Abstract
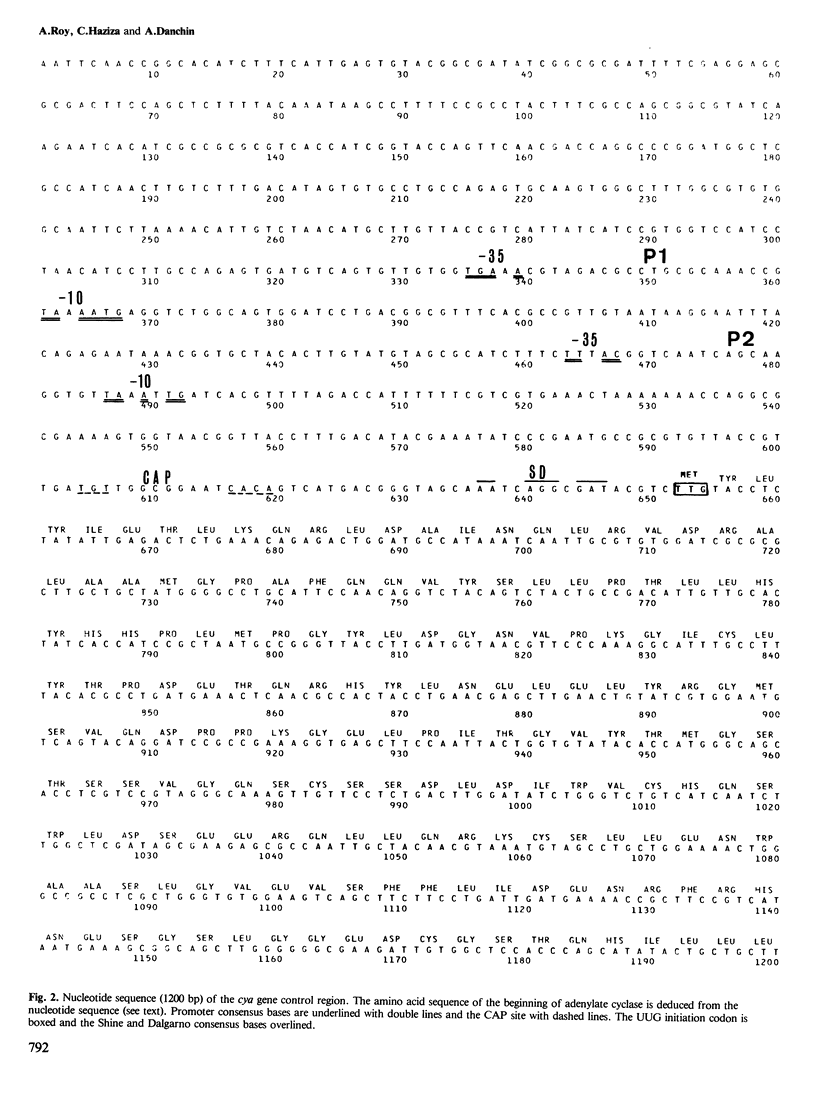
The regulatory region of the cya gene from Escherichia coli has been characterized by nucleotide sequence analysis and genetic approaches. Two promoters, P1 and P2, organized in that order with respect to the beginning of the cya open reading frame, were identified. Using cya-lac operon and protein fusions, it was possible to show that both promoters are active in vivo. P1 activity seemed sensitive to catabolite repression whereas activity of the stronger promoter, P2, did not respond to inhibition by glucose. No effect of cAMP or its receptor, catabolite activator protein (CAP), could be found although the DNA sequence reveals a consensus CAP site downstream of P2. The 548 nucleotides situated at the 3' end of the sequence carry an open reading frame which can tentatively be assigned to the beginning of adenylate cyclase. Among noteworthy features of the corresponding sequence are an UUG codon as the putative start site of cyclase, and a long hydrophobic stretch of amino acids resembling leader peptides in secreted or membrane proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankaitis V. A., Bassford P. J., Jr Regulation of adenylate cyclase synthesis in Escherichia coli: studies with cya-lac operon and protein fusion strains. J Bacteriol. 1982 Sep;151(3):1346–1357. doi: 10.1128/jb.151.3.1346-1357.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Botsford J. L., Drexler M. The cyclic 3',5'-adenosine monophosphate receptor protein and regulation of cyclic 3',5'-adenosine monophosphate synthesis in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):47–56. doi: 10.1007/BF00270375. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenais J., Richaud C., Ronceray J., Cherest H., Surdin-Kerjan Y., Patte J. C. Construction of hybrid plasmids containing the lysA gene of Escherichia coli: studies of expression in Escherichia coli and Saccharomyces cerevisiae. Mol Gen Genet. 1981;182(3):456–461. doi: 10.1007/BF00293935. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Sullivan P., Cunningham C., Hader P., Kofoid E. C., Neilson T. Effect of bases contiguous to AUG on translation initiation. J Biol Chem. 1982 Jul 25;257(14):8228–8232. [PubMed] [Google Scholar]
- Greenlee D. V., Andreasen T. J., Storm D. R. Calcium-independent stimulation of Bordetella pertussis adenylate cyclase by calmodulin. Biochemistry. 1982 May 25;21(11):2759–2764. doi: 10.1021/bi00540a028. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Isolation and characterization of an Escherichia coli mutant affected in the regulation of adenylate cyclase. J Bacteriol. 1981 Dec;148(3):753–761. doi: 10.1128/jb.148.3.753-761.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiso N., Blazy B. Regulatory aspects of the cyclic AMP receptor protein in Escherichia coli K-12. Biochem Biophys Res Commun. 1980 May 14;94(1):278–283. doi: 10.1016/s0006-291x(80)80217-8. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecek J., Náprstek J., Dobrová Z., Jiresová M., Spízek J. Characterization of adenylate cyclase from Escherichia coli. Folia Microbiol (Praha) 1980;25(5):361–368. doi: 10.1007/BF02876688. [DOI] [PubMed] [Google Scholar]
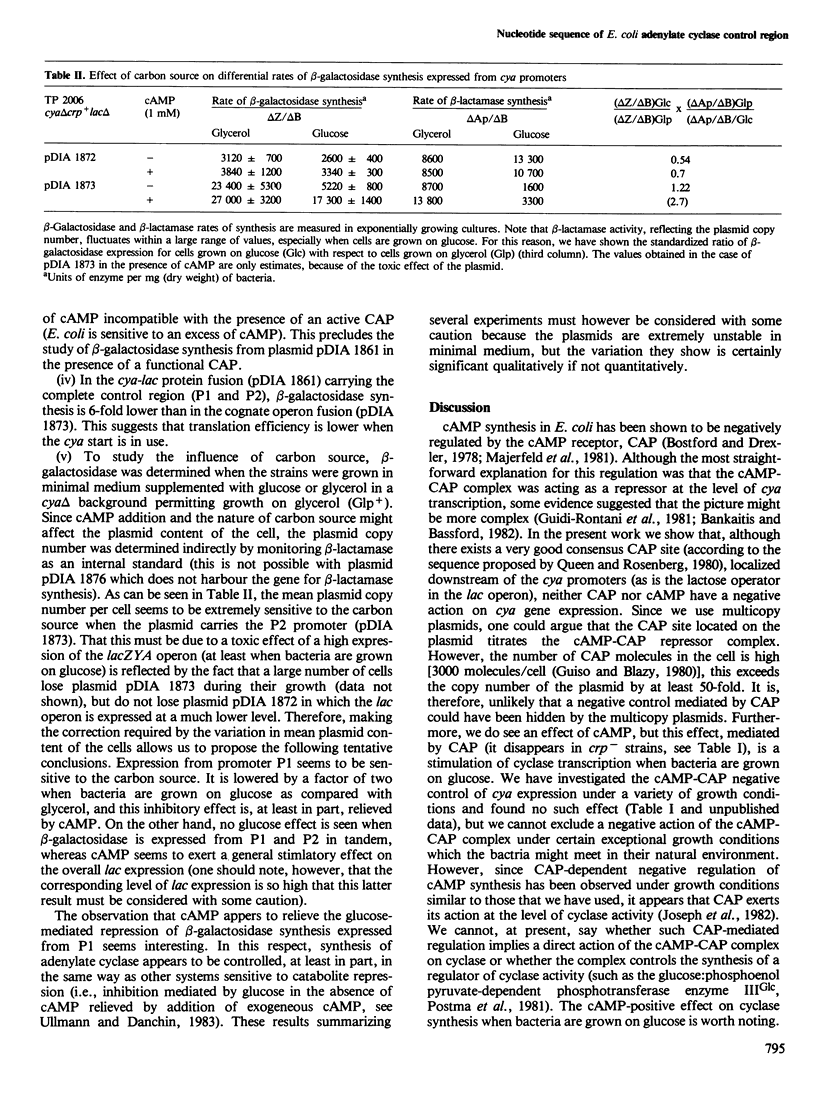
- Joseph E., Bernsley C., Guiso N., Ullmann A. Multiple regulation of the activity of adenylate cyclase in Escherichia coli. Mol Gen Genet. 1982;185(2):262–268. doi: 10.1007/BF00330796. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerfeld I. H., Miller D., Spitz E., Rickenberg H. V. Regulation of the synthesis of adenylate cyclase in Escherichia coli by the cAMP -- cAMP receptor protein complex. Mol Gen Genet. 1981;181(4):470–475. doi: 10.1007/BF00428738. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Schuitema A., Kwa C. Regulation of methyl beta-galactoside permease activity in pts and crr mutants of Salmonella typhimurium. Mol Gen Genet. 1981;181(4):448–453. doi: 10.1007/BF00428734. [DOI] [PubMed] [Google Scholar]
- Poulis M. I., Shaw D. C., Campbell H. D., Young I. G. In vitro synthesis of the respiratory NADH dehydrogenase of Escherichia coli. Role of UUG as initiation codon. Biochemistry. 1981 Jul 7;20(14):4178–4185. doi: 10.1021/bi00517a035. [DOI] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. A promoter of pBR322 activated by cAMP receptor protein. Nucleic Acids Res. 1981 Jul 24;9(14):3365–3377. doi: 10.1093/nar/9.14.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
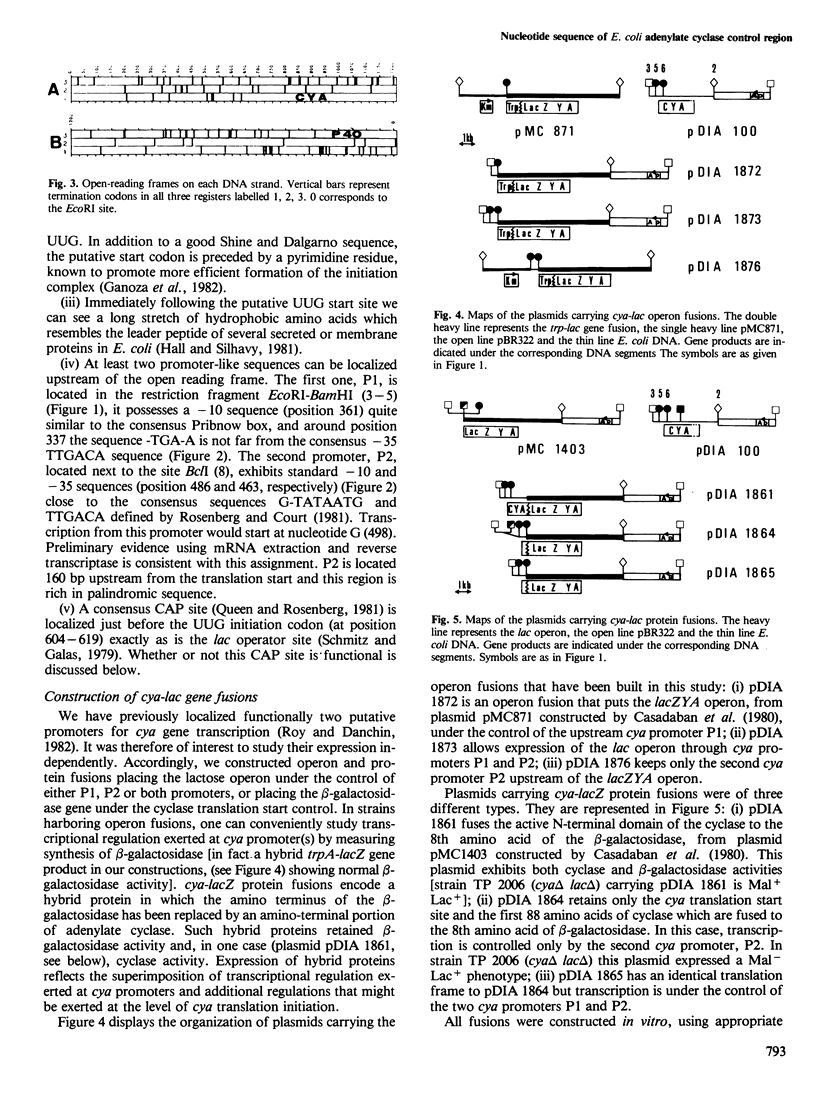
- Roy A., Danchin A. The cya locus of Escherichia coli K12: organization and gene products. Mol Gen Genet. 1982;188(3):465–471. doi: 10.1007/BF00330050. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L., Ehrenfeucht A. Use of the 'Perceptron' algorithm to distinguish translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2997–3011. doi: 10.1093/nar/10.9.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber I. T., Takio K., Titani K., Steitz T. A. The cAMP-binding domains of the regulatory subunit of cAMP-dependent protein kinase and the catabolite gene activator protein are homologous. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7679–7683. doi: 10.1073/pnas.79.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welply J. K., Fowler A. V., Beckwith J. R., Zabin I. Positions of early nonsense and deletion mutations in lacZ. J Bacteriol. 1980 May;142(2):732–734. doi: 10.1128/jb.142.2.732-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]