Abstract
We studied the evolutionary relationships among basal metazoan lineages by using complete large subunit (LSU) and small subunit (SSU) ribosomal RNA sequences for 23 taxa. After identifying competing hypotheses, we performed maximum likelihood searches for trees conforming to each hypothesis. Kishino–Hasegawa tests were used to determine whether the data (LSU, SSU, and combined) reject any of the competing hypotheses. We also conducted unconstrained tree searches, compared the resulting topologies, and calculated bootstrap indices. Shimodaira–Hasegawa tests were applied to determine whether the data reject any of the topologies resulting from the constrained and unconstrained tree searches. LSU, SSU, and the combined data strongly contradict two assertions pertaining to sponge phylogeny. Hexactinellid sponges are not likely to be the basal lineage of a monophyletic Porifera or the sister group to all other animals. Instead, Hexactinellida and Demospongia form a well-supported clade of siliceous sponges, Silicea. It remains unclear, on the basis of these data alone, whether the calcarean sponges are more closely related to Silicea or to nonsponge animals. The SSU and combined data reject the hypothesis that Bilateria is more closely related to Ctenophora than it is to Cnidaria, whereas LSU data alone do not refute either hypothesis. LSU and SSU data agree in supporting the monophyly of Bilateria, Cnidaria, Ctenophora, and Metazoa. LSU sequence data reveal phylogenetic structure in a data set with limited taxon sampling. Continued accumulation of LSU sequences should increase our understanding of animal phylogeny.
A significant advance in the field of metazoan evolution would be brought about by having a better understanding of animal relationships. Comparative zoology is predicated on the assumption of a phylogenetic history. Therefore, a well-founded phylogeny is key for the development and assessment of hypotheses dealing with the rise of metazoan complexity (1), the body architecture and life history traits of major clade ancestors (2–7), and the buildup of body plans during the Cambrian radiation (8, 9). Masses of data pertinent to questions of animal evolution are being accumulated by studies of the fossil record, genomics, and the molecular basis of development. Synthetic treatments of these data will be enhanced by reducing uncertainties in our understanding of metazoan relationships (10, 11).
Analyses of nuclear small subunit (SSU) rRNA sequences have greatly influenced current thinking about the phylogeny of Metazoa despite abundant criticisms of SSU rRNA data for phylogenetic reconstruction. Among the limitations noted are nucleotide compositional bias, among site rate variation, and heterogeneous rates of evolution across lineages (12–16). Nevertheless, the best measure of the validity of any hypothesis is provided by the accord or incongruence of alternative lines of evidence. Indeed, intensive investigation of animal phylogeny by using alternative sets of data is under way. Many inconsistencies remain, but the outlines of a consensus view are beginning to emerge. For example, there is nearly universal support for the triploblastic bilaterally symmetric animals forming the clade Bilateria. Agreement has also developed for the proposal that Deuterostomia is a clade composed of just Chordata, Urochordata, Hemichordata, and Echinodermata (17, 18). Presently, somewhat less confidence is warranted for assertions that the majority of the remaining bilaterian phyla, which may or may not form a monophyletic Protostomia, can be naturally grouped into three major alliances, Ecdysozoa, Lophotrochozoa, and Platyzoa.
Less effort has been directed toward understanding the earlier phylogenetic history of Metazoa, presumably involving the divergences among and between cnidarians, ctenophores, placozoans, sponges, and bilaterians as a whole. Several hypotheses for the phylogenetic positions of these groups remain at odds. Cladistic analyses of morphology tend to suggest a step-wise arrangement where either Porifera or Placozoa diverge first, then Cnidaria, and finally Ctenophora, from the lineage leading to Bilateria (19, 20). This view is contradicted by SSU rRNA data, which weakly suggest that sponges are paraphyletically arranged at the base of Metazoa (21, 22) and that Cnidaria, Placozoa, and Bilateria form a well-supported clade to the exclusion of Ctenophora (22, 23). Additional data from Protein Kinase C and Heat-Shock Protein 70 sequences have been used to assess phylogenetic questions concerning sponges (24–26), but these data have not yet been gathered for any ctenophore taxa. Therefore, additional data are needed to improve our understanding of the early phylogenetic history of animals.
The nuclear large subunit (LSU) rRNA gene may have great potential as a phylogenetic marker for animals. Unlike for some protein coding genes, determining orthologs is not problematic for ribosomal genes because of the mechanisms of concerted evolution. Moreover, LSU is much larger than SSU and contains a greater proportion of variable regions. The size of LSU and the lack of established sequencing primers have made it difficult to obtain complete sequences. In addition, it potentially is subject to the same limitations as those noted for SSU rRNA because of similar functional constraints and genetic linkage. However, the analytical scrutiny under which SSU rRNA data have been submitted could be an advantage, as it should allow for a more appropriate handling of LSU rRNA data.
In this study, we have compiled a data set of complete LSU and SSU rRNA sequences for 23 taxa. Seventeen of the LSU and three of the SSU sequences are new. We evaluate the utility of these data for elucidating early animal phylogeny by using a somewhat unorthodox approach. We choose sets of competing hypotheses that pertain to a particular question (e.g., is Ctenophora or Cnidaria more closely related to Bilateria?). Then, by using maximum likelihood, we estimate how probable the data are, given each of the alternative hypotheses. Those hypotheses for which the data are significantly improbable can be rejected. Our confidence that a given hypothesis is false would be most enhanced if it were rejected by both LSU and SSU. On the other hand, if neither data set rejects any in a set of competing hypotheses, then our ability to discern among them is low, in the absence of any other information. This approach of hypothesis elimination should efficiently achieve the goal of minimizing uncertainties about animal phylogeny. Having said that, engaging in the more standard approach of phylogenetic analysis is also useful. By using LSU, SSU, and the data combined, we construct optimal trees and estimate support for the various clades within them. This type of analysis has the potential to generate novel hypotheses for future consideration.
Materials and Methods
We compiled sequences for the LSU and SSU genes of 23 taxa (Table 1). We sequenced the LSU gene from 17 organisms, mainly nonbilaterian animals (one mesomycetozoan, two choanoflagellates, four sponges, five cnidarians, three ctenophores, and two bilaterians) and the SSU gene from three cnidarians (M. franksi, H. circumcincta, and Nectopyramis sp.). We isolated total DNA by standard SDS/Proteinase K digestion (27). We performed DNA amplifications by long PCR (94°C, 5 min (94°C, 30 sec/45°C, 1 min/65°C, 12 min) ×30–72°C, 10 min). The enzyme used was a combination of rTth (Applied Biosystems) and vent polymerases (New England Biolabs). After A-tailing with Taq polymerase, all PCR products were cloned into a TOPO vector (Invitrogen). We developed sets of primers to specifically amplify and sequence approximately 4 kb of the eukaryotic LSU rRNA gene (Table 2). SSU fragments were amplified and sequenced by using the universal eukaryotic primers of Medlin et al. (28). We obtained SSU and LSU sequences from GenBank for three fungi to use as an outgroup.
Table 1.
Species, taxonomic classification, and accession numbers
| Species | Classification | LSU Acc. | SSU Acc. |
|---|---|---|---|
| Saccharomyces cerevisiae | Fungi, Ascomycota | J01355 | M27607 |
| Tricholoma matsutake | Fungi, Basidiomycota | U62964 | U62538 |
| Mucor racemosus | Fungi, Zygomycota | AJ271061 | AJ271061 |
| Ichthyophonus hoferi | Mesomycetozoa | AY026370* | U25637 |
| Monosiga brevicolis | Choanoflagellida | AY026374* | AF100940 |
| Salpingoeca infusionum | Choanoflagellida | AY026380* | AF100941 |
| Leucosolenia sp. | Porifera, Calcarea | AY026372* | AF100945 |
| Mycale fibrexilis | Porifera, Demospongia | AY026376* | AF100946 |
| Suberites ficus | Porifera, Demospongia | AY026381* | AF100947 |
| Rhabdocalyptus dawsoni | Porifera, Hexactinellida | AY026379* | AF100949 |
| Montastraea franksi | Cnidaria, Anthozoa | AY026375* | AY026382* |
| Antipathes galapagensis | Cnidaria, Anthozoa | AY026365* | AF100943 |
| Atolla vanhoeffeni | Cnidaria, Scyphozoa | AY026368* | AF100942 |
| Hydra circumcincta | Cnidaria, Hydrozoa | AY026371* | AF358080* |
| Nectopyramis sp. | Cnidaria, Hydrozoa | AY026377* | AF358068* |
| Pleurobrachia bachei | Ctenophora, Pleurobrachiidae | AY026378* | AF293677 |
| Mnemiopsis leidyi | Ctenophora, Lobata | AY026373* | AF293700 |
| Beroe ovata | Ctenophora, Beroida | AY026369* | AF293694 |
| Aplysia californica | Bilateria, Mollusca | AY026366* | AY039804 |
| Dugesia tigrina | Bilateria, Platyhelminthes | U78718 | AF013157 |
| Arbacia punctulata | Bilateria, Echinodermata | AY026367* | AH001568 |
| Styela plicata | Bilateria, Urochordata | AF158724 | M97577 |
| Xenopus borealis/X. laevis | Bilateria, Chordata | X59733 | K01373 |
New sequence.
Table 2.
PCR and sequencing primers used in this study
| Forward PCR |
| 5.8SF, GGATCACTCGGCTCRTGNRTCGATGAAG (Universal) |
| F63mod, ACCCGCTGAAYTTAAGCATATHANTMAG (Eukaryota) |
| F1586, GTGCAGATCTTGGTDGNAGTAGCAAATATTC (Eukaryota) |
| Reverse PCR |
| R1630, CCYTTCYCCWCTCRGYCTTC (Eukaryota) |
| R3264, TTCYGACTTAGAGGCGTTCAG (Universal) |
| 28S amp, GAGCTGGGTTYAGAMCGTCGTGAGACAGGT (Eukaryota) |
| Forward sequencing |
| F63sq, AATAAGCGGAGGAAAAGAAAC (most Eukaryota) |
| F635sq, CCGTCTTGAAACACGGACC |
| F1379sq, GACAGCAGGACGGTGGYCATGG |
| F2076sq, TAACYTCGGGAWAAGGATTGGCTC |
| F2766sq, AGTTTGGCTGGGGCGGYACA |
| Reverse sequencing |
| R635sq, GGTCCGTGTTTCAAGACGG (Eukaryota) |
| R1411sq, GTTGTTACACACTCCTTAGCGG |
| R2077sq, GAGCCAATCCTTWTCCCGARGTT |
| R2766sq, CAGRTGTRCCGCCCCAGCCAAACT |
Alignments for both SSU and LSU sequences were refined by eye by using a multiple sequence alignment editor. According to the model for yeast, we encoded secondary structure in the alignment, identifying stems, loops, and bulges in both molecules (29). We manually excluded regions of ambiguous alignment from the final dataset, including the LSU structural features coded as B15, C1, E9_1, E20_1, E20_2, and G5_2, according to the nomenclature developed by De Rijk et al. (29). The final alignment of both genes, which is available on request, includes 4,003 characters, 1,595 sites from the SSU gene and 2,408 sites from LSU (Table 3).
Table 3.
Total, variable, and parsimony informative characters
| SSU | 1,595, | 705, | 466 |
| LSU | 2,408, | 1,074, | 750 |
| Combined | 4,003, | 1,779, | 1,216 |
We performed nested likelihood ratio tests (LRT) by using modeltest version 3.0 (30) to determine the best available model of sequence evolution for the SSU, LSU, and combined data, as well as the stem and loop regions of each gene. We chose sets of competing hypotheses for evaluation based on published views (Table 4). We used paup* 4.0 (31) for all phylogenetic analyses. For LSU, SSU, and the combined data, we performed maximum likelihood (ML) searches for optimal trees congruent with each a priori hypothesis, assuming the model of nucleotide evolution identified by LRT. For each set of competing hypotheses, we performed Kishino–Hasegawa (KH) tests (32) to determine whether any are rejected by the LSU, SSU, or combined data.
Table 4.
Comparison of competing hypotheses–KH and SH test P values
| Competing phylogenetic hypotheses | KH
test
|
SH test
|
||||
|---|---|---|---|---|---|---|
| SSU | LSU | Combined | SSU | LSU | Combined | |
| Sister group to Metazoa? | ||||||
| Choanoflagellida | 0.049* | 0.600 | 0.232 | 0.451 | 0.881 | 0.861 |
| Mesomycetozoa | 0.105 | 1.000 | 0.707 | 0.525 | 1.000 | 0.940 |
| Choanoflagellida Plus Mesomycetozoa | 1.000 | 0.172 | 1.000 | 1.000 | 0.740 | 1.000 |
| Sponge phylogeny | ||||||
| Porifera monophyletic with Hexactinellida basal | 0.021* | 0.002* | 0.000* | 0.164 | 0.010* | 0.018* |
| Porifera monophyletic with Calcarea basal | 0.302 | 1.000 | 1.000 | 0.719 | 0.785 | 1.000 |
| Porifera paraphyletic: Calcarea sister to Eumetazoa | 1.000 | 0.116 | 0.606 | 1.000 | 0.320 | 0.918 |
| Hexactinellida sister to all other Metazoans | 0.028* | 0.005* | 0.000* | 0.374 | 0.020* | 0.005* |
| Cnidarian phylogeny | ||||||
| Cnidaria monophyletic with Hydrozoa basal | 1.000 | 0.004* | 0.016* | 1.000 | 0.036* | 0.161 |
| Cnidaria monophyletic with Anthozoa basal | 0.282 | 1.000 | 1.000 | 0.592 | 1.000 | 1.000 |
| Sister group to Bilateria? | ||||||
| Ctenophora sister to Bilateria | 0.022* | 1.000 | 0.033* | 0.178 | 0.506 | 0.563 |
| Cnidaria sister to Bilateria | 1.000 | 0.658 | 1.000 | 1.000 | 0.411 | 1.000 |
| Optimal topologies (a posteriori) | ||||||
| Small subunit ML tree | 1.000 | 0.000* | 0.064 | |||
| Large subunit ML tree | 0.090 | 1.000 | 0.452 | |||
| Combined ML tree | 0.094 | 0.152 | 1.000 | |||
| Combined MP tree 1 | 0.320 | 0.092 | 0.612 | |||
| Combined MP tree 2 | 0.133 | 0.095 | 0.630 | |||
Significant rejection.
We also conducted ML and maximum parsimony (MP) searches without any constraints. The MP analyses assumed a weighting of 2:1 for transversions to transitions to account for the bias estimated by using ML (1.88, 2.14, and 2.04 for SSU, LSU, and combined, respectively). To estimate branch support, we performed 100 and 1,000 bootstrap pseudoreplicates under the ML and MP criteria, respectively, and calculated Bremer indices for each of the nodes present in the strict consensus of the MP trees. The KH test is often inappropriately used to discern among hypotheses chosen a posteriori (33). Thus, we used another nonparametric test, the Shimodaira–Hasegawa (SH) test (34), to compare the likelihood scores of trees directly derived from the data at hand. We applied the SH test to all potential topologies, including those resulting from unconstrained ML and MP searches. Finally, we repeated most of the analyses described above with one taxon (D. tigrina) excluded to determine the impact of its relatively elevated rate of rRNA evolution.
Results
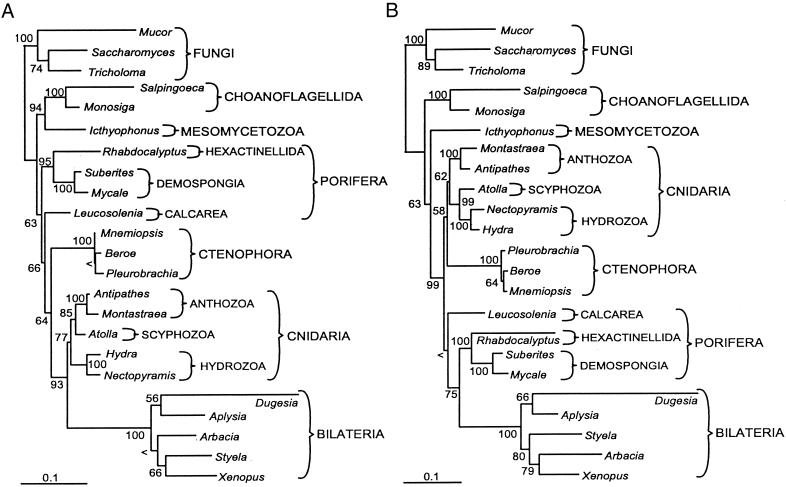
For the taxa we sampled, the pattern and overall base composition of the LSU gene is very similar to that of the SSU gene (Table 5). Within each gene, the loops were markedly more A-T rich than the stems. The likelihood ratio test implemented in modeltest (30) indicated that very similar models of nucleotide evolution best fit the LSU, SSU, and combined data (Table 5). A comparison of branch lengths in the unconstrained ML topologies based on SSU and LSU data suggests that there are lineage specific factors affecting the rate of evolution in these two genes (Fig. 1). For instance, relatively accelerated substitution rates in both genes are evident for the bilaterian taxa whereas rates are relatively low for cnidarians and ctenophores.
Table 5.
Summary of the different model parameters for the three datasets
| Gene | Model | PINV | α | A | C | G | T |
|---|---|---|---|---|---|---|---|
| SSU | TrN | 0.2567 | 0.5370 | 0.2663 | 0.2039 | 0.2693 | 0.2606 |
| LSU | TrN | 0.2768 | 0.5248 | 0.2611 | 0.2086 | 0.2957 | 0.2346 |
| Combined | TrN | 0.2685 | 0.5257 | 0.2637 | 0.2061 | 0.2859 | 0.2442 |
| Structural domains | |||||||
| SSU loops | GTR | 0.2620 | 0.5114 | 0.3649 | 0.1607 | 0.2170 | 0.2575 |
| LSU loops | TIM | 0.3561 | 0.5017 | 0.3673 | 0.1499 | 0.2615 | 0.2213 |
| SSU stems | K81uf | — | 0.3402 | 0.1850 | 0.2467 | 0.2951 | 0.2732 |
| LSU stems | HKY | 0.2031 | 0.5269 | 0.1740 | 0.2640 | 0.3087 | 0.2533 |
PINV, proportion of invariant sites; α, gamma distribution shape parameter; A-C-G-T, base frequencies; TrN, Tamura–Nei; GTR, General Time Reversible; HKY, Hasegawa–Kishino–Yano; TIM, transition; K81uf, Kimura-unequal base frequencies.
Figure 1.
Comparison of ML SSU and LSU trees (A and B, respectively). ML bootstrap (100 replicates) values are shown at the nodes. < indicates bootstrap less than 50%. (Bar = 0.1 substitutions per site.)
KH tests reject a number of a priori metazoan hypotheses (Table 4). That Choanoflagellida is the sister group to Metazoa is rejected by SSU data. LSU, SSU, and the combined data reject two of four hypotheses dealing with sponge phylogeny. The LSU and combined data reject the idea that Hydrozoa is the sister group to other cnidarians. The combined and SSU data reject the hypothesis that Ctenophora is more closely related to Bilateria than is Cnidaria. Results of KH tests for all other hypotheses chosen a priori were not significant (Table 4).
The optimal SSU and LSU ML trees contain topological consistencies and inconsistencies (Fig. 1). They are consistent in suggesting monophyletic Choanoflagellida, Metazoa, Bilateria, Cnidaria, and Ctenophora, with ML bootstraps of 100 except for Cnidaria (77 and 62) and Metazoa (63 and 63). In addition, both topologies contradict the hypothesis that sponges form a monophyletic Porifera and reveal a well-supported (bootstraps of 95 and 100) grouping of the siliceous sponges, Hexactinellida and Demospongia (Silicea). The LSU tree depicts Ctenophora plus Cnidaria as the sister group to a clade that includes sponges paraphyletically arranged at the base of the bilaterian animals. In contrast, the SSU topology has Hexactinellida plus Demospongia as the sister group to all other animals, Calcarea as the sister to nonsponge animals (Eumetazoa), and Ctenophora as the sister group to Cnidaria plus Bilateria. Finally, the layout among cnidarians differs between the two topologies, with Hydrozoa being the sister group of Anthozoa plus Scyphozoa in the SSU topology and Anthozoa as the sister group to the medusa-bearing cnidarians in the LSU topology.
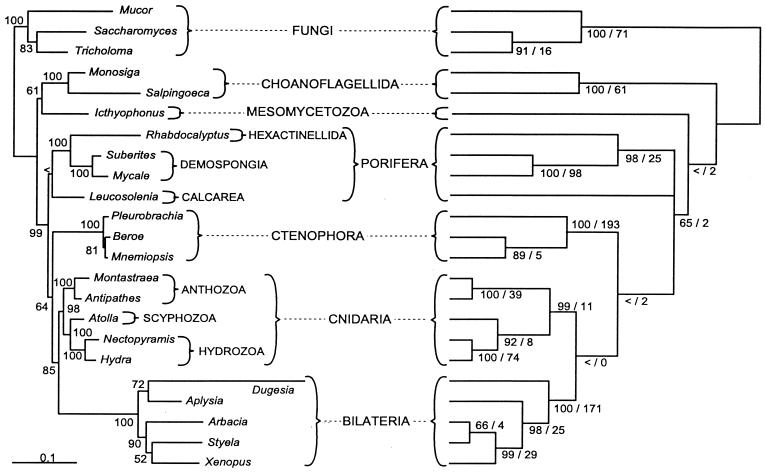
Not surprisingly, all clades in common to the SSU and LSU topologies are also present in the combined ML tree (Fig. 2). By combining the data, bootstrap support for monophyly of Cnidaria and Metazoa increased to 98 and 99, respectively. The combined data produce a topology that is more similar to the SSU ML tree than to the LSU reconstruction. The symmetric difference, or the number of clades present in only one of two trees being compared, is 6 between the combined ML topology and the SSU tree, whereas the symmetric difference between the combined and LSU trees is 10. In the combined ML topology, Mesomycetozoa plus Choanoflagellida compose the sister group to Metazoa. A poorly supported (bootstrap < 50) monophyletic Porifera is the sister to all other animals, which are broadly arranged as seen in the SSU topology. One difference, however, is in the arrangement among cnidarian taxa. The combined tree agrees with the LSU topology in having Anthozoa as sister to the medusa-bearing cnidarians. Although not identical, there is a strong correspondence between the ML and MP topologies based on the combined data (Fig. 2).
Figure 2.
Comparison of ML tree and strict consensus of two MP trees based on combined SSU and LSU data (Left and Right, respectively). ML bootstrap values are shown at the nodes of the ML tree. MP bootstrap values and Bremer support indices are shown at the nodes of the MP tree. < indicates bootstrap of less than 50%. (Bar = 0.1 substitutions per site.)
The SH test, which was applied to all hypotheses that resulted from the constrained and unconstrained tree searches, did not yield many significant P-values. The SSU data do not refute any hypotheses by using this test. However, the LSU and combined data both reject the two hypotheses pertaining to sponge phylogeny that were rejected by the KH tests. In addition, the SH test with LSU data reject the hypothesis that Hydrozoa is sister to the other cnidarians. The LSU data also reject the SSU ML topology, presumably because it contains the cnidarian hypothesis that is rejected. Finally, removing the relatively fast evolving sequences of D. tigrina had no substantive impact on the results (not shown) of the constrained and unconstrained tree searches, as well as the KH and SH tests.
Discussion
The hypothesis that Choanoflagellida is the nearest relative of Metazoa has long been posited on the basis of morphological comparisons (ref. 35 and references therein). More recently, SSU data have buttressed this view (36). However, another group of unicellular eukaryotes that may be more closely related to animals is the recently named Mesomycetozoa (37). Originally identified by SSU data as a small group of fish parasites closely related to animals and choanoflagellates (38–40), the clade has grown to include a number of different species with diverse animal hosts (37, 41–43). The analysis presented here is the first, to our knowledge, to address explicitly the conflicting hypotheses of whether Choanoflagellida, Mesomycetozoa, or the two combined are the closest living relatives of animals. The SSU and combined data ML trees (Figs. 1 and 2) suggest that Choanoflagellida plus Mesomycetozoa is the sister group to animals. In contrast, the LSU data favor the interesting idea that the mesomycetozoan animal parasites are the closest living relatives of animals. Moreover, the SSU data reject the hypothesis that Choanoflagellida is most closely related to Metazoa. Nevertheless, our data set, with its relatively limited taxon sampling (particularly outside the fungal/animal divergence), may be inadequate to resolve this question. A general consideration of morphology and ecology of these two groups would seem to contradict the molecular data by suggesting that choanoflagellates are the true sisters of animals. Choanoflagellates are similar to sponges in possessing cells with a single flagellum surrounded by a microvillar collar and ingesting bacteria, whereas at least some mesomycetozoans have structures like the endospores of fungi and digest extracellularly. Increased taxon sampling and additional data are needed to pursue this interesting question.
Although cladistic analyses of morphological data have concurred in suggesting that Porifera is monophyletic, two opposing views have arisen from these studies (44–46). One hypothesis holds that the two groups of cellular sponges, Calcarea and Demospongia, are the sister group of the syncytial sponges, Hexactinellida (45, 46). The contrasting view is that the two sponge groups with siliceous spicules (Hexactinellida and Demospongia) form a sister group to sponges with calcareous spicules, Calcarea (44). An alternative to both these hypotheses, weakly suggested by SSU data, is that Calcarea is actually more closely related to nonsponge animals than it is to the other sponges (21, 22). Further evidence that Calcarea may be the sister to Eumetazoa comes from amino acid sequences of Protein Kinase C (24, 26). These studies differ from those derived from SSU data by faintly suggesting that Hexactinellida may be the sister group to all other metazoans. Our data unequivocally contradict this hypothesis and the proposal that Calcarea and Demospongia form a clade, leaving two hypotheses pertaining to sponge phylogeny for further consideration. Porifera may be monophyletic, with Calcarea sister to the siliceous sponges, or Porifera may be paraphyletic, with Calcarea forming a clade with Eumetazoa. Nevertheless, three independent sets of molecular data show sponges to be paraphyletic—SSU, LSU, and Protein Kinase C—implying that this alternative is most likely. If true, then an animal with sponge characteristics is in the direct ancestry of nonsponge animals. A sponge can be described as an animal with a feeding system involving rings of microvilli that filter nutrients carried by a unidirectional water flow. This flow is driven by beating flagella that line the surface of chambers connected by a series of canals. Sponge characteristics were presumably lost in conjunction with the transition to feeding on larger food items in the lineage leading to Eumetazoa.
Hyman (47) suggested that Hydrozoa was the ancestral group of cnidarians. That hypothesis was strongly controverted by the determination that hydrozoans and the other medusa-bearing cnidarian groups share linear mitochondrial genomes (48). The mitochondrial genomes of anthozoans are circular like those of other animals. Surprisingly, the optimal ML tree, on the basis of the SSU sequences compiled here, corresponds with Hyman's view. However, the SSU data do not refute either of the competing hypotheses, and the LSU and combined data both strongly contradict the hypothesis that Hydrozoa is sister to the other cnidarian groups. Interestingly, studies with denser taxon sampling of the SSU gene also produce topologies with the sessile anthozoans as the sister group to the medusa-bearing cnidarian groups (22, 23). That LSU is able to reject the hydrozoan basal hypothesis with so few taxa indicates that additional LSU sequences may help resolve a number of outstanding phylogenetic questions, both within and between animal phyla.
Cladistic analyses of morphological characters suggest that Ctenophora is more closely related to Bilateria than is Cnidaria (19, 20). However, SSU data contradict that notion (22, 23). In fact, the SSU and combined data presented here suggest that the hypothetical clade Ctenophora plus Bilateria is significantly less probable than Bilateria plus Cnidaria. This result has profound impact on how best to interpret the burgeoning evidence from comparative morphology, development, and genomics of these early diverging metazoan lineages (22, 23). Nevertheless, in this analysis, bilaterian taxa display the longest branches, which are notoriously difficult for phylogenetic analyses, particularly when taxon sampling is limited. Therefore, the position of Bilateria in any of the topologies suggested by our data may potentially be artifactual. Additional evidence is needed to satisfactorily answer the question of what extant group of animals is sister to the bilaterians, as well as where the other basal animal lineages branch within Metazoa.
This study is, to our knowledge, the first attempt to assess the usefulness of complete LSU sequences as an indicator of metazoan relationships. The LSU and SSU data that we present suggest that a number of hypotheses concerning early animal phylogeny are unlikely to be true. Purging these hypotheses from further consideration focuses research attention on discerning among the remaining alternatives. LSU data, especially when combined with SSU data, have significant potential to further resolve questions dealing with the phylogenetic relationships within and among animal phyla. Additional taxon sampling of the LSU gene should increase the ability of the data to reveal phylogenetic history, as has certainly been the case in SSU studies. Generating additional LSU sequences for diverse animal taxa should be a fruitful endeavor.
Acknowledgments
We acknowledge the helpful comments of two anonymous reviewers. We are grateful to T. Collins (Florida International University) and M. Podar (Woods Hole Oceanographic Institution) for contributing 18S sequences for Aplysia californica and ctenophores, respectively, before publication. We also thank M. Ragan (National Research Council of Canada) and M. Podar for sharing DNA templates and H. Morrison (Marine Biological Laboratory) for developing a 28S amplification primer. This research was supported by National Institutes of Health Grant GM32964 and by the National Aeronautics and Space Administration Astrobiology Institute, membership NCC2–1054. A.G.C. was sponsored by National Science Foundation Grant EAR-9814845.
Abbreviations
- LSU
large subunit
- SSU
small subunit
- ML
maximum likelihood
- MP
maximum parsimony
- KH
Kishino–Hasegawa
- SH
Shimodaira–Hasegawa
Footnotes
References
- 1.Valentine J W. Paleobiology. 2000;26:513–519. [Google Scholar]
- 2.Nielsen C, Nørrevang A. In: The Origins and Relationships of Lower Invertebrates. Conway Morris S, George R D, Gibson R, Platt H M, editors. Oxford, U.K.: Claredon; 1985. pp. 28–41. [Google Scholar]
- 3.Davidson E H, Peterson K J, Cameron R A. Science. 1995;270:1319–1325. doi: 10.1126/science.270.5240.1319. [DOI] [PubMed] [Google Scholar]
- 4.Valentine J W. Proc Natl Acad Sci USA. 1994;91:6751–6757. doi: 10.1073/pnas.91.15.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentine J W. Proc Natl Acad Sci USA. 1997;94:8001–8005. doi: 10.1073/pnas.94.15.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins A G, Lipps J H, Valentine J W. Paleobiology. 2000;26:47–55. [Google Scholar]
- 7.Dewel R A. J Morphol. 2000;243:35–74. doi: 10.1002/(SICI)1097-4687(200001)243:1<35::AID-JMOR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Valentine J W, Collins A G. Evol Dev. 2000;2:152–156. doi: 10.1046/j.1525-142x.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 9.Budd G E, Jensen S. Biol Rev. 2000;75:253–295. doi: 10.1017/s000632310000548x. [DOI] [PubMed] [Google Scholar]
- 10.Knoll A H, Carroll S B. Science. 1999;284:2129–2137. doi: 10.1126/science.284.5423.2129. [DOI] [PubMed] [Google Scholar]
- 11.Valentine J W, Jablonski D, Erwin D H. Development (Cambridge, UK) 1999;126:851–859. doi: 10.1242/dev.126.5.851. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigo A G, Bergquist P R, Bergquist P L, Reeves R A. In: Sponges in Time and Space. van Soest R W M, van Kempen T M G, Braekman J-C, editors. Rotterdam, The Netherlands: Balkema; 1994. pp. 47–54. [Google Scholar]
- 13.Aguinaldo A M A, Turbeville J M, Linford L S, Rivera M C, Garey J R, Raff R A, Lake J A. Nature (London) 1997;387:489–493. doi: 10.1038/387489a0. [DOI] [PubMed] [Google Scholar]
- 14.Abouheif E, Zardoya R, Meyer A. J Mol Evol. 1998;47:394–405. doi: 10.1007/pl00006397. [DOI] [PubMed] [Google Scholar]
- 15.Ayala F J, Rzhetsky A, Ayala F J. Proc Natl Acad Sci USA. 1998;95:606–611. doi: 10.1073/pnas.95.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maley L E, Marshall C R. Science. 1998;279:505–506. doi: 10.1126/science.279.5350.505. [DOI] [PubMed] [Google Scholar]
- 17.Zrzavy J, Mihulka S, Kepka P, Bezdek A, Tietz D. Cladistics. 1998;14:249–285. doi: 10.1111/j.1096-0031.1998.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 18.Cameron C B, Garey J R, Swalla B J. Proc Natl Acad Sci USA. 2000;97:4469–4474. doi: 10.1073/pnas.97.9.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schram F R. In: The Early Evolution of Metazoa and the Significance of Problematic Taxa. Simonetta A M, Conway Morris S, editors. Cambridge, U.K.: Cambridge Univ. Press; 1991. pp. 35–46. [Google Scholar]
- 20.Nielsen C, Scharff N, Eibye-Jacobsen D. Biol J Linn Soc. 1996;57:385–410. [Google Scholar]
- 21.Cavalier-Smith T, Allsopp M T E P, Chao E E, Boury-Esnault N, Vacelet J. Can J Zool. 1996;74:2031–2045. [Google Scholar]
- 22.Collins A G. Proc Natl Acad Sci USA. 1998;95:15458–15463. doi: 10.1073/pnas.95.26.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J H, Kim W, Cunningham C W. Mol Biol Evol. 1999;16:423–427. doi: 10.1093/oxfordjournals.molbev.a026124. [DOI] [PubMed] [Google Scholar]
- 24.Kruse M, Leys S P, Mueller I M, Mueller W E G. J Mol Evol. 1998;46:721–728. doi: 10.1007/pl00006353. [DOI] [PubMed] [Google Scholar]
- 25.Borchiellini C, BouryEsnault N, Vacelet J, LeParco Y. Mol Biol Evol. 1998;15:647–655. doi: 10.1093/oxfordjournals.molbev.a025968. [DOI] [PubMed] [Google Scholar]
- 26.Schuetze J, Krasko A, Custodio M R, Efremova S M, Mueller I M, Mueller W E G. Proc R Soc Biol Sci Ser B. 1999;266:63–73. doi: 10.1098/rspb.1999.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 28.Medlin L, Elwood H J, Stickel S, Sogin M L. Gene. 1988;71:491–500. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 29.De Rijk P, Van De Peer Y, Van Den Broeck I, De Wachter R. J Mol Evol. 1995;41:366–375. doi: 10.1007/BF01215184. [DOI] [PubMed] [Google Scholar]
- 30.Posada D, Crandall K A. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 31.Swofford D L. paup* Sunderland, MA: Sinauer; 2000. [Google Scholar]
- 32.Kishino H, Hasegawa M. J Mol Evol. 1989;29:170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- 33.Goldman N, Anderson J P, Rodrigo A G. Syst Biol. 2000;49:652–670. doi: 10.1080/106351500750049752. [DOI] [PubMed] [Google Scholar]
- 34.Shimodaira H, Hasegawa M. Mol Biol Evol. 1999;16:1114–1116. [Google Scholar]
- 35.Salvini-Plawen L V. Z zool Syst Evol Naturrforsch. 1978;16:40–88. [Google Scholar]
- 36.Wainright P O, Hinkle G, Sogin M L, Stickel S K. Science. 1993;260:340–342. doi: 10.1126/science.8469985. [DOI] [PubMed] [Google Scholar]
- 37.Herr R A, Ajello L, Taylor J W, Arseculeratne S N, Mendoza L. J Clin Microbiol. 1999;37:2750–2754. doi: 10.1128/jcm.37.9.2750-2754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerk D, Gee A, Standish M, Wainwright P O, Drum A S, Elston R A, Sogin M L. Mar Biol (Berlin) 1995;122:187–192. [Google Scholar]
- 39.Ragan M A, Goggin C L, Cawthorn R J, Cerenius L, Jamieson A V C, Plourde S M, Rand T G, Soderhall K, Gutell R R. Proc Natl Acad Sci USA. 1996;93:11907–11912. doi: 10.1073/pnas.93.21.11907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spanggaard B, Skouboe P, Rossen L, Taylor J W. Mar Biol (Berlin) 1996;126:109–115. [Google Scholar]
- 41.Baker G C, Beebee T J C, Ragan M A. Microbiology. 1999;145:1777–1784. doi: 10.1099/13500872-145-7-1777. [DOI] [PubMed] [Google Scholar]
- 42.Figueras A, Lorenzo G, Ordas M C, Gouy M, Novoa B. Marine Biotechnol (New York) 2000;2:419–428. doi: 10.1007/s101260000015. [DOI] [PubMed] [Google Scholar]
- 43.Ustinova I, Krienitz L, Huss V A R. Protist. 2000;151:253–262. doi: 10.1078/1434-4610-00023. [DOI] [PubMed] [Google Scholar]
- 44.Böger H. Meyniana. 1988;40:67–90. [Google Scholar]
- 45.Mehl D, Reiswig H M. Z zool Syst Evolutionsforsch. 1991;29:312–319. [Google Scholar]
- 46.Reitner J, Mehl D. Verh Naturwiss Ver Hamburg. 1996;36:5–32. [Google Scholar]
- 47.Hyman L H. The Invertebrates. New York: McGraw–Hill; 1940. p. 726. [Google Scholar]
- 48.Bridge D, Cunningham C W, Schierwater B, Desalle R, Buss L W. Proc Natl Acad Sci USA. 1992;89:8750–8753. doi: 10.1073/pnas.89.18.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]