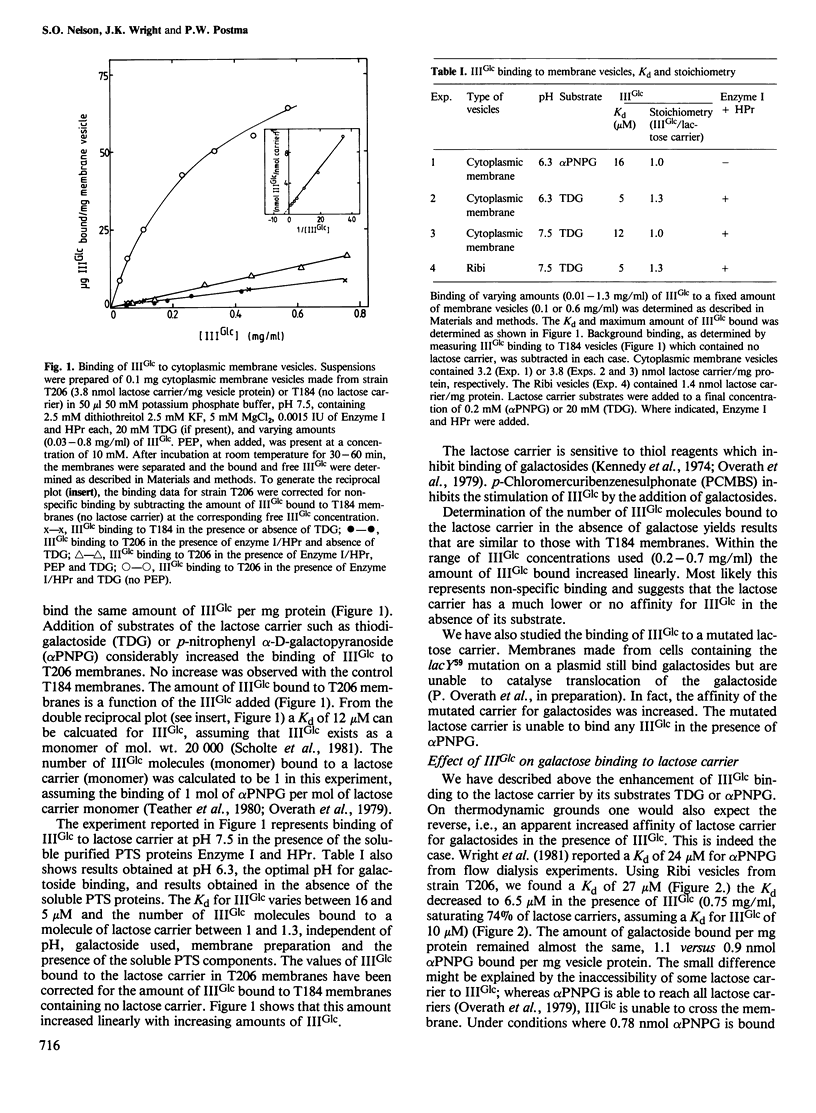
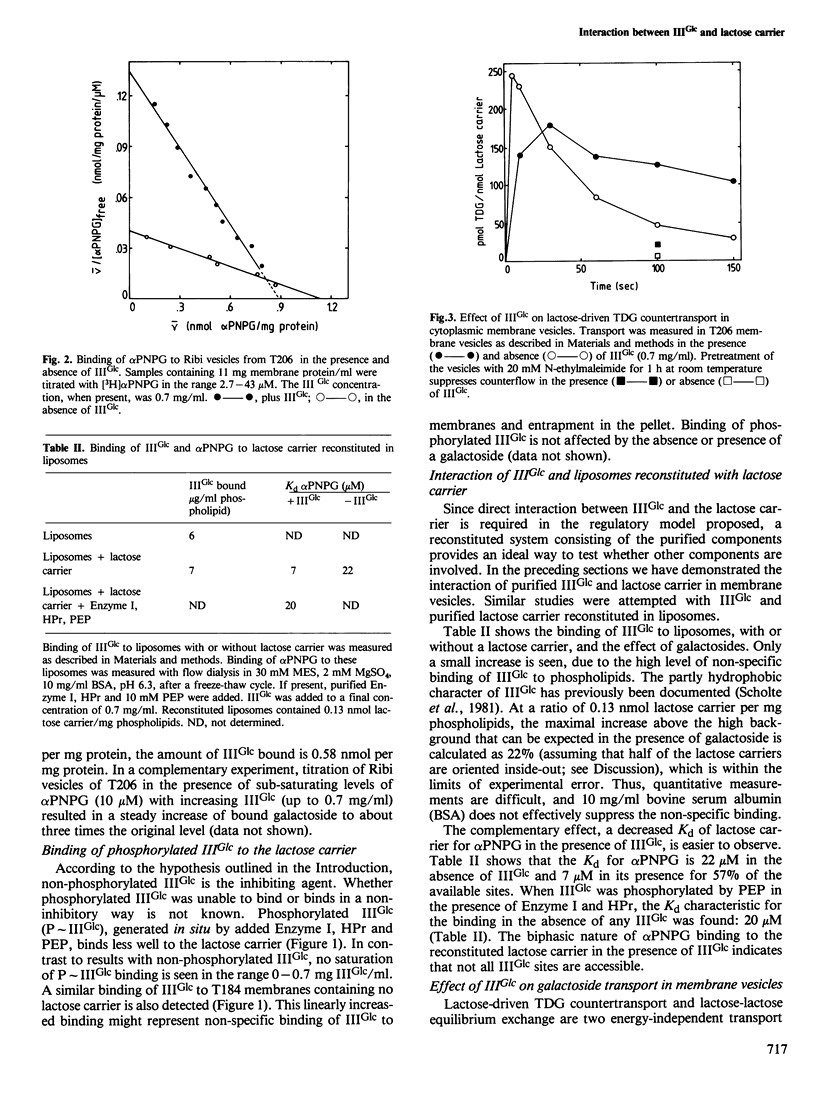
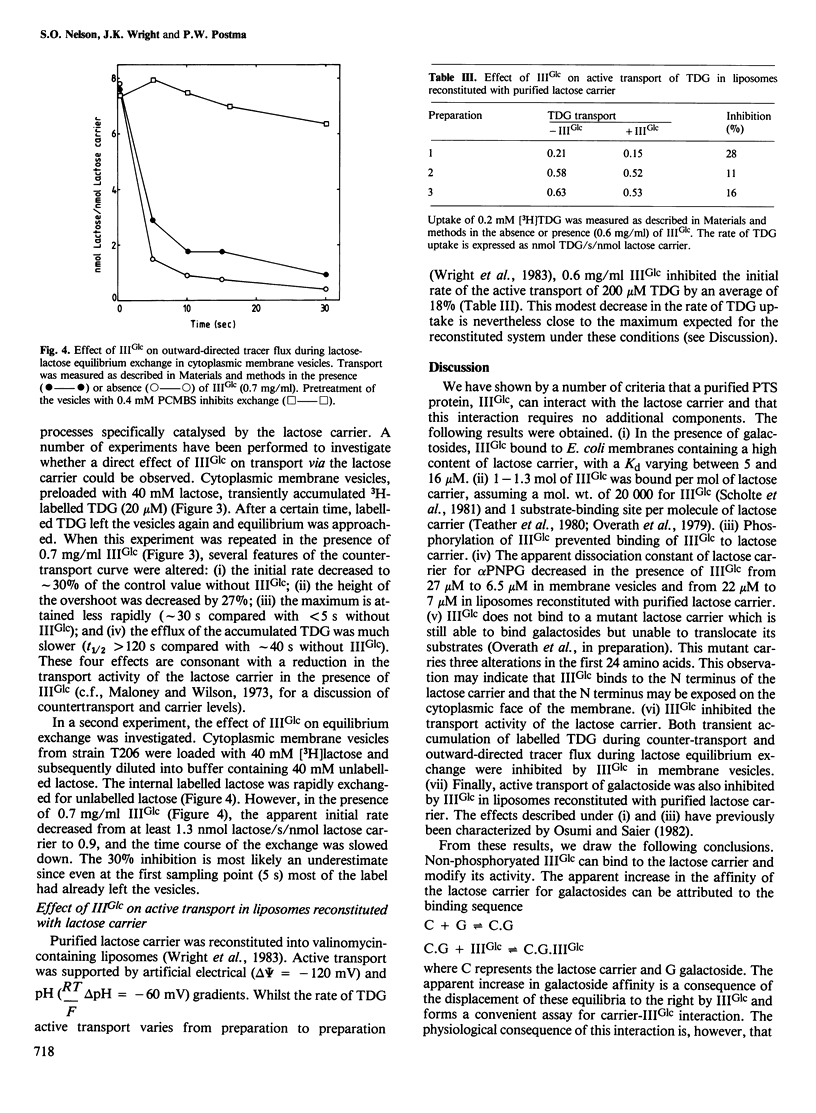
Abstract
A hypothesis for the regulation of some sugar transport systems by the bacterial phosphoenolpyruvate:sugar transport system postulates an interaction between IIIGlc of this system and the carrier whose activity is regulated. We have studied this interaction in more detail, employing one of these transport systems, the lactose carrier of Escherichia coli. Purified IIIGlc of the phosphotransferase system interacted directly with the lactose carrier. The binding of IIIGlc to lactose carrier required the presence of the non-phosphorylated form of IIIGlc and substrates of the carrier and exhibited a stoichiometry of 1.2± 0.2 mol IIIGlc/mol lactose carrier. The Kd of lactose carrier for IIIGlc was 10 ± 5 µM. IIIGlc is apparently unable to interact with a mutant lactose carrier which still binds but does not transport galactosides. The binding of IIIGlc to the lactose carrier results in a 3.5-fold increase in the apparent affinity of galactosides for the carrier. Significantly, the binding of IIIGlc to the lactose carrier results in an inhibition of galactoside translocation both in membrane vesicles and liposomes reconstituted with the purified lactose carrier. This inhibition may thus be the basis for the well-documented phenomenon of inducer exclusion.
Keywords: inducer exclusion, lactose carrier, phosphotransferase system, reconstitution, regulation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Beyreuther K., Sobek H. M., Stüber K., Hengstenberg W. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: factor IIIlac, a trimeric phospho-carrier protein that also acts as a phase transfer catalyst. Biochemistry. 1982 Sep 28;21(20):4867–4873. doi: 10.1021/bi00263a006. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Armstrong J. B. Dierect measurement of the binding of labeled sugars to the lactose permease M protein. J Biol Chem. 1974 Jan 10;249(1):33–37. [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hinkle P. C. Studies of the beta-galactoside transporter in inverted membrane vesicles of Escherichia coli. II. Symmetrical binding of a dansylgalactoside induced by an electrochemical proton gradient and by lactose efflux. J Biol Chem. 1977 Nov 10;252(21):7662–7666. [PubMed] [Google Scholar]
- Maloney P. C., Wilson T. H. Quantitative aspects of active transport by the lactose transport system of Escherichia coli. Biochim Biophys Acta. 1973 Dec 13;330(2):196–205. doi: 10.1016/0005-2736(73)90225-3. [DOI] [PubMed] [Google Scholar]
- Nelson S. O., Scholte B. J., Postma P. W. Phosphoenolpyruvate:sugar phosphotransferase system-mediated regulation of carbohydrate metabolism in Salmonella typhimurium. J Bacteriol. 1982 May;150(2):604–615. doi: 10.1128/jb.150.2.604-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osumi T., Saier M. H., Jr Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1457–1461. doi: 10.1073/pnas.79.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Teather R. M., Simoni R. D., Aichele G., Wilhelm U. Lactose carrier protein of Escherichia coli. Transport and binding of 2'-(N-dansyl)aminoethyl beta-D-thiogalactopyranoside and p-nitrophenyl alpha-d-galactopyranoside. Biochemistry. 1979 Jan 9;18(1):1–11. doi: 10.1021/bi00568a001. [DOI] [PubMed] [Google Scholar]
- Overath P., Wright J. K. Lactose permease and the molecular biology of transport. Hoppe Seylers Z Physiol Chem. 1982 Dec;363(12):1409–1414. doi: 10.1515/bchm2.1982.363.2.1409. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Stimulation of Escherichia coli adenylate cyclase by lactose in strains carrying mutations in lactose permease. Biosci Rep. 1981 Jan;1(1):53–60. doi: 10.1007/BF01115149. [DOI] [PubMed] [Google Scholar]
- Postma P. W. Regulation of sugar transport in Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):261–267. [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. The crr mutation: its effect on repression of enzyme synthesis. J Biol Chem. 1976 Nov 10;251(21):6598–6605. [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Characterization of factor IIIGLc in catabolite repression-resistant (crr) mutants of Salmonella typhimurium. J Bacteriol. 1982 Feb;149(2):576–586. doi: 10.1128/jb.149.2.576-586.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Isolation of IIIGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Salmonella typhimurium. J Bacteriol. 1981 Oct;148(1):257–264. doi: 10.1128/jb.148.1.257-264.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Hamelin O., Schwarz H., Overath P. Functional symmetry of the beta-galactoside carrier in Escherichia coli. Biochim Biophys Acta. 1977 Jun 16;467(3):386–395. doi: 10.1016/0005-2736(77)90316-9. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Riede I., Overath P. Lactose carrier protein of Escherichia coli: interaction with galactosides and protons. Biochemistry. 1981 Oct 27;20(22):6404–6415. doi: 10.1021/bi00525a019. [DOI] [PubMed] [Google Scholar]


