Abstract
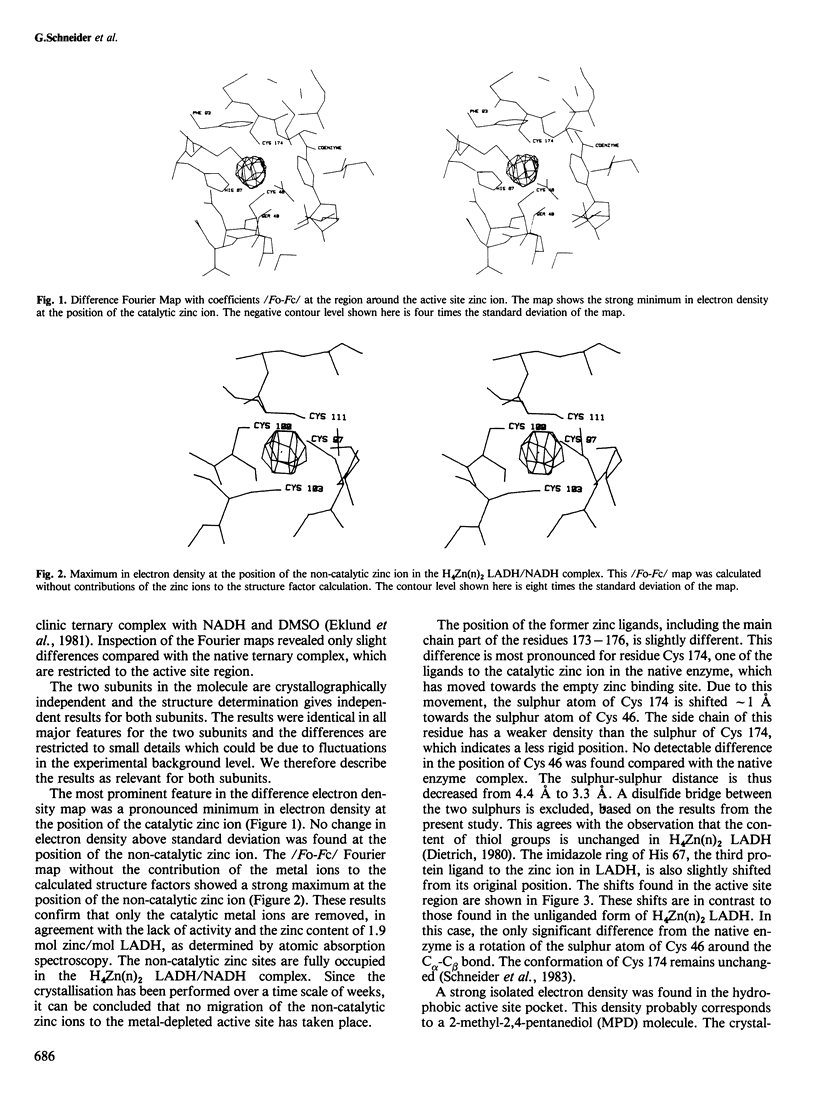
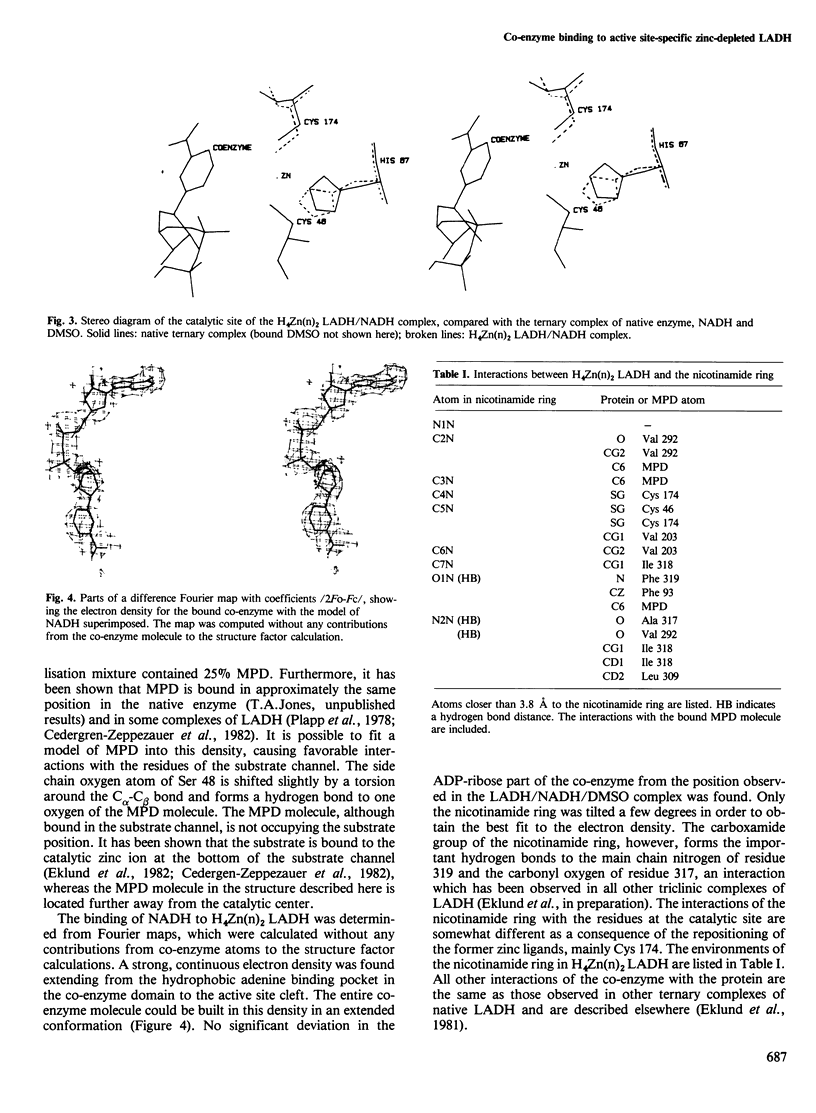
The complex between active site-specific metal-depleted horse liver alcohol dehydrogenase and NADH has been studied with X-ray crystallographic methods to 2.9 A resolution. The electron density maps revealed that only the catalytic zinc ions are removed, whereas the non-catalytic zinc sites ae fully occupied. A gross conformational change in the protein induced by co-enzyme binding takes place in this enzyme species despite the absence of the metal ion in the catalytic center. This circumstance is of great importance in the understanding and further analysis of the trigger mechanisms operating during the conformation transition in alcohol dehydrogenase, since the catalytic center is located at the hinge region for a domain rotation in the subunit, and the metal atom is essential for catalysis. The overall protein structure is the same as that of an NADH complex of the native zinc enzyme and the co-enzyme is bound in a similar manner. The local structural changes observed are restricted to the empty metal binding site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeson A. On the zinc content of horse liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1964 Oct 14;17(3):211–214. doi: 10.1016/0006-291x(64)90385-7. [DOI] [PubMed] [Google Scholar]
- Cedergren-Zeppezauer E., Samama J. P., Eklund H. Crystal structure determinations of coenzyme analogue and substrate complexes of liver alcohol dehydrogenase: binding of 1,4,5,6-tetrahydronicotinamide adenine dinucleotide and trans-4-(N,N-dimethylamino)cinnamaldehyde to the enzyme. Biochemistry. 1982 Sep 28;21(20):4895–4908. doi: 10.1021/bi00263a011. [DOI] [PubMed] [Google Scholar]
- Coleman P. L., Iweibo I., Weiner H. Role of zinc in horse liver alcohol dehydrogenase. Influence on structure and conformational changes. Biochemistry. 1972 Mar 14;11(6):1010–1018. doi: 10.1021/bi00756a010. [DOI] [PubMed] [Google Scholar]
- Eklund H., Brändén C. I. Structural differences between apo- and holoenzyme of horse liver alcohol dehydrogenase. J Biol Chem. 1979 May 10;254(9):3458–3461. [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Eklund H., Plapp B. V., Samama J. P., Brändén C. I. Binding of substrate in a ternary complex of horse liver alcohol dehydrogenase. J Biol Chem. 1982 Dec 10;257(23):14349–14358. [PubMed] [Google Scholar]
- Eklund H., Samma J. P., Wallén L., Brändén C. I., Akeson A., Jones T. A. Structure of a triclinic ternary complex of horse liver alcohol dehydrogenase at 2.9 A resolution. J Mol Biol. 1981 Mar 15;146(4):561–587. doi: 10.1016/0022-2836(81)90047-4. [DOI] [PubMed] [Google Scholar]
- Hoagstrom C. W., Iweibo I., Weiner H. Interaction of coenzyme with differently prepared zinc-free (apo) horse liver alcohol dehydrogenases. J Biol Chem. 1969 Nov 10;244(21):5967–5971. [PubMed] [Google Scholar]
- Iweibo I., Weiner H. Role of zinc in horse liver alcohol dehydrogenase. Coenzyme and substrate binding. Biochemistry. 1972 Mar 14;11(6):1003–1010. doi: 10.1021/bi00756a009. [DOI] [PubMed] [Google Scholar]
- Klinman J. P. Probes of mechanism and transition-state structure in the alcohol dehydrogenase reaction. CRC Crit Rev Biochem. 1981;10(1):39–78. doi: 10.3109/10409238109114635. [DOI] [PubMed] [Google Scholar]
- Maret W., Andersson I., Dietrich H., Schneider-Bernlöhr H., Einarsson R., Zeppezauer M. Site-specific substituted cobalt(II) horse liver alcohol dehydrogenases. Preparation and characterization in solution, crystalline and immobilized state. Eur J Biochem. 1979 Aug 1;98(2):501–512. doi: 10.1111/j.1432-1033.1979.tb13211.x. [DOI] [PubMed] [Google Scholar]
- Plapp B. V., Eklund H., Brändén C. I. Crystallography of liver alcohol dehydrogenase complexed with substrates. J Mol Biol. 1978 Jun 15;122(1):23–32. doi: 10.1016/0022-2836(78)90105-5. [DOI] [PubMed] [Google Scholar]
- Rudolph R., Gerschitz J., Jaenicke R. Effect of zinc(II) on the refolding and reactivation of liver alcohol dehydrogenase. Eur J Biochem. 1978 Jul 3;87(3):601–606. doi: 10.1111/j.1432-1033.1978.tb12412.x. [DOI] [PubMed] [Google Scholar]
- Samama J. P., Zeppezauer E., Biellmann J. F., Brändén C. I. The crystal structure of complexes between horse liver alcohol dehydrogenase and the coenzyme analogues 3-iodopyridine-adenine dinucleotide and pyridine-adenine dinucleotide. Eur J Biochem. 1977 Dec 1;81(2):403–409. doi: 10.1111/j.1432-1033.1977.tb11965.x. [DOI] [PubMed] [Google Scholar]


