Abstract
Recently, we identified harvest moon (hmn), a fully penetrant and expressive recessive zebrafish mutant with hepatic steatosis. Larvae showed increased triacylglycerol in the absence of other obvious defects. When we attempted to raise these otherwise normal-appearing mutants to adulthood, we observed a developmental arrest and death in the early juvenile period. In this study, we report the positional cloning of the hmn locus and characterization of the defects caused by the mutation. Using bulk segregant analysis and fine mapping, we find that hmn mutants harbor a point mutation in an invariant residue within the sugar isomerase 1 domain of the gene encoding the rate-limiting enzyme of the hexosamine biosynthetic pathway (HBP) glutamine-fructose-6-phosphate transamidase (Gfpt1). The mutated protein shows increased abundance. The HBP generates β-N-acetyl-glucosamine (GlcNAc) as a spillover pathway from glucose. GlcNAc can be O-linked to seryl and threonyl residues of diverse cellular proteins (O-GlcNAc modification). Although some of these O-GlcNAc modifications serve an essential structural role, many others are dynamically generated on signaling molecules, including several impacting insulin signaling. We find that gfpt1 mutants show global increase in O-GlcNAc modification, and, surprisingly, lower fasting blood glucose in males. Taken together with our previously reported work, the gfpt1 mutant we isolated demonstrates that global increase in O-GlcNAc modification causes some severe insulin resistance phenotypes (hepatic steatosis and runting) but does not cause hyperglycemia. This animal model will provide a platform for dissecting how O-GlcNAc modification alters insulin responsiveness in multiple tissues.
An activating mutation in the gfpt1 gene was identified in a zebrafish strain found to have complex phenotypes suggestive of altered insulin signaling.
Previously, we identified hmn, a zebrafish mutant with two distinguishing phenotypes: hepatic steatosis at a point in development when maternally deposited yolk lipids are exhausted, and developmental arrest in the late larval period (1). Although we were initially interested in identifying mutants with hepatic steatosis to isolate new genes involved in the liver’s regulation of the transition from fed to fasted states (2), we found that neither feeding nor fasting ameliorated or exacerbated the developmental arrest of hmn mutants (1).
In this study, we found that neither feeding nor fasting altered the hepatic steatosis phenotype of hmn mutants. This finding suggested that a permanent defect in metabolic regulation was present in the mutants. Thus, we positionally cloned and functionally characterized the hmn locus. We found a recessive activating mutation in the gfpt1 gene that increases enzymatic flux through the hexosamine biosynthetic pathway (HBP). Because it is well established that increased HBP flux causes multiorgan insulin resistance (IR), the gfpt1 mutant we have isolated could serve as a useful tool for revealing the molecular mechanisms of IR.
Materials and Methods
Zebrafish mutagenesis, screening, and histological analyses
Animal studies were approved by the Institutional Animal Care and Use Committee of the University of Utah. The hmnz110 mutant was identified, as we described previously (1). In brief, F2 larvae from males mutagenized with N-ethyl-N-nitorosurea were fixed and stained with Oil Red O (ORO) 7 days postfertilization (dpf) and scored for the presence of hepatic steatosis. The agtpbp1sa17482 allele was purchased from the Zebrafish International Resource Center (3). ORO staining and histological analyses were done exactly as described previously; samples were stained with Sudan Black and eosin (4).
Positional cloning
A map cross of heterozygous hmn carriers to the polymorphic WIK line was performed (5). A single pair of heterozygous progeny was bred to generate all the individuals used to positionally clone the locus. First, larvae were fixed and stained with ORO and then sorted into two pools. DNA from 21 wild-type (WT) and 21 hmn mutant larvae were examined by bulk segregant analysis using a standard set of simple sequence-length polymorphism microsatellite markers (z markers) on a meiotic map (6, 7). This allowed us to assign the mutated gene to the south subtelomeric region of chromosome 8. Examination of 51 additional progeny from this single pair allowed us to narrow the locus further to a single contig. Complementary DNAs (cDNAs) were cloned for all protein-coding genes in the locus and the indicated genes just outside the locus, and sequenced. Genomic DNA was obtained from individual 7-dpf larvae after fixation and staining in ORO by digesting at 65°C with proteinase K (10 μg/mL final) in 10 mM Tris, pH 9, 50 mM KCl, 1.5 mM MgCl2, 0.3 Tween 20, and 0.3% IGEPAL-CA630. Following heat inactivation of proteinase K, DNA was diluted 1:10 in water and used in polymerase chain reaction–based genotyping (4).
cDNA synthesis and sequencing
To extract RNA, 7-dpf larvae were sorted following live staining with Nile Red (8). Total RNA was extracted by homogenization with a tissue grinder into Qiagen (Germantown, MD) RNeasy MINI lysis buffer (RTL with 2-mercaptoethanol) and purified, following the manufacturer’s protocol, for tissue samples. cDNA was prepared using RNA to cDNA EcoDry Premix (Takara Clontech, Mountainview, CA). Two forward and two reverse primers were used in all possible combinations to successfully amplify the gfpt1 message: forward primer, 5′-CGCCTTCTTGACCAATACCA-3′ and reverse primer, 5′- GGTGTTGGTCAGGAAGCATT-3′. After purification of the products, GoTaq (Promega) was added for one cycle to create A-overhangs for T-A cloning into pGEMT (Promega, Madison, WI). SP6 and T7 primers were used for Sanger sequencing of cloned cDNAs.
Immunoblot analyses
Immunoblot analyses were performed exactly as we described previously using dissected zebrafish livers (4). Rabbit anti-human GFPT1 was from Proteintech (Rosemont, IL). Mouse monoclonal IgG RL2 against O-β-N-acetyl-glucosamine (GlcNAc) modification (9) was purchased from Abcam (Cambridge, MA). Rabbit anti–β-tubulin (Tubb) was purchased from Abcam. Catalog numbers, dilutions, and RRIDs are shown in Table 1.
Table 1.
Antibodies Used
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Gfpt1 | Human GFPT1 | Gfpt1 | Proteintech, 14132-1-AP | Rabbit; polyclonal | 1 to 1000 | AB_2110155 |
| O-GlcNAc modification | O-GlcNAc | RL2 | Abcam, ab2730 | Mouse; monoclonal | 1 to 1000 | AB_531200 |
| β-Tubulin | Human β-tubulin | β-Tubulin | Abcam, ab6046 | Rabbit; polyclonal | 1 to 1000 | AB_2210370 |
Abbreviation: RRID, research resource identifier.
Blood glucose measurements
Blood glucose was measured exactly as described previously in animals 3 months postfertilization (10).
Statistical analysis
The two-tailed Student t test was used to compare plasma glucose levels. Values are reported as mean ± standard error of the mean.
Results
Positional cloning of the hmn locus reveals a mutation in gfpt1
The hmn z110 mutant shows hepatic lipid accumulation 7 dpf [Fig. 1(a)]. This steatosis phenotype is not modified by prolonged fasting [Fig. 1(b)] or feeding [Fig. 1(c)]. These findings suggested that the mutation causes a metabolically inflexible change in liver metabolism. Importantly, the WT animals used are derived from a heterozygous cross of hmn−/z110 adults: one-third is hmn+/+ and two-thirds are hmn−+/z110.
Figure 1.
Hepatic steatosis in hmn mutants is not modified by nutritional status. (a) Diffuse accumulation of neutral lipid droplets in never-fed 7-dpf livers stained with eosin following osmium impregnation is seen in hmn mutant livers. (b) Never-fed larvae were live-sorted at 7 dpf with Nile Red staining. They were fixed and stained with ORO at the indicated ages and scored for hepatic steatosis. (c) Larvae were live-sorted at 7 dpf and fed until fixation and staining with ORO at the indicated ages.
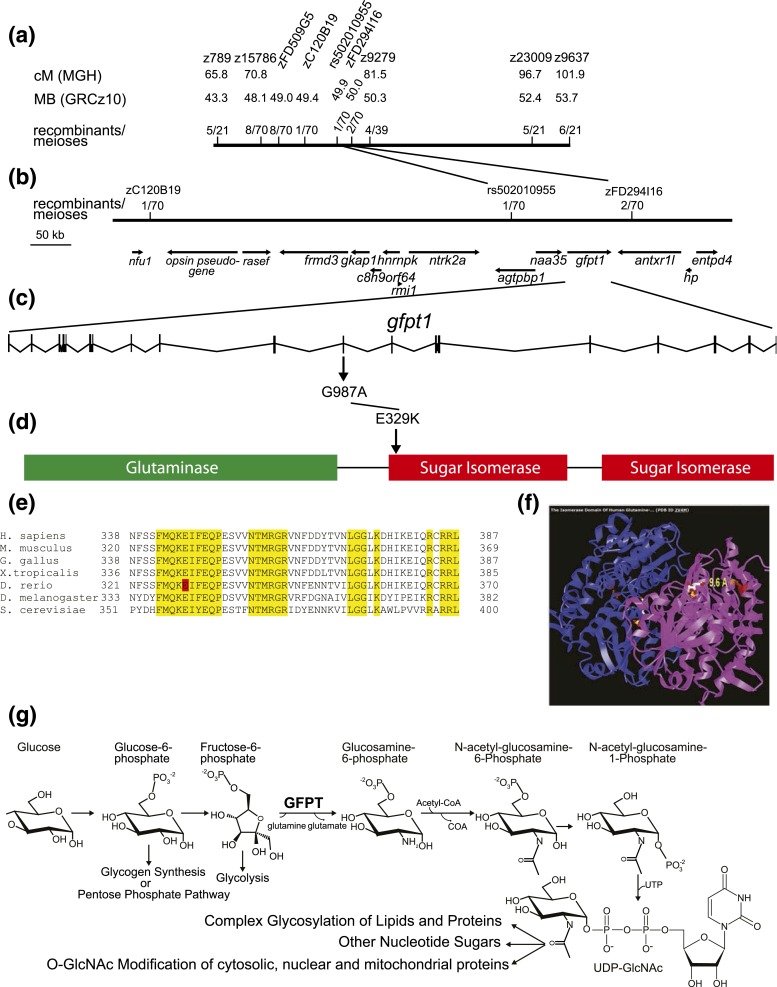
We used standard methods to isolate the mutated gene. Bulk segregant analysis established linkage to chromosome 8 [Fig. 2(a)]. Fine mapping narrowed the critical interval to four candidate genes: agtpbp1, naa35, gfpt1, and antxr1l [Fig. 2(b)]. Full-length cDNAs were cloned and sequenced from transcripts for all four candidates. There were no coding changes in naa35 or antxr11 cDNAs cloned from hmnz110 mutants. Because the rs5020109055 polymorphism causes a L450H coding change in agtpbp1, we performed a complementation test with the null allele agtpbp1sa17482. This agtpbp1 allele complemented the hmn z110 mutant, indicating agtpbp1 loss-of-function does not give rise to the hmn phenotype (data not shown).
Figure 2.
Positional cloning of the hmn mutant reveals a substitution mutation in an invariant residue of Gfpt1 protein within the first sugar isomerase domain. (a) A map cross of heterozygous hmn carriers to the polymorphic WIK line was performed. A single pair of heterozygous progeny was bred to generate all the individuals used to positionally clone the locus. First, larvae were fixed and stained with ORO and then sorted into two pools. DNA from 21 WT and 21 hmn mutant larvae were examined by bulk segregant analysis using a standard set of simple sequence-length polymorphism microsatellite markers (SSLP, z markers) on a meiotic map (6). This allowed us to assign the mutated gene to the south subtelomeric region of chromosome 8. (b) Examination of 51 additional progeny from this single pair allowed us to narrow the locus further to a single contig. cDNAs were cloned and sequenced for all protein-coding genes within the critical interval. (c) At position 987 of the gfpt1 coding sequence, a G-to-A transition mutation was identified in hmn mutants (and was confirmed to have been induced in the mutagenized male through sequencing genomic DNA). (d) The mutation changed codon 329 from an E to a K residue. (e) Comparison among multiple species from yeast to humans revealed that E329 (zebrafish numbering, shaded in red) is an invariant residue. Many neighboring residues are also invariant (yellow) or highly conserved among the species examined. (f) Inspection of the crystal structure of human GFPT1 (a homodimer, with both chains shown in different colors) shows that the invariant E239 residue is in close proximity (9.6 Å) to the fructose-6-phosphate substrate. (g) The HBP is shown, with the rate-limiting enzyme GFPT, catalyzing the conversion of fructose-6-phosphate to glucosamine-6-phosphate via a transamidation-requiring glutamine.
A mutagen-induced G to A transition was found in exon 11 of gfpt1, causing an E to K substitution at codon 329 [Fig. 2(c)]. Residue E329 of Gfpt1 falls in the start of the first isomerase domain [Fig. 2(d)], and is invariant among orthologs from Saccharomyces cerevisiae to Homo sapiens [Fig. 2(e)]. In the crystal structure of human GFPT1, residue E329 is within 10 Å of the fructose-6-phosphate substrate that receives the NH4+ from the glutamine substrate [Fig. 2(f) and 2(g)]. The location of the mutated residue and the substitution of a negatively charged side chain with a positively charged side chain suggest that substrate binding, catalytic activity, competitive inhibition by glucosamine 6-phosphate, allosteric inhibition by uridine diphosphate, or a combination of these biochemical processes is altered (11). The last two possibilities are particularly attractive because this mutation is recessive: a homodimer of E329K mutants is most likely required for the observed phenotypes.
The gfpt1E329K mutation increases Gfpt1 protein expression, global O-GlcNAc modification, and lower blood glucose
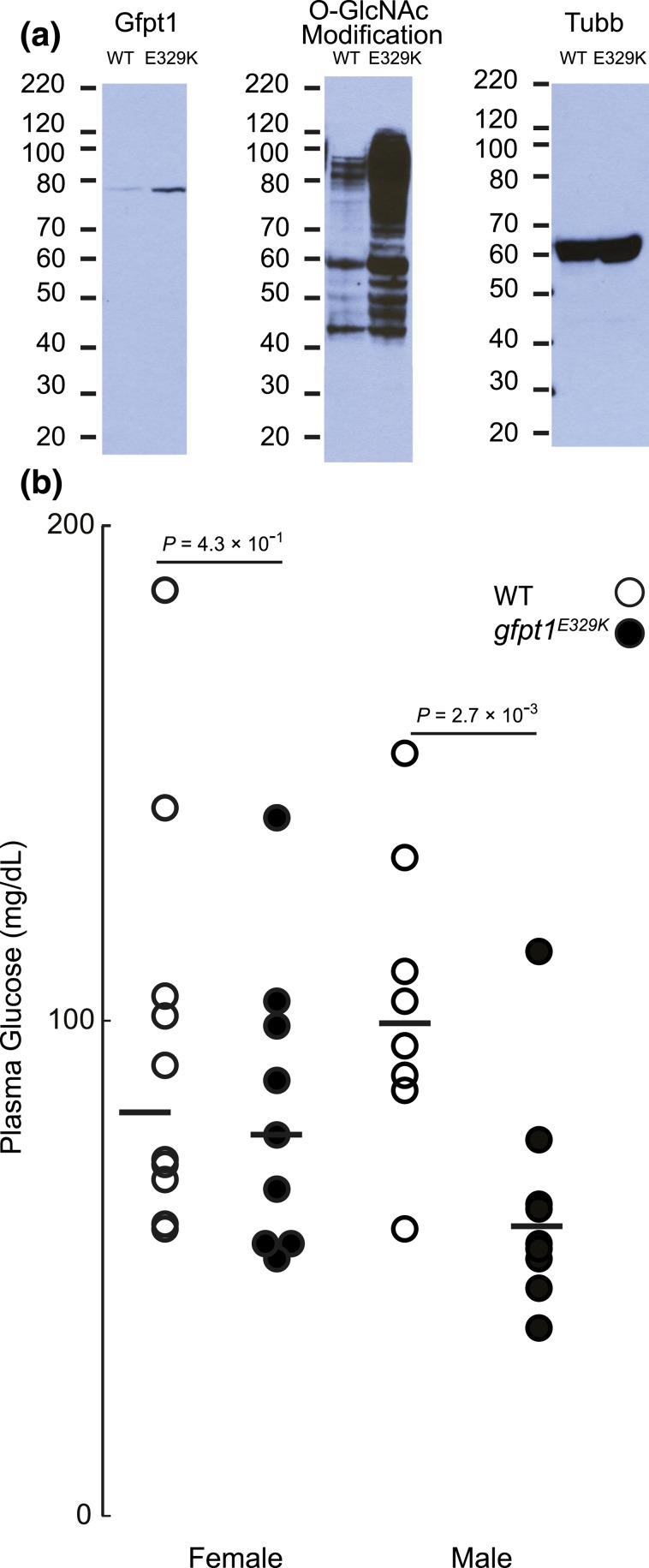
Gfpt1 is the rate-limiting enzyme of the HBP [Fig. 2(g)]. This nutrient-sensing pathway diverts excess sugars from glycolysis to the production of signaling molecules in the form of O-GlcNAc modification of diverse proteins that alter protein function (12, 13). To establish the consequence of the gfpt1E329K mutation on Gfpt1 protein expression and O-GlcNAc modification (a direct measure of HBP flux), we performed immunoblot analyses for Gfpt1 protein and O-GlcNAc modification. Compared with WT siblings, adult homozygous gfpt1E329K mutants showed increased Gfpt1 protein and global O-GlcNAc modification of proteins in their livers [Fig. 3(a)]. These two findings, and the observation that heterozygous carriers [two-thirds of the WT cohorts in Fig. 1(b) and 1(c)] do not develop hepatic steatosis, strongly suggest that the gfpt1E329K mutation causes increased HBP flux (i.e., the mutation is a recessive, gain-of-function) by increasing Gfpt1 protein abundance, activity, or both. Finally, gfpt1 transcript abundance was unchanged in livers of hmn mutants (data not shown), indicating additional mutagen-induced gene expression changes are unlikely to account for the observed phenotypes (data not shown).
Figure 3.
Gfpt1E329K mutants have higher Gfpt1 protein abundance and increased O-GlcNAc modification, but lower fasting blood glucose. (a) Livers from three adult WT and gfpt1E329K mutants were dissected following an overnight fast. The livers were pooled and homogenized in protein extraction buffer. Proteins (15 μg per sample) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (molecular weight standards shown in kDa) and transferred to polyvinylidene fluoride membranes, and immunoblot analyses were performed with anti-GFPT1, anti–O-GlcNAc modification, and anti-Tubb IgGs. As expected, the anti-GFPT1 IgG detected a single band of nearly 80 kDa; multiple O-GlcNAc modified proteins were detected in both groups. (b) Animals were fasted overnight prior to measurement of blood glucose.
Ten to 15% of homozygous gfpt1E329K mutants survive to adulthood (1). This observation allowed us to measure fasting blood glucose. In both sexes, blood glucose was lower in gfpt1E329K mutants, although the difference was statistically significant in males only [Fig. 3(b)].
Discussion
Type 2 diabetes mellitus (DM2) has reached pandemic proportions (14, 15). This multifactorial disease has high direct and indirect burdens on individuals and societies (16). It is widely recognized that environmental factors that drive IR in multiple cell types are a prerequisite for the development of DM2 in genetically susceptible individuals (17–19). Although multiple IR mechanisms have been identified, an incomplete understanding of these pathogenic metabolic and signaling pathways remains. This state has hindered the development of novel diagnostic and therapeutic modalities to address DM2.
Increased HBP flux is a major driver of IR. First, increased flux through the HBP inhibits adipocyte glucose disposal (20). Second, skeletal muscle biopsies from humans with DM2 show increased flux through the HBP pathway, and this flux correlates with decreased glucose disposal rates (21). Third, transgenic overexpression of Gfpt1 in mouse liver increases O-GlcNAc modification and is sufficient to cause IR, marked by hyperglycemia, hypertriglyceridemia, and increased hepatic glycogen stores (22–26). Other O-GlcNAc modification pathway transgenic overexpression models (27) and pharmacological manipulations to increase HPF flux in vivo (28, 29) confirm that this pathway is sufficient to drive IR. Cell-based models confirm these results (12).
What remains unclear is whether increased HBP flux is necessary for the development of IR (30). This is an important question because its answer would reveal whether (and where) attempts to alter HBP flux could be used to reverse IR. At present, a viable GFPT1 loss-of-function model is not available. This reflects the key role of HBP in early skeletal development and in neuromuscular maturation (31, 32). Through phenotype-driven forward genetic screening, we have isolated a zebrafish mutant with increased HBP flux, and two distinguishing phenotypes of severe, lifelong insulin resistance: hepatic steatosis and runting (1, 33). The mutant protein we identified should be studied in vitro to establish the biochemical basis for global increase in O-GlcNAc modification. It is also possible that the mutation decreases the rate of protein degradation in vivo.
A minority of homozygous gfpt1E329K mutants is viable to adulthood; we found that lifelong, global activation of HBP flux did not cause fasting hyperglycemia when animals are fed their normal diets. Indeed, the lower fasting blood glucose observed in gfpt1E329K mutant males suggests that there are most likely multiple metabolic alterations present in animals with global increase in HBP flux. Because homozygous loss-of-function gfpt1j23e1 mutants have lethal craniofacial defects (32), conventional genetic approaches to dissecting the function of the gfpt1E329K mutation in adults might not be possible; however, if transheterozygous gfpt1j23e1/ gfpt1E329K mutants prove viable, then it would be informative to assess glucose homeostasis in animals carrying a single recessive gain-of-function Gfpt1 allele. Additionally, it is nominally possible that the gfpt1E329K mutant harbors additional gene-regulatory lesions that contribute to the phenotype of increased HBP flux. Blood glucose should be evaluated at additional ages during adulthood under normal and high-fat feeding. Likewise, insulin tolerance tests could be performed on the few surviving mutants (34). In summary, the Gfpt1E329K mutant affords the opportunity to examine the role of HBP in glucose homeostasis on an integrated physiological level and at organ- and cell-type resolution.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health Grant R01-DK096710 (to A.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cDNA
- complementary DNA
- DM2
- type 2 diabetes mellitus
- dpf
- days postfertilization
- GlcNAc
- β-N-acetyl-glucosamine
- HBP
- hexosamine biosynthetic pathway
- IR
- insulin resistance
- ORO
- Oil Red O
- WT
- wild-type.
References
- 1.Hugo SE, Schlegel A. A genetic screen for zebrafish mutants with hepatic steatosis identifies a locus required for larval growth. J Anat. 2017;230(3):407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlegel A. Studying non-alcoholic fatty liver disease with zebrafish: a confluence of optics, genetics, and physiology. Cell Mol Life Sci. 2012;69(23):3953–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fényes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, Stemple DL. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496(7446):494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hugo SE, Cruz-Garcia L, Karanth S, Anderson RM, Stainier DY, Schlegel A. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev. 2012;26(3):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauch G-J, Granato M, Haffter P. A polymorphic zebrafish line for genetic mapping. Tech Tips Online. 1997;2(1):148–150. [Google Scholar]
- 6.Knapik EW, Goodman A, Ekker M, Chevrette M, Delgado J, Neuhauss S, Shimoda N, Driever W, Fishman MC, Jacob HJ. A microsatellite genetic linkage map for zebrafish (Danio rerio). Nat Genet. 1998;18(4):338–343. [DOI] [PubMed] [Google Scholar]
- 7.Bahary N, Davidson A, Ransom D, Shepard J, Stern H, Trede N, Zhou Y, Barut B, Zon LI. The Zon laboratory guide to positional cloning in zebrafish. Methods Cell Biol. 2004;77:305–329. [DOI] [PubMed] [Google Scholar]
- 8.Flynn EJ III, Trent CM, Rawls JF. Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). J Lipid Res. 2009;50(8):1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snow CM, Senior A, Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987;104(5):1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karanth S, Zinkhan EK, Hill JT, Yost HJ, Schlegel A. FOXN3 regulates hepatic glucose utilization. Cell Reports. 2016;15(12):2745–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broschat KO, Gorka C, Page JD, Martin-Berger CL, Davies MS, Huang Hc HC, Gulve EA, Salsgiver WJ, Kasten TP. Kinetic characterization of human glutamine-fructose-6-phosphate amidotransferase I: potent feedback inhibition by glucosamine 6-phosphate. J Biol Chem. 2002;277(17):14764–14770. [DOI] [PubMed] [Google Scholar]
- 12.Teo CF, Wollaston-Hayden EE, Wells L. Hexosamine flux, the O-GlcNAc modification, and the development of insulin resistance in adipocytes. Mol Cell Endocrinol. 2010;318(1-2):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol. 2015;208(7):869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) . National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. [DOI] [PubMed] [Google Scholar]
- 15.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown JC, Wang H, Duber HC, Naghavi M, Dicker D, Dandona L, Salomon JA, Heuton KR, Foreman K, Phillips DE, Fleming TD, Flaxman AD, Phillips BK, Johnson EK, Coggeshall MS, Abd-Allah F, Abera SF, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Adeyemo AO, Adou AK, Adsuar JC, Agardh EE, Akena D, Al Kahbouri MJ, Alasfoor D, Albittar MI, Alcalá-Cerra G, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allen PJ, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA, Amare AT, Amini H, Ammar W, Anderson BO, Antonio CA, Anwari P, Arnlöv J, Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Badawi A, Balakrishnan K, Banerjee A, Basu S, Beardsley J, Bekele T, Bell ML, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta ZA, Abdulhak AB, Binagwaho A, Blore JD, Basara BB, Bose D, Brainin M, Breitborde N, Castañeda-Orjuela CA, Catalá-López F, Chadha VK, Chang JC, Chiang PP, Chuang TW, Colomar M, Cooper LT, Cooper C, Courville KJ, Cowie BC, Criqui MH, Dandona R, Dayama A, De Leo D, Degenhardt L, Del Pozo-Cruz B, Deribe K, Des Jarlais DC, Dessalegn M, Dharmaratne SD, Dilmen U, Ding EL, Driscoll TR, Durrani AM, Ellenbogen RG, Ermakov SP, Esteghamati A, Faraon EJ, Farzadfar F, Fereshtehnejad SM, Fijabi DO, Forouzanfar MH, Fra Paleo U, Gaffikin L, Gamkrelidze A, Gankpé FG, Geleijnse JM, Gessner BD, Gibney KB, Ginawi IA, Glaser EL, Gona P, Goto A, Gouda HN, Gugnani HC, Gupta R, Gupta R, Hafezi-Nejad N, Hamadeh RR, Hammami M, Hankey GJ, Harb HL, Haro JM, Havmoeller R, Hay SI, Hedayati MT, Pi IB, Hoek HW, Hornberger JC, Hosgood HD, Hotez PJ, Hoy DG, Huang JJ, Iburg KM, Idrisov BT, Innos K, Jacobsen KH, Jeemon P, Jensen PN, Jha V, Jiang G, Jonas JB, Juel K, Kan H, Kankindi I, Karam NE, Karch A, Karema CK, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Keren A, Kereselidze M, Khader YS, Khalifa SE, Khan EA, Khang YH, Khonelidze I, Kinfu Y, Kinge JM, Knibbs L, Kokubo Y, Kosen S, Defo BK, Kulkarni VS, Kulkarni C, Kumar K, Kumar RB, Kumar GA, Kwan GF, Lai T, Balaji AL, Lam H, Lan Q, Lansingh VC, Larson HJ, Larsson A, Lee JT, Leigh J, Leinsalu M, Leung R, Li Y, Li Y, De Lima GM, Lin HH, Lipshultz SE, Liu S, Liu Y, Lloyd BK, Lotufo PA, Machado VM, Maclachlan JH, Magis-Rodriguez C, Majdan M, Mapoma CC, Marcenes W, Marzan MB, Masci JR, Mashal MT, Mason-Jones AJ, Mayosi BM, Mazorodze TT, Mckay AC, Meaney PA, Mehndiratta MM, Mejia-Rodriguez F, Melaku YA, Memish ZA, Mendoza W, Miller TR, Mills EJ, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montico M, Moore AR, Mori R, Moturi WN, Mukaigawara M, Murthy KS, Naheed A, Naidoo KS, Naldi L, Nangia V, Narayan KM, Nash D, Nejjari C, Nelson RG, Neupane SP, Newton CR, Ng M, Nisar MI, Nolte S, Norheim OF, Nowaseb V, Nyakarahuka L, Oh IH, Ohkubo T, Olusanya BO, Omer SB, Opio JN, Orisakwe OE, Pandian JD, Papachristou C, Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pereira DM, Pervaiz A, Pesudovs K, Petzold M, Pourmalek F, Qato D, Quezada AD, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Ur Rahman S, Raju M, Rana SM, Razavi H, Reilly RQ, Remuzzi G, Richardus JH, Ronfani L, Roy N, Sabin N, Saeedi MY, Sahraian MA, Samonte GM, Sawhney M, Schneider IJ, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Sheikhbahaei S, Shibuya K, Shin HH, Shiue I, Shivakoti R, Sigfusdottir ID, Silberberg DH, Silva AP, Simard EP, Singh JA, Skirbekk V, Sliwa K, Soneji S, Soshnikov SS, Sreeramareddy CT, Stathopoulou VK, Stroumpoulis K, Swaminathan S, Sykes BL, Tabb KM, Talongwa RT, Tenkorang EY, Terkawi AS, Thomson AJ, Thorne-Lyman AL, Towbin JA, Traebert J, Tran BX, Dimbuene ZT, Tsilimbaris M, Uchendu US, Ukwaja KN, Uzun SB, Vallely AJ, Vasankari TJ, Venketasubramanian N, Violante FS, Vlassov VV, Vollset SE, Waller S, Wallin MT, Wang L, Wang X, Wang Y, Weichenthal S, Weiderpass E, Weintraub RG, Westerman R, White RA, Wilkinson JD, Williams TN, Woldeyohannes SM, Wong JQ, Xu G, Yang YC, Yano Y, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis M, Yu C, Jin KY, El Sayed Zaki M, Zhao Y, Zheng Y, Zhou M, Zhu J, Zou XN, Lopez AD, Vos T. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnefond A, Froguel P. Rare and common genetic events in type 2 diabetes: what should biologists know? Cell Metab. 2015;21(3):357–368. [DOI] [PubMed] [Google Scholar]
- 19.Grarup N, Sandholt CH, Hansen T, Pedersen O. Genetic susceptibility to type 2 diabetes and obesity: from genome-wide association studies to rare variants and beyond. Diabetologia. 2014;57(8):1528–1541. [DOI] [PubMed] [Google Scholar]
- 20.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system: role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266(8):4706–4712. [PubMed] [Google Scholar]
- 21.Yki-Järvinen H, Daniels MC, Virkamäki A, Mäkimattila S, DeFronzo RA, McClain D. Increased glutamine:fructose-6-phosphate amidotransferase activity in skeletal muscle of patients with NIDDM. Diabetes. 1996;45(3):302–307. [DOI] [PubMed] [Google Scholar]
- 22.Hebert LF Jr, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98(4):930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veerababu G, Tang J, Hoffman RT, Daniels MC, Hebert LF Jr, Crook ED, Cooksey RC, McClain DA. Overexpression of glutamine: fructose-6-phosphate amidotransferase in the liver of transgenic mice results in enhanced glycogen storage, hyperlipidemia, obesity, and impaired glucose tolerance. Diabetes. 2000;49(12):2070–2078. [DOI] [PubMed] [Google Scholar]
- 24.Crook ED, McClain DA. Regulation of glycogen synthase and protein phosphatase-1 by hexosamines. Diabetes. 1996;45(3):322–327. [DOI] [PubMed] [Google Scholar]
- 25.Crook ED, Zhou J, Daniels M, Neidigh JL, McClain DA. Regulation of glycogen synthase by glucose, glucosamine, and glutamine:fructose-6-phosphate amidotransferase. Diabetes. 1995;44(3):314–320. [DOI] [PubMed] [Google Scholar]
- 26.Crook ED, Daniels MC, Smith TM, McClain DA. Regulation of insulin-stimulated glycogen synthase activity by overexpression of glutamine: fructose-6-phosphate amidotransferase in rat-1 fibroblasts. Diabetes. 1993;42(9):1289–1296. [DOI] [PubMed] [Google Scholar]
- 27.McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA. 2002;99(16):10695–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest. 1995;96(1):132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virkamäki A, Daniels MC, Hämäläinen S, Utriainen T, McClain D, Yki-Järvinen H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance in multiple insulin sensitive tissues. Endocrinology. 1997;138(6):2501–2507. [DOI] [PubMed] [Google Scholar]
- 30.Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290(1):E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senderek J, Müller JS, Dusl M, Strom TM, Guergueltcheva V, Diepolder I, Laval SH, Maxwell S, Cossins J, Krause S, Muelas N, Vilchez JJ, Colomer J, Mallebrera CJ, Nascimento A, Nafissi S, Kariminejad A, Nilipour Y, Bozorgmehr B, Najmabadi H, Rodolico C, Sieb JP, Steinlein OK, Schlotter B, Schoser B, Kirschner J, Herrmann R, Voit T, Oldfors A, Lindbergh C, Urtizberea A, von der Hagen M, Hübner A, Palace J, Bushby K, Straub V, Beeson D, Abicht A, Lochmüller H. Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet. 2011;88(2):162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C-T, Hindes AE, Hultman KA, Johnson SL. Mutations in gfpt1 and skiv2l2 cause distinct stage-specific defects in larval melanocyte regeneration in zebrafish. PLoS Genet. 2007;3(6):e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semple RK, Savage DB, Cochran EK, Gorden P, O’Rahilly S. Genetic syndromes of severe insulin resistance. Endocr Rev. 2011;32(4):498–514. [DOI] [PubMed] [Google Scholar]
- 34.Safavi-Hemami H, Gajewiak J, Karanth S, Robinson SD, Ueberheide B, Douglass AD, Schlegel A, Imperial JS, Watkins M, Bandyopadhyay PK, Yandell M, Li Q, Purcell AW, Norton RS, Ellgaard L, Olivera BM. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc Natl Acad Sci USA. 2015;112(6):1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]