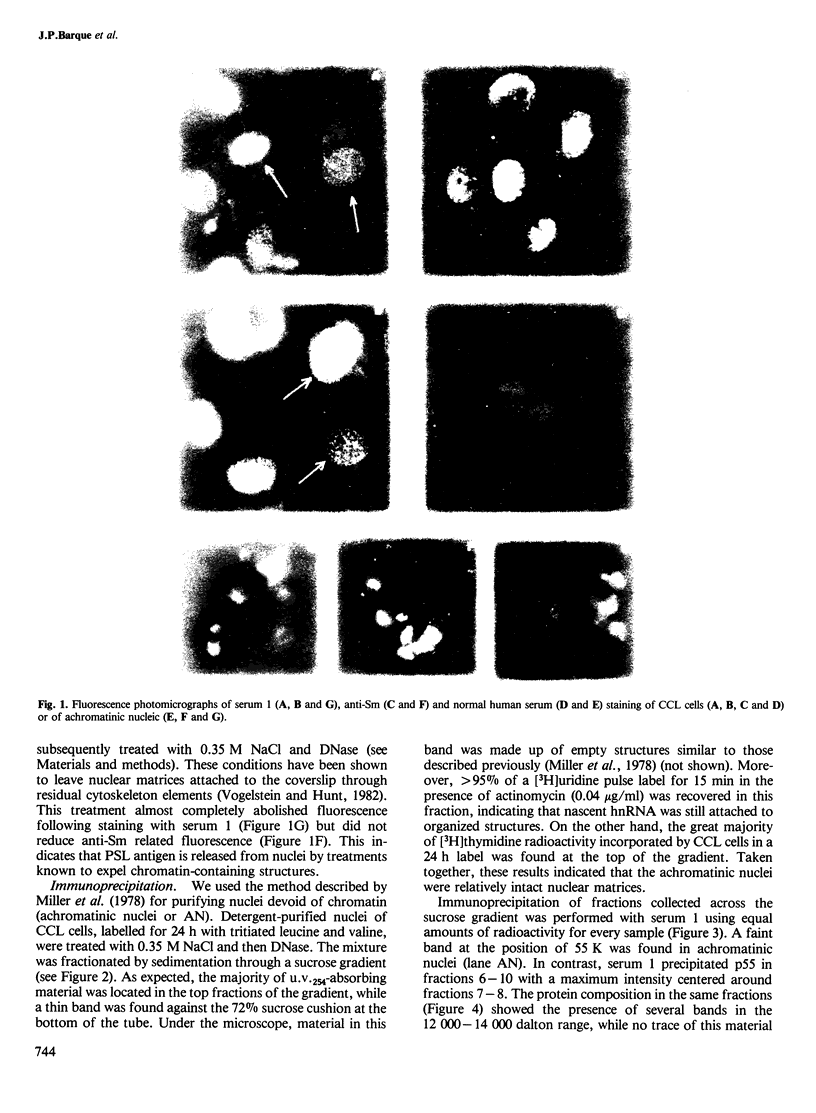
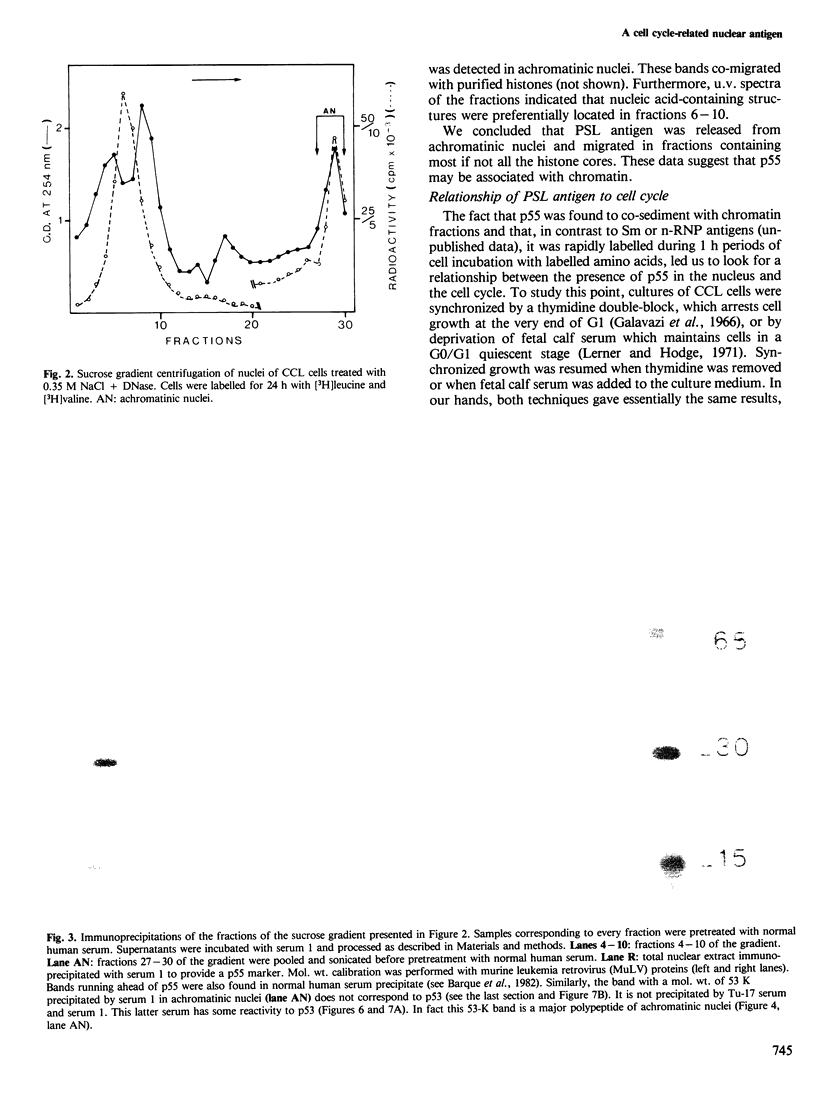
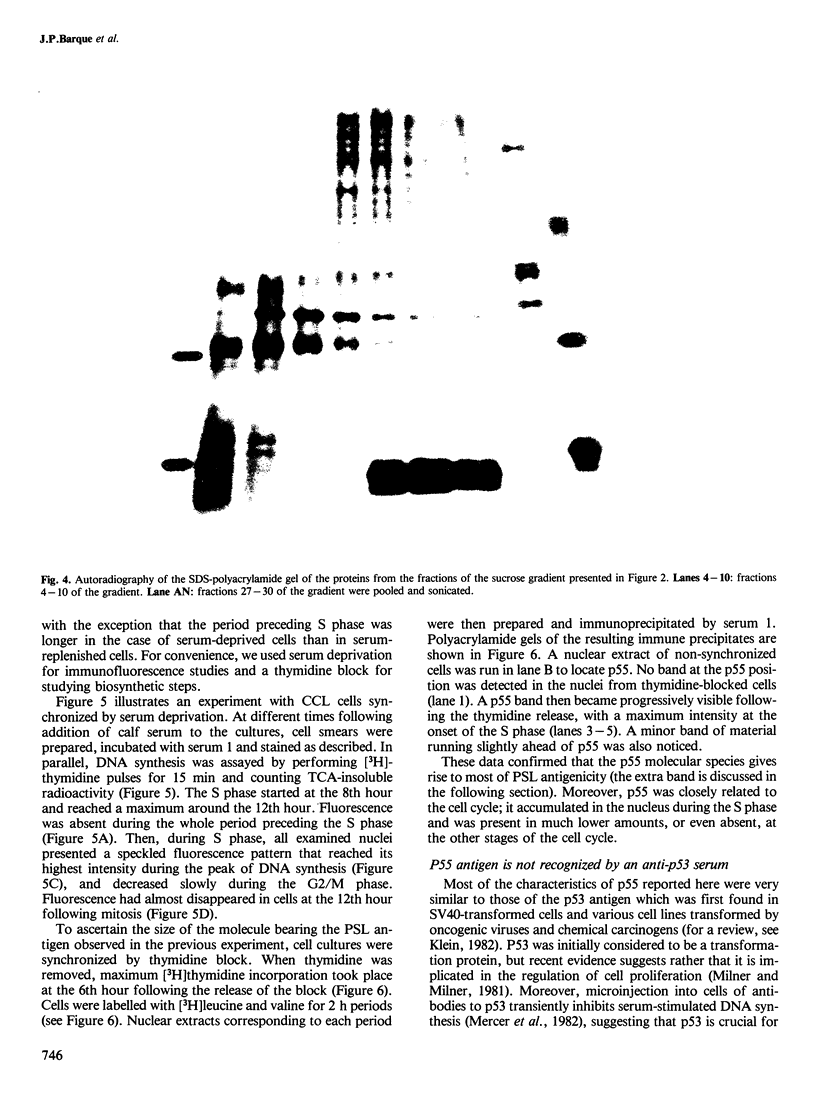
Abstract
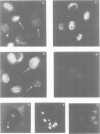
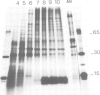
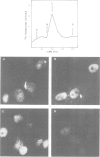
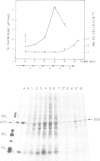
Using a serum from a patient with an autoimmune disease, we have recently described a novel 55 000-dalton antigen (p55) in the nucleus of several animal cells including human ones. This antigen, designated PSL, was not related to the previously defined antigens recognized by sera from patients with systemic rheumatic diseases (Sm, n-RNP, SS-B, Scl-70). We have now found that p55 is associated with chromatin structures as it is released from the nucleus of mink cell fibroblasts by saline + DNase treatments. Analysis by sucrose gradient centrifugation of the nuclear material released in these conditions indicated that p55 co-migrated with core histones. Meanwhile, p55 was absent from the residual nuclear matrices (achromatinic nuclei). Localization of p55 in synchronized cells was performed by indirect immunofluorescence and immunoprecipitation. P55 appeared to accumulate in the nucleus during the S phase. Finally, it was not recognized by an anti-SV40 tumor serum that specifically precipitated the protein p53, which has been recently related to cell proliferation. Thus, PSL an p53, although apparently not antigenically related, appear to be implicated in the same step of the cell cycle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph K. W., Phelps J. P. Role of non-histones in chromosome structure. Cell cycle variations in protein synthesis. J Biol Chem. 1982 Aug 10;257(15):9086–9092. [PubMed] [Google Scholar]
- Barque J. P., Yeni P., Peraudeau L., Danon F., Larsen C. J. Nuclear ribonucleoproteins recognized by human antinuclear antibodies in retrovirus-infected cells. Biochem Biophys Res Commun. 1981 Mar 16;99(1):284–291. doi: 10.1016/0006-291x(81)91743-5. [DOI] [PubMed] [Google Scholar]
- Barque J. P., Yéni P., Péraudeau L., Signoret Y., Danon F., Larsen C. J. Un nouvel antigène nucléaire mis en évidence á l'aide d'auto-anticorps humains. C R Seances Acad Sci III. 1982 Mar 22;294(12):563–566. [PubMed] [Google Scholar]
- Benchimol S., Pim D., Crawford L. Radioimmunoassay of the cellular protein p53 in mouse and human cell lines. EMBO J. 1982;1(9):1055–1062. doi: 10.1002/j.1460-2075.1982.tb01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Lienhard S., Parisot R. F. Intracellular transport of microinjected 5S and small nuclear RNAs. Nature. 1982 Feb 18;295(5850):572–577. doi: 10.1038/295572a0. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Jay G., DeLeo A. B., Khoury G., Old L. J. p53 transformation-related protein: detection by monoclonal antibody in mouse and human cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1695–1699. doi: 10.1073/pnas.78.3.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galavazi G., Schenk H., Bootsma D. Synchronization of mammalian cells in vitro by inhibition of the DNA synthesis. I. Optimal conditions. Exp Cell Res. 1966 Feb;41(2):428–437. doi: 10.1016/s0014-4827(66)80149-0. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lenk R. P., Maizel J. V., Jr, Crouch R. J. Expression of two late adenovirus genes is altered by introducing antibodies against ribonucleoprotein into living HeLa cells. Eur J Biochem. 1982 Jan;121(3):475–482. doi: 10.1111/j.1432-1033.1982.tb05812.x. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Hodge L. D. Gene expression in synchronized lymphocytes: studies on the control of synthesis of immunoglobulin polypeptides. J Cell Physiol. 1971 Apr;77(2):265–276. doi: 10.1002/jcp.1040770215. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Sri Widada J., Brunel C. Particles containing small molecular weight nuclear RNAs (snRNPs). Structure and possible functions. Mol Biol Rep. 1981 May 22;7(1-3):41–45. doi: 10.1007/BF00778731. [DOI] [PubMed] [Google Scholar]
- Luka J., Jörnvall H., Klein G. Purification and biochemical characterization of the Epstein-Barr virus-determined nuclear antigen and an associated protein with a 53,000-dalton subunit. J Virol. 1980 Sep;35(3):592–602. doi: 10.1128/jvi.35.3.592-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray A. J., Carroll A. R., Dahi S., Naxakis G., Sadaie M. R., Wallis C. M., Jing T. The composition of the nuclear antigens Sm and RNP of human rheumatic and connective tissue diseases and the relevance of their autoantibodies as probes for RNA processing mechanisms. FEBS Lett. 1982 May 17;141(2):139–147. doi: 10.1016/0014-5793(82)80033-1. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and ribonucleoprotein complexes containing HnRNA. J Cell Biol. 1978 Mar;76(3):675–691. doi: 10.1083/jcb.76.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Hunt B. F. A subset of small nuclear ribonucleoprotein particle antigens is a component of the nuclear matrix. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1224–1232. doi: 10.1016/0006-291x(82)91099-3. [DOI] [PubMed] [Google Scholar]
- Werner D., Zimmermann H. P., Rauterberg E., Spalinger J. Antibodies to the most tightly bound proteins in eukaryotic DNA. Exp Cell Res. 1981 May;133(1):149–157. doi: 10.1016/0014-4827(81)90365-7. [DOI] [PubMed] [Google Scholar]
- Yang V. W., Lerner M. R., Steitz J. A., Flint S. J. A small nuclear ribonucleoprotein is required for splicing of adenoviral early RNA sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1371–1375. doi: 10.1073/pnas.78.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G., Penman S. Subnuclear particles containing a small nuclear RNA and heterogeneous nuclear RNA. J Mol Biol. 1981 Jan 25;145(3):501–523. doi: 10.1016/0022-2836(81)90542-8. [DOI] [PubMed] [Google Scholar]