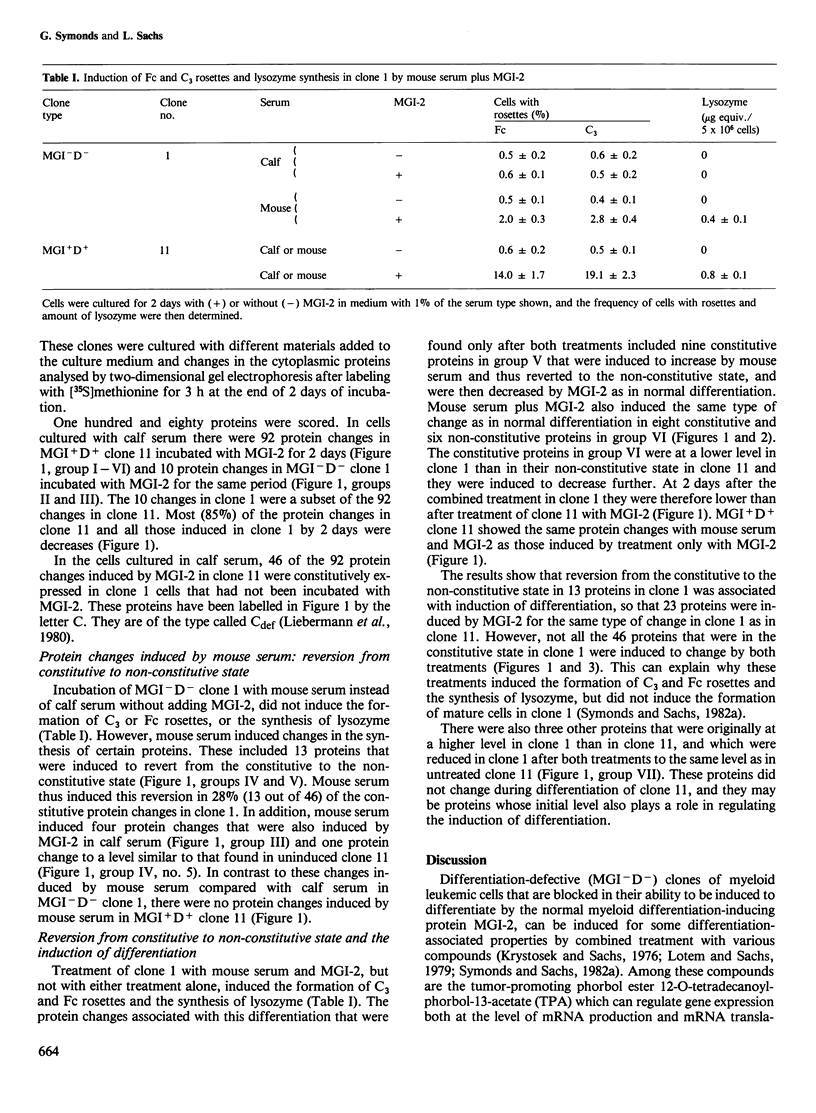
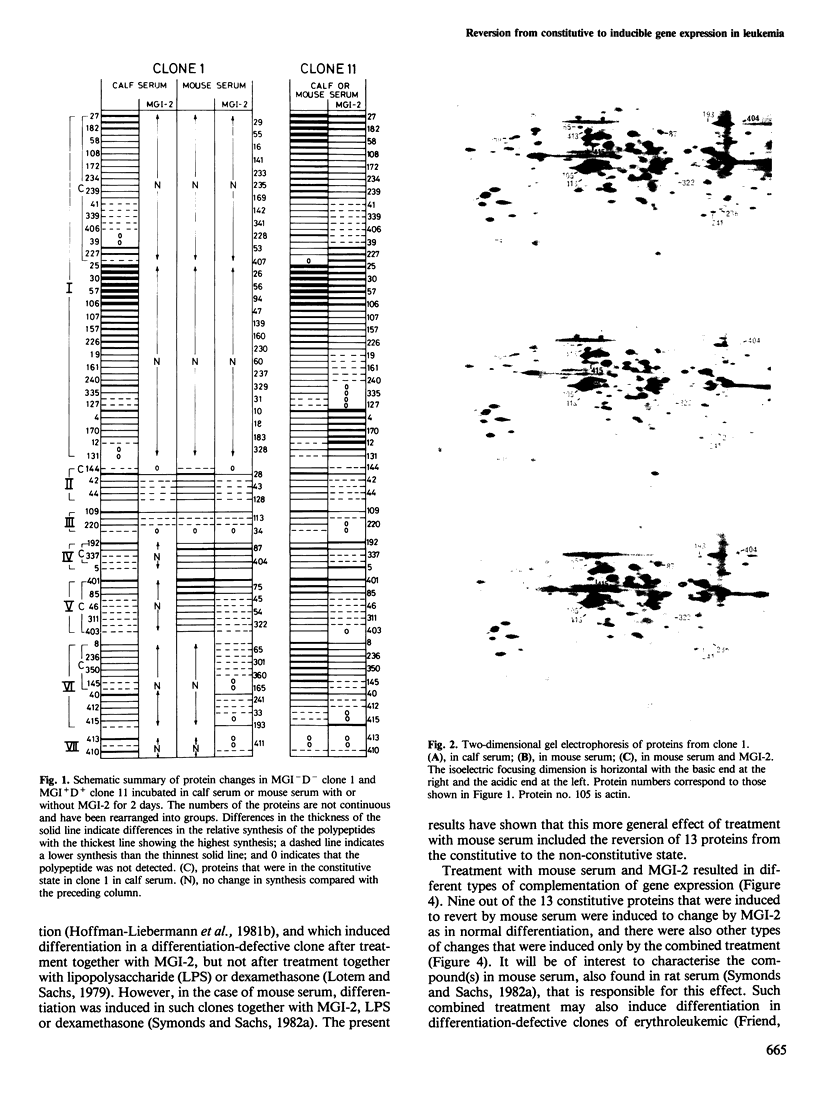
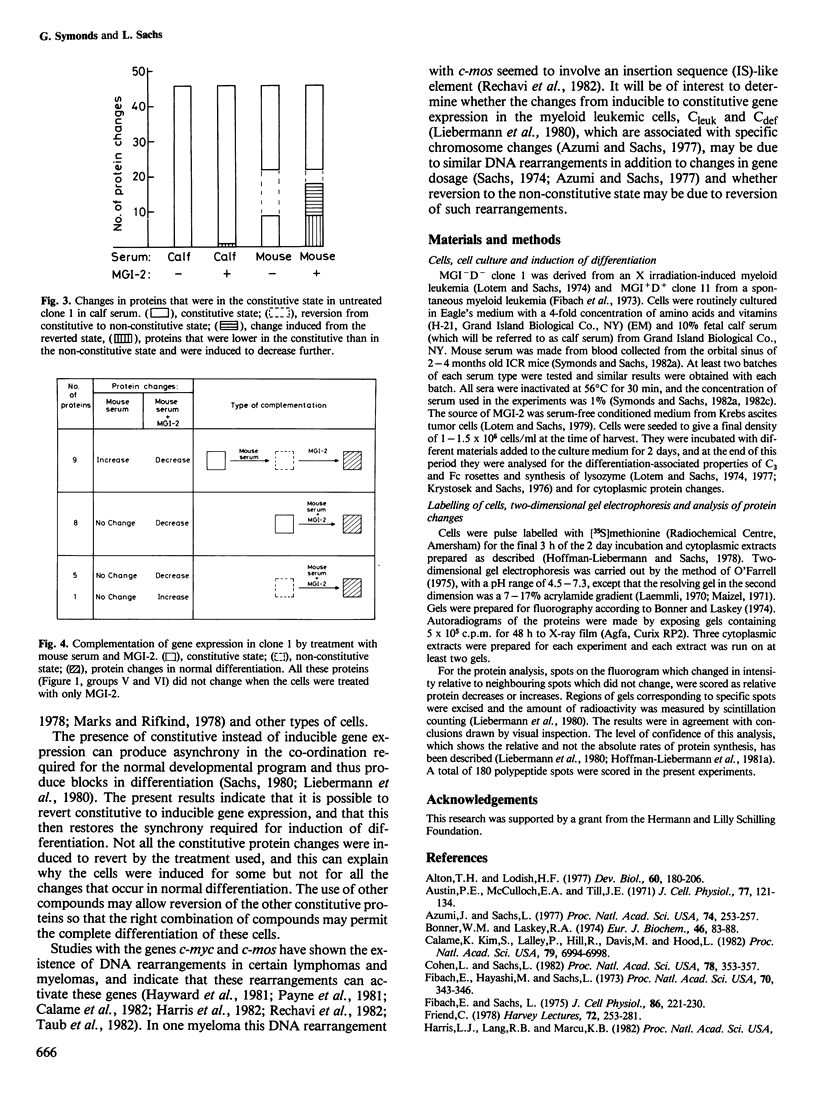
Abstract
There are mutant myeloid leukemic cells that cannot be induced to differentiate in serum-free culture medium, or medium with calf serum by the macrophage and granulocyte differentiation-inducing protein (MGI-2) that induces differentiation in normal myeloid cells. These mutants can be induced to differentiate by MGI-2 in medium with mouse serum. The mechanism of this induction of differentiation has been analysed by using two-dimensional gel electrophoresis to study changes in the synthesis of cytoplasmic proteins. In calf serum, 46 of the protein changes that were induced by MGI-2 in normally differentiating cells were constitutive in the differentiation-defective mutant cells. Treatment with mouse serum reverted 13 of these proteins from the constitutive to the non-constitutive state. This reversion was associated with a gain of inducibility for various differentiation-associated properties, so that 23 proteins were induced by MGI-2 for the same type of change as in normal differentiation. A normal developmental program requires synchrony of gene expression. The existence of constitutive instead of inducible gene expression can produce asynchrony in this program and thus produce blocks in differentiation. The results indicate that it is possible to treat these mutant cells so as to induce the reversion of specific proteins from the constitutive to the non-constitutive state, and that this can then restore the synchrony required for induction of differentiation. It is suggested that this mechanism may also allow induction of differentiation in other types of differentiation-defective cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Developmental changes in messenger RNAs and protein synthesis in Dictyostelium discoideum. Dev Biol. 1977 Oct 1;60(1):180–206. doi: 10.1016/0012-1606(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Austin P. E., McCulloch E. A., Till J. E. Characterization of the factor in L-cell conditioned medium capable of stimulating colony formation by mouse marrow cells in culture. J Cell Physiol. 1971 Apr;77(2):121–134. doi: 10.1002/jcp.1040770202. [DOI] [PubMed] [Google Scholar]
- Azumi J. I., Sachs L. Chromosome mapping of the genes that control differentiation and malignancy in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):253–257. doi: 10.1073/pnas.74.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Calame K., Kim S., Lalley P., Hill R., Davis M., Hood L. Molecular cloning of translocations involving chromosome 15 and the immunoglobulin C alpha gene from chromosome 12 in two murine plasmacytomas. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6994–6998. doi: 10.1073/pnas.79.22.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L., Sachs L. Constitutive gene expression in myeloid leukemia and cell competence for induction of differentiation by the steroid dexamethasone. Proc Natl Acad Sci U S A. 1981 Jan;78(1):353–357. doi: 10.1073/pnas.78.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Hayashi M., Sachs L. Control of normal differentiation of myeloid leukemic cells to macrophages and granulocytes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):343–346. doi: 10.1073/pnas.70.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Sachs L. Control of normal differentiation of myeloid leukemic cells. VIII. Induction of differentiation to mature granulocytes in mass culture. J Cell Physiol. 1975 Oct;86(2 Pt 1):221–230. doi: 10.1002/jcp.1040860205. [DOI] [PubMed] [Google Scholar]
- Friend C. The phenomenon of differentiation in murine erythroleukemic cells. Harvey Lect. 1978;72:253–281. [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hoffman-Liebermann B., Liebermann D., Sachs L. Control mechanisms regulating gene expression during normal differentiation of myeloid leukemic cells: differentiation defective mutants blocked in mRNA production and mRNA translation. Dev Biol. 1981 Jan 30;81(2):255–265. doi: 10.1016/0012-1606(81)90289-x. [DOI] [PubMed] [Google Scholar]
- Hoffman-Liebermann B., Liebermann D., Sachs L. Regulation of gene expression by tumor promoters. III. Complementation of the developmental program in myeloid leukemic cells by regulating mRNA production and mRNA translation. Int J Cancer. 1981 Nov 15;28(5):615–620. doi: 10.1002/ijc.2910280514. [DOI] [PubMed] [Google Scholar]
- Hoffman-Liebermann B., Sachs L. Regulation of actin and other proteins in the differentiation of myeloid leukemic cells. Cell. 1978 Aug;14(4):825–834. doi: 10.1016/0092-8674(78)90338-0. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Pluznik D. H., Sachs L. Feedback inhibition of the development of macrophage and granulocyte colonies. I. Inhibition by macrophage. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1480–1486. doi: 10.1073/pnas.58.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Sachs L. Control of lysozyme induction in the differentiation of myeloid leukemic cells. Cell. 1976 Dec;9(4 Pt 2):675–684. doi: 10.1016/0092-8674(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landau T., Sachs L. Characterization of the inducer required for the development of macrophage and granulocyte colonies. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2540–2544. doi: 10.1073/pnas.68.10.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson J., Goodfellow P., vadeboncoeur M., McDevitt H. Identification of stage-specific polypeptides synthesized during murine preimplantation development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3332–3336. doi: 10.1073/pnas.75.7.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebermann D., Hoffman-Liebermann B., Sachs L. Molecular dissection of differentiation in normal and leukemic myeloblasts: separately programmed pathways of gene expression. Dev Biol. 1980 Sep;79(1):46–63. doi: 10.1016/0012-1606(80)90072-x. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Different blocks in the differentiation of myeloid leukemic cells. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3507–3511. doi: 10.1073/pnas.71.9.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Genetic dissection of the control of normal differentiation in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5554–5558. doi: 10.1073/pnas.74.12.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. In vivo inhibition of the development of myeloid leukemia by injection of macrophage- and granulocyte-inducing protein. Int J Cancer. 1981 Sep 15;28(3):375–386. doi: 10.1002/ijc.2910280318. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Mechanisms that uncouple growth and differentiation in myeloid leukemia cells: restoration of requirement for normal growth-inducing protein without restoring induction of differentiation-inducing protein. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4347–4351. doi: 10.1073/pnas.79.14.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. I. Continuous cluster formation and relation of clusters to colonies. J Cell Physiol. 1969 Dec;74(3):323–332. doi: 10.1002/jcp.1040740313. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Reeves R., Cserjesi P. Sodium butyrate induces new gene expression in Friend erythroleukemic cells. J Biol Chem. 1979 May 25;254(10):4283–4290. [PubMed] [Google Scholar]
- Sachs L. Constitutive uncoupling of pathways of gene expression that control growth and differentiation in myeloid leukemia: a model for the origin and progression of malignancy. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6152–6156. doi: 10.1073/pnas.77.10.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Sachs L. Regulation of membrane changes, differentiation, and malignancy in carcinogenesis. Harvey Lect. 1974;68:1–35. [PubMed] [Google Scholar]
- Storti R. V., Horovitch S. J., Scott M. P., Rich A., Pardue M. L. Myogenesis in primary cell cultures from Drosophila melanogaster: protein synthesis and actin heterogeneity during development. Cell. 1978 Apr;13(4):589–598. doi: 10.1016/0092-8674(78)90210-6. [DOI] [PubMed] [Google Scholar]
- Symonds G., Sachs L. Autoinduction of differentiation in myeloid leukemic cells: restoration of normal coupling between growth and differentiation in leukemic cells that constitutively produce their own growth-inducing protein. EMBO J. 1982;1(11):1343–1346. doi: 10.1002/j.1460-2075.1982.tb01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds G., Sachs L. Cell competence for industion of differentiation by insulin and other compounds in myeloid leukemic clones continuously cultured in serum-free medium. Blood. 1982 Jul;60(1):208–212. [PubMed] [Google Scholar]
- Symonds G., Sachs L. Modulation of cell competence for induction of differentiation in myeloid leukemic cells. J Cell Physiol. 1982 Apr;111(1):9–14. doi: 10.1002/jcp.1041110103. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring G. L., Mahowald A. P. Identification and time of synthesis of chorion proteins in Drosophila melanogaster. Cell. 1979 Mar;16(3):599–607. doi: 10.1016/0092-8674(79)90033-3. [DOI] [PubMed] [Google Scholar]