively followed by specialists in systemic auto-immune diseases. Women of childbearing age who are diagnosed with systemic autoimmune disease should be tested for anti-Ro and anti-La antibodies 6 months prior to pregnancy.
Figure 6.
Autoantibody sequential studies and clinical evaluation of women who have babies with CHB of any degree, EFE or congenital valvular disease in an otherwise structurally-normal fetal heart. For women with negative results in Ro/La commercial tests, we recommend extending immunological studies to include anti-Ro52 and anti-Ro60 antibodies using a native antigen. *Systemic autoimmune diseases more frequently identified in CHB-affected mothers. Abbreviations: CHB, congenital heart block; EFE, endocardial fibroelastosis, pSS, primary Sjögren syndrome; SLE, systemic lupus erythematosus.
Fetal screening
Pregnant women who test positive for anti-Ro and anti-La antibodies are considered to have a high-risk pregnancy, especially those with a previous history of neonatal lupus or CHB, and should therefore be referred to a highly specialized obstetric unit. Fetal echocardiography is a safe, noninvasive method for screening CHB that offers an accurate assessment of the fetal heart rate, rhythm and ventricular function.91,92 Although we found in our systematic review that all reported cases of CHB were diagnosed after week 18 of gestation, we identified four isolated cases diagnosed at 16–17 weeks; therefore, we recommend screening from 16 weeks of gestation. Other diagnostic tests (electrocardiogram plus Holter monitor, magnetocardiography, gated-pulsed Doppler technique, velocity-based fetal kinetocardiogram) might detect subtle signs of the development of CHB, but their use is not standard practice.93–96 Serial echocardiograms and obstetric sonograms should be performed weekly from 16 weeks of gestation onwards, although the frequency might be reduced in the absence of CHB after week 26, as we found that <20% of cases are diagnosed after week 30 of gestation. Serial echocardiograms should also evaluate EFE, which might be subclinical, like type I CHB, but with a worse prognosis. Also, it would be reasonable for neonates to be observed during the first month of life, as only 2% of reported cases of CHB are diagnosed postnatally (<27 days after birth). However, as a general rule, postnatal heart monitoring is not recommended unless there are abnormalities detected in utero or during the first month of life.
Diagnosis of heart damage
Complete AV block is irreversible and has high morbidity and mortality rates, making it the most-severe manifestation of neonatal lupus. Signs or symptoms are mainly associated with the ventricular rate, which is usually 30–100 bpm,97 but can also involve cardiac contractility or an aberrant ratio between atrial and ventricular contractions. However, a ventricular rate <55 bpm is critical as it often indicates a poor fetal prognosis. Hydrops fetalis, an increased cardiothoracic index, AV valve regurgitation, or a low aortic flow velocity are also associated with poor prognosis.
Several studies have attempted to find electrophysiological abnormalities that predict the development of autoimmune CHB, searching for a potential ‘therapeutic window’ in which to prevent progression to an irreversible, complete AV block. A PR interval that exceeds the expected 95% confidence interval of a normal population has been suggested as the principal predictive sign for the development of autoimmune CHB, but unfortunately conclusive proof does not exist, as a prolonged PR interval (Figure 7) might be transient, sustained or progressive.98 The development of complete autoimmune CHB after early detection of a prolonged PR interval seems to depend on the addition of previously unidentified fetal and environmental factors.98
Figure 7.
Electrocardiogram. Typical electrocardiogram of a healthy person.
The spectrum of cardiac disease associated with maternal anti-Ro and anti-La autoantibodies mostly, but not exclusively, involves AV block. The immune-mediated damage is located principally at the conduction system and involves the AV node, but also other cardiac structures, such as the adjacent myocardium or the papillary valvular muscles, which might be fragile and susceptible to rupture (Figure 1). Therefore, babies (of anti-Ro-positive or anti-La-positive mothers) who develop cardiac failure in the absence of heart block should be screened for other cardiac abnormalities, such as EFE and valvular disease. In addition, mothers delivering babies with EFE or valvular insufficiency should be tested for anti-Ro and anti-La autoantibodies, even in the absence of AV block.
Precounselling advice
Physicians caring for anti-Ro or anti-La-antibody- positive women of childbearing age must offer accurate updated information when mothers ask about the risk of having a baby with autoimmune CHB (currently 1.7%).82 However, women might also ask about maternal features, other than anti-Ro and anti-La antibodies, that are associated with an increased risk. Current evidence shows that the obstetric history of the mother is the principal factor that increases this risk; mothers with a previous history of autoimmune-CHB-affected pregnancy have ninefold the risk (16%) in a subsequent pregnancy, and mothers with a previous history of noncardiac neonatal lupus have a sevenfold higher risk (13%) than those without this history.99 In addition, some maternal antibody patterns (double or triple positivity, or high serum levels of anti-Ro52, anti-Ro60 or anti-La autoantibodies) are associated with a higher risk of autoimmune CHB, although information from specific prospective studies is required before informing mothers with these autoimmune profiles that they are at a higher risk than mothers with single positivity or low serum levels of autoantibodies. With respect to the underlying maternal disease, the data indicate that mothers with pSS or SLE might have a higher risk than asymptomatic mothers and those with incomplete or undifferentiated diseases, but formal statistical analysis has not shown a significant influence of maternal disease status. The influence of other maternal factors that might increase the risk of CHB (maternal age, low vitamin D levels, hypothyroidism) have not been confirmed by prospective studies.
Conclusions
Autoimmune CHB is a severe, potentially life-threatening, disorder associated with the passive transfer of maternal anti-Ro and anti-La autoantibodies. However, the true incidence of autoimmune CHB is unknown as these antibodies are not always associated with clinical disease in the mother. Autoimmune CHB is the cardiac component of a cluster of fetal and neonatal manifestations that are pathogenically, epidemiologically and clinically related to these autoantibodies. However, some studies support a different point of view, suggesting that the pathogenesis of CHB involves allogenicity and that the anti-Ro and anti-La antibodies are less important biomarkers than maternal autoimmune disease per se.100 We support the idea that autoimmune CHB should be defined strictly, with two mandatory features (maternal anti-Ro and anti-La autoantibodies plus diagnosis of AV block in utero or neonatally) that conform to a specific disease phenotype, thus preserving the homogeneity of the populations studied.13 This definition will facilitate the launch of practical recommendations for a specific and well-defined disease entity and will help differentiate autoimmune CHB from other CHBs with nonautoimmune aetiologies. Unfortunately, irreversible complete AV block is the principal cardiac manifestation of CHB, and some babies might develop more severe cardiac complications (EFE, valvular insufficiency) even in the absence of cardiac block. The severity of autoimmune CHB is clear, with a 20% global mortality rate and 64% of live births requiring pacemakers. These dramatic numbers might be explained by the absence of confirmed effective preventive or therapeutic strategies for complete AV block, which is diagnosed in >80% of affected babies. Research on the aetiopathogenic mechanisms of autoimmune CHB should continue in order to identify potential biomarkers that might help to prevent (or diagnose before irreversible block occurs) this autoantibody-induced congenital heart disease.
Supplementary Material
Key points.
Autoimmune congenital heart block (CHB) is part of a cluster of fetal and neonatal manifestations associated with placental transference of autoantibodies
Maternal autoantibodies specific for Ro and La, and diagnosis in utero or neonatally, are key features of CHB
Irreversible complete atrioventricular (AV) block is the principal cardiac manifestation in >80% of reported cases
Some babies might develop other, more severe, cardiac complications, including endocardial fibroelastosis or valvular insufficiency
20% of cases of autoimmune CHB are fatal and 64% of live births require a pacemaker
1.7% of pregnant women positive for anti-Ro or anti-La antibodies have a baby with autoimmune CHB, but the risk climbs to 16% for mothers who have had previous CHB-affected pregnancies
Acknowledgments
M.R.-C. is supported by Grants La Marató de TV3 (071810) and Fondo de Investigaciones Sanitarias (080103/1201009). J.P.B. is supported by NIH Grants 3R37AR042455 and 3R03HD069986. P.B.-Z. is supported by ‘Ajut per a la Recerca Josep Font’ from Hospital Clinic-Barcelona.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
P.B.-Z., M.R.-C. and M.A.K. contributed to the conception and design of the Review. All authors contributed to acquisition and/or analysis of the systematic review data, and to writing the article and to review and/or editing of the manuscript before submission.
Supplementary information is linked to the online version of the paper at www.nature.com/nrrheum.
References
- 1.Capone C, Buyon JP, Friedman DM, Frishman WH. Cardiac manifestations of neonatal lupus: a review of autoantibody-associated congenital heart block and its impact in an adult population. Cardiol Rev. 2012;20:72–76. doi: 10.1097/CRD.0b013e31823c808b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brucato A, Cimaz R, Caporali R, Ramoni V, Buyon J. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol. 2011;40:27–41. doi: 10.1007/s12016-009-8190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morquio L. Sur une maladie infantile et familiale characterisee par des modifications permanentes du pouls, des attaques syncopales et epileptiformes et la mort subite [French] Arch Med Enfants. 1901;4:467–475. [Google Scholar]
- 4.Aylward RD. Congenital heart block. Br Med J. 1928;1:943. [Google Scholar]
- 5.Hull D, Binns BAO, Joyce D. Congenital heart block and widespread fibrosis due to maternal lupus erythematosus. Arch Dis Child. 1966;41:688–690. doi: 10.1136/adc.41.220.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCue CM, Mantakas ME, Tingelstad JB, Ruddy S. Congenital heart block in newborns of mothers with connective tissue disease. Circulation. 1977;56:82–90. doi: 10.1161/01.cir.56.1.82. [DOI] [PubMed] [Google Scholar]
- 7.Chameides L, et al. Association of maternal systemic lupus erythematosus with congenital complete heart block. N Eng J Med. 1977;297:1204–1207. doi: 10.1056/NEJM197712012972203. [DOI] [PubMed] [Google Scholar]
- 8.Brucato A, et al. Proposal for a new definition of congenital complete atrioventricular block. Lupus. 2003;12:427–435. doi: 10.1191/0961203303lu408oa. [DOI] [PubMed] [Google Scholar]
- 9.Landtman B, Linder E, Hjelt L, Tuuteri L. Congenital complete heart block. A clinical study of 27 cases. Ann Paediatr Fenn. 1964;10:99–104. [PubMed] [Google Scholar]
- 10.Gochberg SH. Congenital heart block. Am J Obstet Gynecol. 1964;88:238–241. doi: 10.1016/0002-9378(64)90262-5. [DOI] [PubMed] [Google Scholar]
- 11.Sirén MK, Julkunen H, Kaaja R. The increasing incidence of isolated congenital heart block in Finland. J Rheumatol. 1998;25:1862–1864. [PubMed] [Google Scholar]
- 12.Ambrosi A, Wahren-Herlenius M. Congenital heart block: evidence for a pathogenic role of maternal autoantibodies. Arthritis Res Ther. 2012;14:208. doi: 10.1186/ar3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costedoat-Chalumeau N, et al. Letter to the Editor in response to the article “Preventing congenital neonatal heart block in offspring of mothers with anti-SSA/Ro and SSB/La antibodies: a review of published literature and registered clinical trials by Gleicher N, Elkayam U, Autoimmun Rev 2013 Sep;12(11): 1039–1045. Autoimmun Rev. 2013;13:70–72. doi: 10.1016/j.autrev.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Anami A, et al. The predictive value of anti-SS-A antibodies titration in pregnant women with fetal congenital heart block. Mod Rheumatol. 2013;23:653–658. doi: 10.1007/s10165-012-0704-z. [DOI] [PubMed] [Google Scholar]
- 15.Behan WM, Behan PO, Reid JM, Doig W, Gairns J. Family studies of congenital heart block associated with Ro antibody. Br Heart J. 1989;62:320–324. doi: 10.1136/hrt.62.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brucato A, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44:1832–1835. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.Brucato A, et al. Congenital heart block not associated with anti-Ro/La antibodies: comparison with anti-Ro/La-positive cases. J Rheumatol. 2009;36:1744–1748. doi: 10.3899/jrheum.080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buyon JP, et al. Identification of mothers at risk for congenital heart block and other neonatal lupus syndromes in their children. Comparison of enzyme-linked immunosorbent assay and immunoblot for measurement of anti-SS-A/Ro and anti-SS-B/La antibodies. Arthritis Rheum. 1993;36:1263–1273. doi: 10.1002/art.1780360911. [DOI] [PubMed] [Google Scholar]
- 19.Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr. 2003;142:678–683. doi: 10.1067/mpd.2003.233. [DOI] [PubMed] [Google Scholar]
- 20.Colombo G, et al. DNA typing of maternal HLA in congenital complete heart block: comparison with systemic lupus erythematosus and primary Sjögren’s syndrome. Arthritis Rheum. 1999;42:1757–1764. doi: 10.1002/1529-0131(199908)42:8<1757::AID-ANR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Costedoat-Chalumeau N, et al. Questions about dexamethasone use for the prevention of anti-SSA related congenital heart block. Ann Rheum Dis. 2003;62:1010–1012. doi: 10.1136/ard.62.10.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costedoat-Chalumeau N, et al. Outcome of pregnancies in patients with anti-SSA/Ro antibodies: a study of 165 pregnancies, with special focus on electrocardiographic variations in the children and comparison with a control group. Arthritis Rheum. 2004;50:3187–3194. doi: 10.1002/art.20554. [DOI] [PubMed] [Google Scholar]
- 23.Dörner T, et al. Significantly increased maternal and fetal IgG autoantibody levels to 52 kD Ro (SS-A) and La (SS-B) in complete congenital heart block. J Autoimmun. 1995;8:675–684. doi: 10.1006/jaut.1995.0050. [DOI] [PubMed] [Google Scholar]
- 24.Fesslova V, et al. The impact of treatment of the fetus by maternal therapy on the fetal and postnatal outcomes for fetuses diagnosed with isolated complete atrioventricular block. Cardiol Young. 2009;19:282–290. doi: 10.1017/S1047951109004053. [DOI] [PubMed] [Google Scholar]
- 25.Gardiner HM, et al. Fetal ECG: a novel predictor of atrioventricular block in anti-Ro positive pregnancies. Heart. 2007;93:1454–1460. doi: 10.1136/hrt.2006.099242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerosa M, et al. Electrocardiographic abnormalities in infants born from mothers with autoimmune diseases--a multicentre prospective study. Rheumatology (Oxford) 2007;46:1285–1289. doi: 10.1093/rheumatology/kem073. [DOI] [PubMed] [Google Scholar]
- 27.Gordon P, et al. Anti-52 kDa Ro, anti-60 kDa Ro, and anti-La antibody profiles in neonatal lupus. J Rheumatol. 2004;31:2480–2487. [PubMed] [Google Scholar]
- 28.Grava C, et al. Isolated congenital heart block in undifferentiated connective tissue disease and in primary Sjögren’s syndrome: a clinical study of 81 pregnancies in 41 patients. Reumatismo. 2005;57:180–186. doi: 10.4081/reumatismo.2005.180. [DOI] [PubMed] [Google Scholar]
- 29.Groves AM, Allan LD, Rosenthal E. Outcome of isolated congenital complete heart block diagnosed in utero. Heart. 1996;75:190–194. doi: 10.1136/hrt.75.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izmirly PM, et al. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124:1927–1935. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution’s experience of 30 years. J Am Coll Cardiol. 2002;39:130–137. doi: 10.1016/s0735-1097(01)01697-7. [DOI] [PubMed] [Google Scholar]
- 32.Jaeggi ET, et al. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110:1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]
- 33.Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol. 2010;55:2778–2784. doi: 10.1016/j.jacc.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 34.Jaeggi ET, et al. Prolongation of the atrioventricular conduction in fetuses exposed to maternal anti-Ro/SSA and anti-La/SSB antibodies did not predict progressive heart block. A prospective observational study on the effects of maternal antibodies on 165 fetuses. J Am Coll Cardiol. 2011;57:1487–1492. doi: 10.1016/j.jacc.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Julkunen H, Miettinen A, Walle TK, Chan EK, Eronen M. Autoimmune response in mothers of children with congenital and postnatally diagnosed isolated heart block: a population based study. J Rheumatol. 2004;31:183–189. [PubMed] [Google Scholar]
- 36.Kaaja R, Julkunen H. Prevention of recurrence of congenital heart block with intravenous immunoglobulin and corticosteroid therapy: comment on the editorial by Buyon et al. Arthritis Rheum. 2003;48:280–281. doi: 10.1002/art.10716. [DOI] [PubMed] [Google Scholar]
- 37.Meilof JF, et al. Maternal autoantibodies and congenital heart block: no evidence for the existence of a unique heart block-associated anti-Ro/SS-A autoantibody profile. Lupus. 1993;2:239–246. doi: 10.1177/096120339300200406. [DOI] [PubMed] [Google Scholar]
- 38.Miyagawa S, et al. Neonatal lupus erythematosus: HLA-DR and -DQ distributions are different among the groups of anti-Ro/SSA-positive mothers with different neonatal outcomes. J Invest Dermatol. 1997;108:881–885. doi: 10.1111/1523-1747.ep12292592. [DOI] [PubMed] [Google Scholar]
- 39.Motta M, Rodriguez-Perez C, Tincani A, Lojacono A, Chirico G. Outcome of infants from mothers with anti-SSA/Ro antibodies. J Perinatol. 2007;27:278–283. doi: 10.1038/sj.jp.7211688. [DOI] [PubMed] [Google Scholar]
- 40.Pisoni CN, et al. Failure of intravenous immunoglobulin to prevent congenital heart block: Findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010;62:1147–1152. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- 41.Press J, et al. Long-term outcome of mothers of children with complete congenital heart block. Am J Med. 1996;100:328–332. doi: 10.1016/S0002-9343(97)89492-2. [DOI] [PubMed] [Google Scholar]
- 42.Rein AJ, et al. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: a prospective, observational, fetal kinetocardiogram-based study. Circulation. 2009;119:1867–1872. doi: 10.1161/CIRCULATIONAHA.108.773143. [DOI] [PubMed] [Google Scholar]
- 43.Salomonsson S, et al. A serologic marker for fetal risk of congenital heart block. Arthritis Rheum. 2002;46:1233–1241. doi: 10.1002/art.10232. [DOI] [PubMed] [Google Scholar]
- 44.Salomonsson S, et al. A population-based investigation of the autoantibody profile in mothers of children with atrioventricular block. Scand J Immunol. 2011;74:511–517. doi: 10.1111/j.1365-3083.2011.02610.x. [DOI] [PubMed] [Google Scholar]
- 45.Shinohara K, Miyagawa S, Fujita T, Aono T, Kidoguchi K. Neonatal lupus erythematosus: results of maternal corticosteroid therapy. Obstet Gynecol. 1999;93:952–957. doi: 10.1016/s0029-7844(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 46.Silverman ED, et al. Autoantibody response to the Ro/La particle may predict outcome in neonatal lupus erythematosus. Clin Exp Immunol. 1995;100:499–505. doi: 10.1111/j.1365-2249.1995.tb03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strandberg L, Salomonsson S, Bremme K, Sonesson S, Wahren-Herlenius M. Ro52, Ro60 and La IgG autoantibody levels and Ro52 IgG subclass profiles longitudinally throughout pregnancy in congenital heart block risk pregnancies. Lupus. 2006;15:346–353. doi: 10.1191/0961203306lu2309oa. [DOI] [PubMed] [Google Scholar]
- 48.Tunks RD, Clowse ME, Miller SG, Brancazio LR, Barker PC. Maternal autoantibody levels in congenital heart block and potential prophylaxis with antiinflammatory agents. Am J Obstet Gynecol. 2013;208:64. doi: 10.1016/j.ajog.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 49.Eliasson H, et al. Fetal Working Group of the European Association of Pediatric Cardiology. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011;124:1919–1926. doi: 10.1161/CIRCULATIONAHA.111.041970. [DOI] [PubMed] [Google Scholar]
- 50.Llanos C, et al. Anatomical and pathological findings in hearts from fetuses and infants with cardiac manifestations of neonatal lupus. Rheumatology (Oxford) 2012;51:1086–1092. doi: 10.1093/rheumatology/ker515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopes LM, et al. Perinatal outcome of fetal atrioventricular block: one-hundred-sixteen cases from a single institution. Circulation. 2008;118:1268–1275. doi: 10.1161/CIRCULATIONAHA.107.735118. [DOI] [PubMed] [Google Scholar]
- 52.Reed JH, et al. Umbilical cordblood levels of maternal antibodies reactive with p200 and full-length Ro 52 in the assessment of risk for cardiac manifestations of neonatal lupus. Arthritis Care Res (Hoboken) 2012;64:1373–1381. doi: 10.1002/acr.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eftekhari P, et al. Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. Eur J Immunol. 2000;30:2782–2790. doi: 10.1002/1521-4141(200010)30:10<2782::AID-IMMU2782>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Fritsch C, et al. 52-kDa Ro/SSA epitopes preferentially recognized by antibodies from mothers of children with neonatal lupus and congenital heart block. Arthritis Res Ther. 2006;8:R4. doi: 10.1186/ar1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clancy RM, et al. Maternal antibody responses to the 52-kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–3086. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 56.Strandberg L, et al. Antibodies to amino acid 200–239 (p200) of Ro52 as serological markers for the risk of developing congenital heart block. Clin Exp Immunol. 2008;154:30–37. doi: 10.1111/j.1365-2249.2008.03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramos-Casals M, et al. Systemic involvement in primary Sjögren syndrome evaluated by the EULAR-SS disease activity index (ESSDAI): analysis of 921 Spanish patients (GEAS-SS registry) Rheumatology (Oxford) 2014;53:321–331. doi: 10.1093/rheumatology/ket349. [DOI] [PubMed] [Google Scholar]
- 58.Tseng CE, Di Donato F, Buyon JP. Stability of immunoblot profile of anti-SSA/Ro-SSB/La antibodies over time in mothers whose children have neonatal lupus. Lupus. 1996;5:212–215. doi: 10.1177/096120339600500308. [DOI] [PubMed] [Google Scholar]
- 59.Acherman RJ, et al. Doppler fetal mechanical PR interval prolongation with positive maternal anti-RNP but negative SSA/Ro and SSB/La autoantibodies. Prenat Diagn. 2010;30:797–799. doi: 10.1002/pd.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salomonsson S, Strandberg L. Autoantibodies associated with congenital heart block. Scand J Immunol. 2010;72:185–188. doi: 10.1111/j.1365-3083.2010.02442.x. [DOI] [PubMed] [Google Scholar]
- 61.Strandberg LS, et al. Congenital heart block maternal sera autoantibodies target an extracellular epitope on the a1G T-type calcium channel in human fetal hearts. PLoS ONE. 2013;8:e72668. doi: 10.1371/journal.pone.0072668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedman DM, et al. PRIDE Investigators. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 63.Friedman DM, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62:1138–1146. doi: 10.1002/art.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sonesson SE, Salomonsson S, Jacobsson LA, Bremme K, Wahren–Herlenius M. Signs of first-degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52-kd antibodies. Arthritis Rheum. 2004;50:1253–1261. doi: 10.1002/art.20126. [DOI] [PubMed] [Google Scholar]
- 65.Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 66.Jonsson R, Theander E, Sjöström B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA. 2013;310:1854–1855. doi: 10.1001/jama.2013.278448. [DOI] [PubMed] [Google Scholar]
- 67.Brito-Zerón P, Ramos-Casals M. Advances in the understanding and treatment of systemic complications in Sjögren’s syndrome. Curr Opin Rheumatol. 2014;26:520–527. doi: 10.1097/BOR.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 68.Buyon JP, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–1666. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi T, et al. Outcome of prenatally diagnosed isolated congenital complete atrioventricular block treated with transplacental betamethasone or ritodrine therapy. Pediatr Cardiol. 2009;30:35–40. doi: 10.1007/s00246-008-9273-5. [DOI] [PubMed] [Google Scholar]
- 70.Buyon JP, Waltuck J, Kleinman C, Copel J. In utero identification and therapy of congenital heart block. Lupus. 1995;4:116–121. doi: 10.1177/096120339500400207. [DOI] [PubMed] [Google Scholar]
- 71.Ayed K, Gorgi Y, Sfar I, Khrouf M. Congenital heart block associated with maternal anti SSA/SSB antibodies: a report of four cases. Pathol Biol (Paris) 2004;52:138–147. doi: 10.1016/j.patbio.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Westwood M, Harris R, Burn JL, Barson AJ. Heredity in primary endocardial fibroelastosis. Br Heart J. 1975;37:1077–1084. doi: 10.1136/hrt.37.10.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eronen M, Miettinen A, Walle TK, Chan EK, Julkunen H. Relationship of maternal autoimmune response to clinical manifestations in children with congenital complete heart block. Acta Paediatr. 2004;93:803–809. doi: 10.1111/j.1651-2227.2004.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 74.Llanos C, et al. Antibody reactivity to alpha-enolase in mothers of children with congenital heart block. J Rheumatol. 2009;36:565–569. doi: 10.3899/jrheum.080860. [DOI] [PubMed] [Google Scholar]
- 75.Guettrot-Imbert G, et al. A new presentation of neonatal lupus: 5 cases of isolated mild endocardial fibroelastosis associated with maternal Anti-SSA/Ro and Anti-SSB/La antibodies. J Rheumatol. 2011;38:378–386. doi: 10.3899/jrheum.100317. [DOI] [PubMed] [Google Scholar]
- 76.Nield LE, et al. Maternal anti-Ro and anti-La antibody-associated endocardial fibroelastosis. Circulation. 2002;105:843–848. doi: 10.1161/hc0702.104182. [DOI] [PubMed] [Google Scholar]
- 77.Pedra SR, Hornberger LK, Leal SM, Taylor GP, Smallhorn JF. Cardiac function assessment in patients with family history of nonhypertrophic cardiomyopathy: a prenatal and postnatal study. Pediatr Cardiol. 2005;26:543–552. doi: 10.1007/s00246-004-0688-3. [DOI] [PubMed] [Google Scholar]
- 78.Chockalingam P, et al. Persistent fetal sinus bradycardia associated with maternal anti-SSA/Ro and anti-SSB/La antibodies. J Rheumatol. 2011;38:2682–2685. doi: 10.3899/jrheum.110720. [DOI] [PubMed] [Google Scholar]
- 79.Trucco SM, et al. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody-mediated cardiomyopathy. J Am Coll Cardiol. 2011;57:715–723. doi: 10.1016/j.jacc.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 80.Killen SA, Buyon JP, Friedman DM. Discordant spectrum of cardiac manifestations of neonatal lupus in twins. Lupus. 2012;21:559–562. doi: 10.1177/0961203311430512. [DOI] [PubMed] [Google Scholar]
- 81.Cuneo BF, et al. Spontaneous rupture of atrioventricular valve tensor apparatus as late manifestation of anti-Ro/SSA antibody-mediated cardiac disease. Am J Cardiol. 2011;107:761–766. doi: 10.1016/j.amjcard.2010.10.059. [DOI] [PubMed] [Google Scholar]
- 82.Brucato A, et al. Pregnancy outcome in 100 women with autoimmune diseases and anti-Ro/SSA antibodies: a prospective controlled study. Lupus. 2002;11:716–721. doi: 10.1191/0961203302lu252oa. [DOI] [PubMed] [Google Scholar]
- 83.Gladman G, et al. Fetal echocardiographic screening of pregnancies of mothers with anti-Ro and/or anti-La antibodies. Am J Perinatol. 2002;19:73–80. doi: 10.1055/s-2002-23555. [DOI] [PubMed] [Google Scholar]
- 84.Meisgen S, et al. The HLA locus contains novel foetal susceptibility alleles for congenital heart block with significant paternal influence. J Intern Med. 2014;275:640–651. doi: 10.1111/joim.12179. [DOI] [PubMed] [Google Scholar]
- 85.Clancy RM, et al. Identification of candidate loci at 6p21 and 21q22 in a genome-wide association study of cardiac manifestations of neonatal lupus. Arthritis Rheum. 2010;62:3415–3424. doi: 10.1002/art.27658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ambrosi A, et al. Development of heart block in children of SSA/SSB-autoantibody-positive women is associated with maternal age and displays a season-of-birth pattern. Ann Rheum Dis. 2012;71:334–340. doi: 10.1136/annrheumdis-2011-200207. [DOI] [PubMed] [Google Scholar]
- 87.Askanase AD, Iloh I, Buyon JP. Hypothyroidism and antithyroglobulin and antithyroperoxidase antibodies in the pathogenesis of autoimmune associated congenital heart block. J Rheumatol. 2006;33:2099. [PubMed] [Google Scholar]
- 88.Spence D, Hornberger L, Hamilton R, Silverman ED. Increased risk of complete congenital heart block in infants born to women with hypothyroidism and anti-Ro and/or anti-La antibodies. J Rheumatol. 2006;33:167–170. [PubMed] [Google Scholar]
- 89.Julkunen H, Eronen M. The rate of recurrence of isolated congenital heart block: a population-based study. Arthritis Rheum. 2001;44:487–488. doi: 10.1002/1529-0131(200102)44:2<487::AID-ANR70>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 90.Solomon DG, Rupel A, Buyon JP. Birth order, gender and recurrence rate in autoantibody-associated congenital heart block: implications for pathogenesis and family counseling. Lupus. 2003;12:646–647. doi: 10.1191/0961203303lu425xx. [DOI] [PubMed] [Google Scholar]
- 91.Kleinman CS, et al. Fetal echocardiography: a tool for the evaluation of in utero cardiac arrhythmias and monitoring of in utero therapy: analysis of 71 patients. Am J Cardiol. 1983;51:237–243. doi: 10.1016/s0002-9149(83)80042-3. [DOI] [PubMed] [Google Scholar]
- 92.Callan NA, Maggio M, Steger S, Kan JS. Fetal echocardiography: indications for referral, prenatal diagnosis, and outcomes. Am J Perinatol. 1991;8:390–394. doi: 10.1055/s-2007-999423. [DOI] [PubMed] [Google Scholar]
- 93.Wacker–Guβmann A, et al. Atrioventricular conduction delay in fetuses exposed to anti-SSA/Ro and anti-SSB/La antibodies: a magnetocardiography study. Clin Dev Immunol. 2012;2012:432176. doi: 10.1155/2012/432176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phoon CK, Kim MY, Buyon JP, Friedman DM. Finding the “PR-fect” solution: what is the best tool to measure fetal cardiac PR intervals for the detection and possible treatment of early conduction disease? Congenit Heart Dis. 2012;7:349–360. doi: 10.1111/j.1747-0803.2012.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monsarrat N, et al. Fetal ultrasonography and Doppler in isolated congenital heart block. Gynecol Obstet Fertil. 2009;37:633–644. doi: 10.1016/j.gyobfe.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 96.Srinivasan S, Strasburger J. Overview of fetal arrhythmias. Curr Opin Pediatr. 2008;20:522–531. doi: 10.1097/MOP.0b013e32830f93ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saxena A, Izmirly PM, Mendez B, Buyon JP, Friedman DM. Prevention and treatment in utero of autoimmune associated congenital heart block. Cardiol Rev. 2014;22:263–267. doi: 10.1097/CRD.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buyon JP, Clancy RM, Friedman DM. Cardiac manifestations of neonatal lupus erythematosus: guidelines to management, integrating clues from the bench and bedside. Nat Clin Pract Rheumatol. 2009;5:139–148. doi: 10.1038/ncprheum1018. [DOI] [PubMed] [Google Scholar]
- 99.Izmirly PM, et al. Cutaneous manifestations of neonatal lupus and risk of subsequent congenital heart block. Arthritis Rheum. 2010;62:1153–1157. doi: 10.1002/art.27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gleicher N, Elkayam U. Preventing congenital neonatal heart block in offspring of mothers with anti-SSA/Ro and SSB/La antibodies: a review of published literature and registered clinical trials. Autoimmun Rev. 2013;12:1039–1045. doi: 10.1016/j.autrev.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Tseng CE, Di Donato F, Buyon JP. Stability of immunoblot profile of anti-SSA/Ro-SSB/La antibodies over time in mothers whose children have neonatal lupus. Lupus. 1996;5:212–215. doi: 10.1177/096120339600500308. [DOI] [PubMed] [Google Scholar]
- 102.Eronen M, et al. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics. 2000;106:86–91. doi: 10.1542/peds.106.1.86. [DOI] [PubMed] [Google Scholar]
- 103.Saleeb S, Copel J, Friedman D, Buyon JP. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody-associated congenital heart block: retrospective review of the research registry for neonatal lupus. Arthritis Rheum. 1999;42:2335–2345. doi: 10.1002/1529-0131(199911)42:11<2335::AID-ANR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 104.Udink ten Cate FE, et al. Dilated cardiomyopathy in isolated congenital complete atrioventricular block: early and long-term risk in children. J Am Coll Cardiol. 2001;37:1129–1134. doi: 10.1016/s0735-1097(00)01209-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.