Abstract
The olivary pretectal nucleus (OPT) is a midbrain structure that receives reciprocal bilateral retinal projections, is involved in the pupillary light reflex, and connects reciprocally with the intergeniculate leaflet (IGL), a retinorecipient brain region that mediates behavioral responses to light pulses (i.e., masking) in diurnal Nile grass rats. Here, we lesioned the OPT and evaluated behavioral responses in grass rats to various lighting conditions, as well as their anxiety-like responses to light exposure. While control grass rats remained diurnal, grass rats with OPT lesions exhibited a more night-active pattern under 12h:12h light-dark (LD) conditions. However, when placed in constant darkness, OPT lesioned grass rats became more active during their subjective day, suggesting that an exaggerated masking response to light may be responsible for the effect of OPT lesions on locomotor activity in LD. To test this hypothesis, we presented dark and light pulses to controls and grass rats with OPT lesions; controls increased their activity in response to light, whereas those with OPT lesions significantly increased activity in response to darkness. Further, when placed in a 7-hr ultradian LD cycle, animals with OPT lesions were more active during darkness than controls. OPT lesions also abolished the pupillary light reflex, but did not affect anxiety-like behaviors. Finally, in animals with OPT lesions, light did not induce Fos expression in the ventrolateral geniculate nucleus, as it did in controls. Altogether, these results suggest that masking responses to light and darkness are dependent upon nuclei within the subcortical visual shell in grass rats.
Keywords: olivary pretectal nucleus, circadian, masking, light, diurnality, behaviour
Introduction
Light influences behavior and physiology in mammals by entraining circadian rhythms (Daan and Pittendrigh, 1976a,b), as well as through acute inhibition or stimulation of the animals’ activity, a process called masking (Redlin, 2001). Masking responses enable animals to respond in adaptive ways to changes in illumination that may not be anticipated by the circadian system. Although there has been substantial progress elucidating the mechanisms responsible for the workings of the circadian system in nocturnal species, less is known about the mechanisms that support the diurnal profile of activity of many mammalian species (Smale et al., 2003). We recently showed that the intergeniculate leaflet (IGL), a direct retinorecipient region in the thalamus, is critical for the display of normal activity patterns of diurnal Nile grass rats (Arvicanthis niloticus) (Gall et al., 2013). Specifically, IGL lesions reversed the activity patterns of these animals, such that they became nocturnal through both circadian mechanisms and masking. The IGL receives direct inputs from melanopsin-containing retinal ganglion cells that are intrinsically photosensitive (ipRGCs; (Chen et al., 2011) and encode retinal luminance levels (Allen et al., 2014;). The IGL has reciprocal connections with the olivary pretectal nucleus (OPT) (Klooster et al., 1995, Moore et al., 2000), which also receives inputs from ipRGCs (Hattar et al., 2006). Thus, we hypothesized that together with the IGL, the OPT is part of a neural system that in diurnal mammals shapes the distribution of activity across the light-dark (LD) cycle, as well as responses to pulses of light and darkness.
There is ample evidence that OPT neurons function as luminance detectors and that the OPT mediates the pupillary light reflex (Trejo and Cicerone, 1984; Gamlin et al., 1995; Gamlin and Clarke, 1995; Szkudlarek et al., 2012). In laboratory rats, it also plays a role in the triggering of rapid eye movement (REM) sleep in response to the shift from light to darkness (Miller et al., 1998, Miller et al., 1999), thus suggesting a more comprehensive role for the OPT in mediating responses to changes in illumination. Other than its role mediating the pupillary light reflex (Gamlin et al., 1995), very little is known about the role of the OPT in diurnal species. We recently showed that it exhibits light-induced Fos expression in intact grass rats (Gall et al., 2014), and that this response is reversed by IGL lesions that also reverse the behavioral responses of grass rats to light pulses (Gall et al., 2014). These results suggest that reciprocal connections between the IGL and OPT may play a key role in modulating the direction of behavioral responses to light in grass rats.
Here, we evaluated the contribution of the OPT to entrainment and masking responses to light in the diurnal grass rat. We first tested the hypothesis that in diurnal grass rats, the OPT is involved in regulating the daily distribution of activity, as well as masking responses to different exposures to light. To that end, we monitored general activity in controls and in animals with bilateral OPT lesions under standard 12:12 LD conditions, under constant darkness (DD), under an ultradian LD cycle, and following 2-h dark and light pulses during the subjective day and night, respectively. We then tested the hypothesis that the OPT is involved in the pupillary light reflex in grass rats, as it is the case in other species (Trejo and Cicerone, 1984, Clarke and Ikeda, 1985a, b, Young and Lund, 1994). If OPT lesions indeed abolish the pupillary light reflex in grass rats, then excessive light exposure due to a dilated pupil may be anxiogenic to these animals, and result in behavioral inhibition when the lights are on. Therefore, we assessed the extent to which OPT lesions affect anxiety-like behaviors using an open field test and a light-dark preference box. Finally, using light-induced Fos expression as a marker for neural activation, we examined the pathways through which the OPT might influence masking effects of light in grass rats. Altogether, our results suggest that masking responses to light and darkness and the pupillary light reflex are dependent upon the OPT in a diurnal species.
Experimental Procedures
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee of Michigan State University. All efforts were made to minimize the number of animals used in these experiments.
Subjects
A total of 32 adult female grass rats (Arvicanthis niloticus) from a breeding colony maintained at Michigan State University were used in the experiments in this study. Virgin female grass rats do not exhibit an estrous cycle when singly housed (McElhinny, 1996). We used female grass rats in this study in order to standardize the sex, and also so that we could make comparisons to our previous lesion studies in grass rats, which used only females (Gall et al., 2013, Gall et al., 2014, Gall et al., 2016). All animals were singly housed in plexiglass cages (34 × 28 × 17 cm); food (PMI Nutrition Prolab RMH 2000, Brentwood, MO) and water were available ad libitum. In order to monitor general locomotor activity in the grass rats, infrared motion detectors (IRs; Visonic, Tel Aviv, Israel) were placed on top of each cage. These IRs allowed us to collect behavioral general locomotor activity data every 5-min using the VitalView Program (Mini-Mitter, Bend, OR, USA). Animals were maintained in a 12:12 LD cycle with lights on at Zeitgeber time 0 (ZT 0); (light intensities were always either 300 lux during the light phase or less than 5 lux during the dark phase). General activity was monitored in 12:12 LD for at least 2 weeks prior to surgery.
Surgery
Animals were anesthetized using isoflurane anesthesia (maintained at 1.5–2.5%), and were placed in a stereotaxic apparatus (Stoelting Co., Wood Dale, Illinois, USA). The animals were injected with an anesthetic (Lidocaine, Elkins-Sin, Inc., Cherry Hill, NJ; 0.2 cc, s.c.), and artificial tears (Butler Company, Columbus, OH) were applied over the eyes. After shaving the animals’ scalps, an incision was made and two small holes were drilled in the skull. An insulated tungsten microelectrode (A-M systems, Model 5770, 500 µm Diameter, Sequim, WA, USA) was used to make bilateral electrolytic lesions in experimental animals to destroy tissue within the OPT using the following coordinates, with the tooth bar set at 0: AP: −0.12 cm from bregma, ML: ±0.05 cm from midline, and DV: −0.28 cm ventral to the meningeal surface. A lesion-making device was used (Stoelting, Model 58040, Chicago, Illinois) to deliver 2.0 mA of DC current for 15 s. The incision was closed with autoclips, and antiseptic ointment (Nolvasan, Fort Dodge, IA; 1% chlorihexidine acetate in 10% sterile alcohol base) was applied. In sham animals, the same procedure was performed, except current was not applied. All animals received an injection of sterile saline (Abbott, s.c.; 1.0 mL) and ketoprofen (Fort Dodge Animal Health; s.c.; 0.2 mL dose) immediately following surgery. 24 and 48 h after surgery, all animals were given meloxicam (Boehringer Ingelheim Vetmedica Inc., St. Joseph, MO, USA; 20 µL) infused in a piece of apple (Castillo-Ruiz and Nunez, 2011). Animals recovered in their home cages for at least 1 hour on a warming pad, and were then moved back to the recording room where their activity was continually monitored under standard 12:12 LD conditions. After 7–10 days post-surgery, the autoclips were removed. 24 grass rats received bilateral electrolytic lesions aimed at the OPT, and 20 of them survived (83.3% survival rate). 8 grass rats received sham surgery as controls (100% survival rate).
Light treatment procedure
General activity of all animals was recorded as animals were exposed to the following sequence of lighting conditions: (1) 12:12 LD for at least 4 weeks after surgery, (2) DD for 2 weeks, (3) 12:12 LD for approximately 4 weeks (at which point all animals were entrained). (4) At this point, two-hour masking pulses were administered in 3-day cycles, as described previously (Shuboni et al., 2012): day 1 = maintenance day (12:12 LD); day 2 = baseline day (12:12 LD); and day 3 = pulse day (2 hour light pulse in the dark phase of a 12:12 LD cycle given at ZT 14, 18, or 22, or a 2 hour dark pulse in the light phase of a 12:12 LD cycle given at ZT 2, 6, or 10). All animals received light pulses first and then received dark pulses, with the time of pulse randomized. Cage changes and food and water replenishments were only done on the maintenance days. (5) Following light and dark pulses, animals were placed in an ultradian light-dark cycle, with 3.5 hours of light followed by 3.5 hours of darkness, presented repeatedly for 9 days. (6) Finally, animals were placed back in 12:12 LD conditions.
Assessment of the Pupillary Light Reflex
One major role of the OPT in other species (Trejo and Cicerone, 1984, Clarke and Ikeda, 1985a, b, Young and Lund, 1994) is to mediate the pupillary light reflex. To determine if this is also the case in grass rats, we removed animals from their cages individually, and recorded pupil size in darkness (5 lux of red light) and when an LED fiber optic light (1,000 lux; Unitron Gooseneck Illuminator, Model #16116, Commack, New York) was shone directly on each eye. Pupillary size was recorded with a digital recording device (Swann Communications, SWDVK-8325N8-US, Santa Fe Springs, California) connected to a video camera (WV-BP310; Panasonic, Tokyo, Japan) equipped with a low-light lens (WV-LA408C3; Panasonic, Tokyo, Japan). The distance of the pupil from the camera was standardized at 6 inches. ImageJ was used to calculate the area of the pupil when grass rats were in darkness and following at least 3 seconds of light.
Assessment of Anxiety-like Behavior
We found that OPT lesions did affect pupillary reflexes in grass rats, which raised the possibility that this might have led to heightened anxiety that changed their activity levels during light exposure. We used two standard procedures to assess independently this possibility between ZT4 and ZT8. The first was an open field test, which was done with a plexiglass box (80 cm length × 80 cm width × 60 cm height) that had tape placed on the floor to create 25 squares (5 squares long and 5 squares wide). 16 of these squares were along the outer edge of the box (i.e., “outer squares”), while 9 squares were on the inside of the box (i.e., “inner squares”). At the beginning of the test, grass rats were placed in a corner square with their heads facing the center of the box; the order of the corner was counterbalanced and the illumination level was 300 lux). Grass rats explored the box freely for 5 minutes, while behavior was recorded via a video camera (WV-BP310; Panasonic, Tokyo, Japan) located directly above the box. Behaviors were scored manually for the number of visits to outer squares and inner squares. The box was cleaned with 70% ethanol between each test.
The second procedure we used to assess anxiety-like behaviors involved placing animals into a light-dark box with two separate compartments: a dark one (40 cm length × 80 cm wide × 60 cm high; less than 5 lux) and a light one (40 cm length × 80 cm wide × 60 cm high; 300 lux). The two parts of the box were connected by an opening (15 cm high × 10 cm wide), so that the grass rat could freely enter either compartment. Grass rats were placed in the light portion of the box facing the opening to the dark chamber. Animals were allowed to move freely between the light and dark compartments for 5 minutes while behavior was being recorded by a video camera (WV-BP310; Panasonic, Tokyo, Japan) located above the box. For both the open field test and the light-dark box test, the experimenter was not in the room while behavior was being recorded. Behaviors were manually scored for amount of time spent in the light side and dark side of the box. An animal was considered to be in a compartment if its entire body (excluding the tail) was located within that compartment. At the end of the 5 min recording, animals were placed back in their home cages and the entire box was cleaned with 70% ethanol.
Histology
One week after assessing anxiety-like behavior, half of the animals were given a 2-h light pulse at ZT 22 and sacrificed for histological analysis, while the other half of the animals was sacrificed in darkness at the same time point. Intraperitoneal (i.p.) injections of sodium pentobarbital were used to euthanize the grass rats (Ovation Pharmaceutical, Deerfield, IL, USA) and they were perfused transcardially with 0.01 M phosphate-buffered saline (PBS), pH 7.2, followed by 4% paraformaldehyde (Sigma-Aldrich; PFA) with 75 mM lysine (Sigma-Aldrich) and 10 mM sodium periodate (Sigma-Aldrich) in 0.1 M PB (PLP). We then removed the brains from the animals, and post-fixed them in PLP for 4 hours, then transferred to a 20% sucrose solution (J.T. Baker, Phillipsburg, NJ, USA), and stored at 4°C for at least 48 hours. Brains were sectioned using a cryostat (in 30 µm coronal sections) and organized into three alternate series. One series was stained for Nissl using thionin to determine the size of the lesions. Complete OPT lesions were identified by a lack of Nissl stained cells within the OPT. Partial OPT lesions were identified by partial damage to the OPT bilaterally, and unilateral OPT lesions by an intact OPT on one side and complete damage to the other side.
A second series was processed using immunohistochemical procedures for labeling of Fos. Here, we followed protocols previously established in the grass rat brain (Castillo-Ruiz and Nunez, 2011; Schwartz et al., 2011). Fos antibody raised in rabbit (1:25,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used for this series of brain sections, and processed with avidin-biotin-immunoperoxidase using DAB (3,3'-diaminobenzidine) as the chromogen enhanced with 2.5% nickel ammonium sulfate (Sigma-Aldrich), as described previously (Schwartz et al., 2011). Gelatin-coated glass slides were used to mount the tissue, which was then dehydrated with ethyl alcohol, and coverslipped with dibutyl phthalate xylene (DPX; Sigma-Aldrich, St. Louis, MO, USA).
Cell counting
To assess numbers of Fos-immunoreactive (Fos-ir) cells, observers blind to experimental condition selected at least 3 sections containing each brain region of interest, including the suprachiasmatic nucleus (SCN), intergeniculate leaflet (IGL), and ventrolateral geniculate nucleus (VLG). Sections were examined under a light microscope (Leitz, Laborlux S, Wetzlar, Germany) and photomicrographs were taken. The SCN, IGL, and VLG were outlined using thionin counterstained tissue. ImageJ was used to calculate the number of Fos positive cells for each outlined area. The number of Fos-positive cells for each region were counted bilaterally and divided by 2 to obtain an average of unilateral Fos-ir counts.
Statistical Analysis
Activity data collected in 5-min bins from VitalView software (Mini-Mitter, Bend, OR, USA) were opened in Microsoft Excel for statistical analyses. Actograms were created using Actiview software (Version 1.3, Starr Life Sciences Corp., Oakmont, Pennsylvania) to view general activity patterns in various lighting conditions. All statistical analyses were done using SPSS (Version 21, IBM Corp., Armonk, NY, USA) and for all analyses, differences were considered significant when p < 0.05. To adjust for multiple comparisons, a Bonferroni correction was applied to evaluate the effect on behavior at ZTs 1–24 (see below). Effect sizes were calculated using Cohen’s d to complement the tests for statistical significance. To test for normality, the Shapiro-Wilk test was used. All means are presented with their standard errors (SEM).
12:12 LD Condition
Activity patterns in 12:12 LD conditions presurgery were analyzed by averaging the total activity counts during the day and night for 5 days for each animal immediately prior to surgery; activity patterns postsurgery were analyzed by averaging the total activity counts also for 5 days, starting at 14 days after surgery. These data were collapsed over the day (ZTs 0–12) and at night (ZTs 12–24) in shams and OPT lesioned animals prior to and after surgery. These data were analyzed with a 2-way ANOVA with a 2×2 factorial design [surgical condition (sham vs. OPT lesion; between-group factor) × illumination phase (day vs. night; within-group factor)]. These data were also used to generate day-night ratios by dividing the total average activity during lights on by the total average activity during lights-off. For these day-night ratios, the nonparametric Wilcoxon signed-rank test was used to analyze differences between presurgery and postsurgery day-night ratios within each surgical condition, as these data violated the assumption of normality. For nonparametric data, medians along with their median absolute deviation (MAD) are presented. Effect size for day-night ratios was calculated by taking the z-score and dividing it by the square root of N (r = Z/(√N)). Finally, a 2-way ANOVA was used to evaluate the effect of surgical condition on behavior at specific ZTs using a 2×24 factorial design [surgical condition (sham vs. OPT lesion; between-group factor) × ZT; within-group factor)]; a significant interaction was followed by an independent samples t-test to evaluate the effect of surgical condition within each ZT.
DD Condition
In DD, behavioral patterns were analyzed by averaging the total activity during the subjective day and night in circadian time for 5 days for each animal immediately following placement into DD. A 2-way ANOVA was used to analyze these data with a 2 × 2 factorial design [surgical condition (sham vs. OPT lesion; between-group factor) × circadian phase (i.e., subjective day vs. subjective night; within-group factor)]. Significant interactions were followed up by analyzing the simple main effects of time-of-day (subjective day vs. subjective night) using paired t-tests.
Masking: Light and Dark Pulses in a LD Cycle
To evaluate the effects of dark and light pulses, activity counts from the same 2-h interval from the pulse day and preceding baseline day in 12:12 LD were compared. A 3-way ANOVA was used to analyze the data separately for light pulses and dark pulses, with a 2 × 2 × 3 factorial design [surgical condition (sham vs. OPT lesion) × lighting condition (baseline vs. 2-h pulse) × time of day (ZT2, 6, 10 for DPs; ZT14, 18, 22 for LPs); surgical condition as the between-group factor and both lighting condition and time of day as the within-group factors]. Then, shams and OPT lesioned animals were analyzed separately using a two-way repeated measures ANOVA to assess within-subject effects of lighting condition (darkness vs. light pulse; light vs. dark pulse) and time-of-day (ZT2, 6, 10, 14, 18, and 22). Significant interactions were followed up by evaluating the simple main effects of lighting condition using paired-samples t-tests.
Masking in an Ultradian Cycle
Behavior following an ultradian LD cycle (3.5 h light followed by 3.5 h of darkness continually for 9 days) was analyzed by comparing the total amount of activity during the lights-on phase to the total activity during the lights-off (dark) phase. A 2-way ANOVA was used to assess within-subjects effects of lighting condition (light vs. dark) and the between-subjects effects of surgical condition (sham vs. OPT lesion). Detection off a significant interaction was followed by evaluating the simple main effects of lighting condition and surgical condition using paired-samples t-test and independent-samples t-tests, respectively.
Pupillary Reflexes
To assess pupillary responses, the percent change in the size of the pupil was calculated by taking the area of the pupil in the light and subtracting the area of the pupil in the darkness, dividing this by the area of the pupil in the darkness, and multiplying by 100. This was done separately for each eye, and the percent change was averaged across both eyes. A one-way ANOVA was used to assess the percentage change in the size of the pupil between shams and lesioned grass rats.
Behavior in Open Field and Light-dark Box
For the open field test (OFT), a one-way ANOVA was used to assess the number of visits to the inner squares, time spent in the center of the OFT (sec), and total distance traveled (cm) in shams vs. grass rats with OPT lesions. For the light-dark box, a one-way ANOVA was used to assess the number of visits to the dark chamber and the time (seconds) spent in the darkness in shams vs. grass rats with OPT lesions.
Fos-ir
A two-way ANOVA was used to analyze the Fos data with a 2 × 2 factorial design [surgical condition (sham vs. IGL lesion) × lighting condition (darkness vs. light pulse)]. Significant interactions were followed by evaluation of simple main effects using independent sample t-tests.
Results
Histology
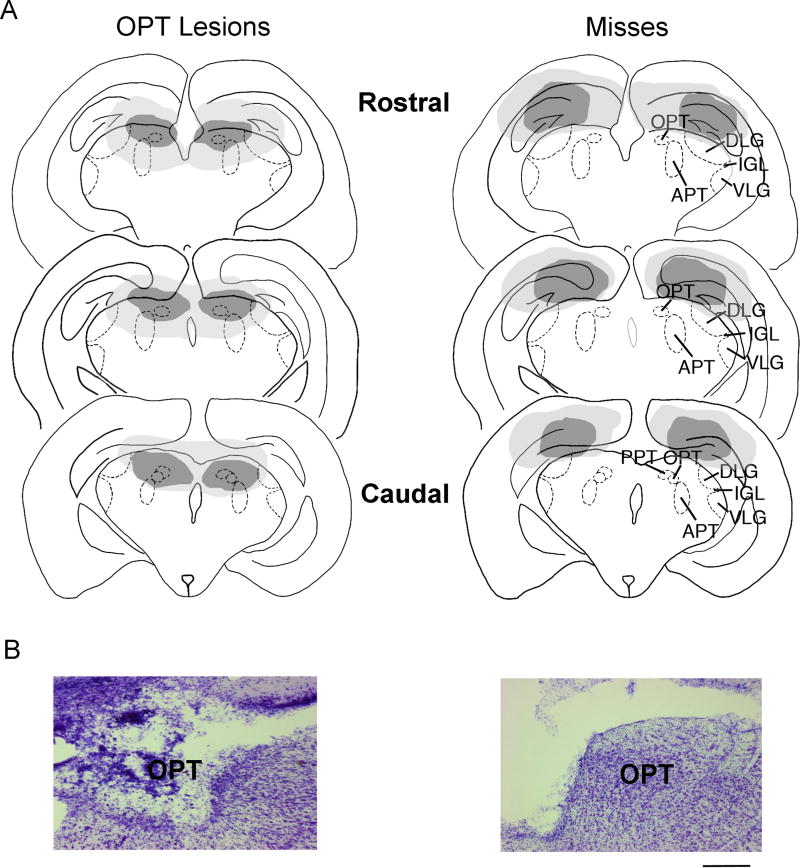
Based upon histology, three groups of grass rats were identified: those with (1) complete, bilateral OPT lesions (n = 15), (2) unilateral, partial, or no damage to the OPT (n = 5), and (3) shams (n = 8). The brain regions that were damaged following electrolytic lesions aimed for the OPT are depicted in Figure 1. The smallest bilateral lesion of the OPT also damaged parts of the anterior pretectal nucleus (APT), posterior pretectal nucleus (PPT), and hippocampus. The largest bilateral lesion of the OPT damaged parts of the dorsolateral geniculate nucleus (DLG), APT, PPT, and hippocampus. Lesions that missed the OPT had damage to the hippocampus and DLG, but the OPT remained intact bilaterally.
Figure 1. Histology from grass rats with bilateral OPT lesions (left column) and misses (right column).
(A) The smallest (dark gray) and largest (light gray) bilateral OPT lesions (left column) and misses (right column). (B) Thionin-stained coronal sections from representative animals with bilateral OPT lesions (left) and a miss that had damage to the hippocampus (right). Scale bar represents 100 um. Abbreviations: APT: anterior pretectal nucleus; DLG: dorsolateral geniculate nucleus; IGL: intergeniculate leaflet; OPT: olivary pretectal nucleus; PPT: posterior pretectal nucleus; VLG: ventrolateral geniculate.
12:12 LD Condition
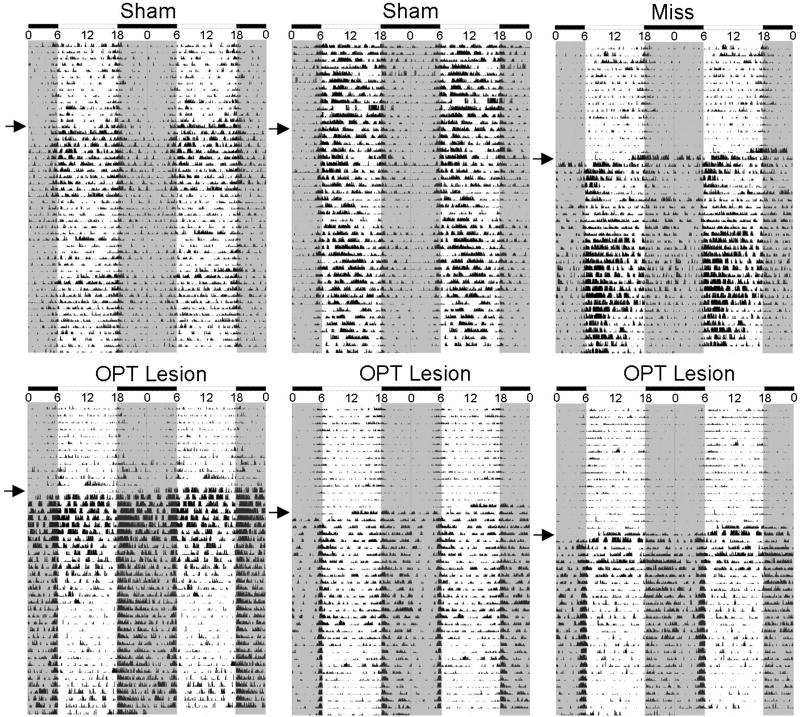
Figure 2 displays actograms from six individual animals in 12:12 LD before and after surgeries (2 shams, 1 “miss” – i.e., an animal with hippocampal damage (but not brainstem damage), and 3 grass rats with bilateral OPT lesions). Prior to surgery in 12:12 LD conditions, all grass rats displayed activity rhythms with significantly more activity occurring during the light phase (i.e., all animals were diurnal; Figure 2). Sham surgery resulted in transient increases in activity in some animals (Figure 2, upper left), but no permanent effect in the basic diurnal pattern of the animals. Lesions that missed the OPT, but that damaged the hippocampus, permanently increased activity without affecting the preferential display of activity during the light phase (Figure 2, upper right). All OPT hits included some damage to the hippocampus that was accompanied by an increase in activity, but the lesions also changed the distribution of activity, such that it occurred predominantly at night with peaks at the beginning and end of the dark phase (Figure 2 bottom panels).
Figure 2. Effects of lesions on behavior in 12:12 LD conditions before and after surgery.
Double-plotted actograms from 2 representative shams, 1 miss with damage to the hippocampus, and 3 grass rats with bilateral damage to the OPT in 12:12 LD conditions before surgery and after surgery. The arrow represents the day of surgery. Gray shaded areas indicate darkness, and white indicates period in which light is present. Although the immediate effects of the OPT lesions on total activity differed across individual cases, the common long term effect of the lesions was an increase in the proportion of daily activity displayed during the dark phase.
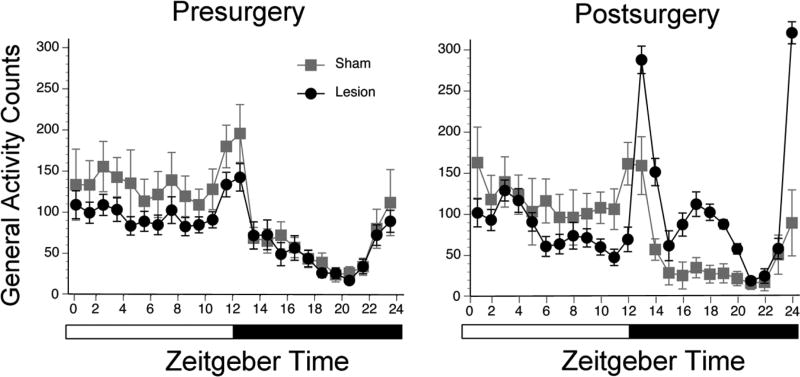
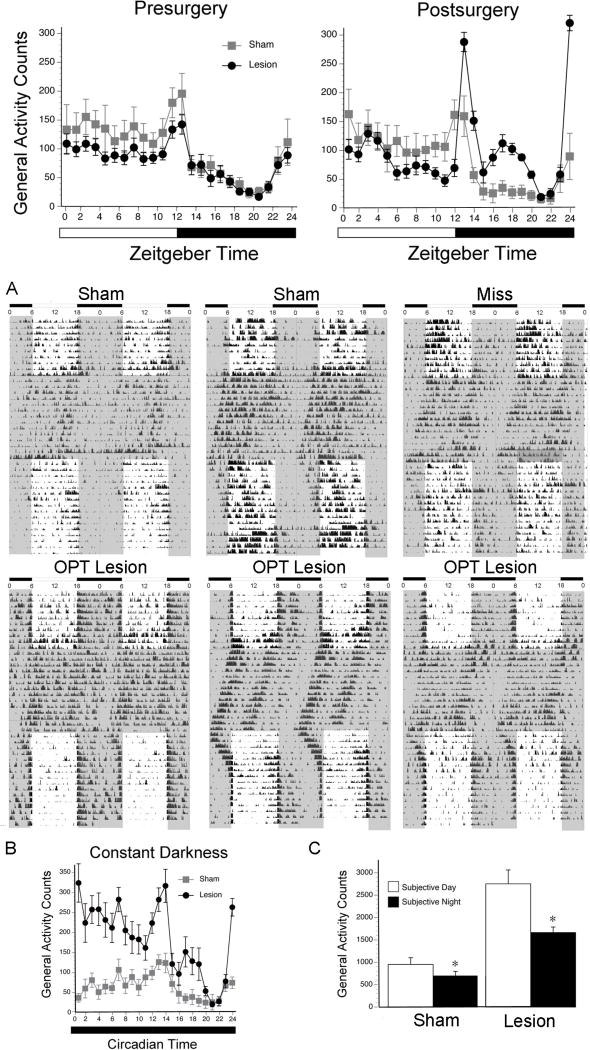
Analyses of the group data based on 5 days before surgery and 5 days starting when the activity patterns stabilized (i.e., two weeks after surgery) confirmed the conclusions drawn from inspection of the actograms. Prior to surgery (Figure 3, left panel), an ANOVA revealed a main effect of illumination phase (F1,21 = 43.0, p < .001), but no main effect of surgical group and no interaction between the two variables (ps > .05). In contrast, following surgery (Figure 3, right panel), there was a significant interaction between illumination phase and surgical condition for general activity counts (F1,21 = 4.9, p = .039). Postsurgical activity averaged over the light phase did not differ between the two surgical groups (t21 = 0.6, p > .05, d = .27), but over the dark phase it was significantly higher for the OPT lesion group (t21 = 5.2, p < .001, d = 2.2). Nonparametric analyses revealed a significant decrease in day-night ratios presurgery vs. postsurgery for OPT lesioned animals (Median ± MADs: 1.8 +/− 0.4 presurgery vs. 0.8 ± 0.5 postsurgery; p < .005; effect size: r = .68), but not for shams (Median ± MADs: 2.1 ± 0.4 vs. 2.6 ± 0.6 p > .05; effect size: r = .02), indicating that the OPT lesioned animals became significantly more night active.
Figure 3. Quantitative analyses of behavior in LD conditions before and after surgery in shams and grass rats with OPT lesions.
Patterns of activity in LD during the day and night are depicted for shams (in gray) and grass rats with complete OPT lesions (in black). Means are presented along with standard error of the mean (SEM) for each value.
An analysis considering the 24 individual ZTs found a significant interaction between ZT (i.e., ZT 1–24) and surgical condition (i.e., sham vs. lesion) (F23,483 = 4.9, p < .001). Using the very conservative Bonferroni correction, the total activity of the OPT lesion group was significantly higher than that of shams only at ZTs 17, 18, and 24 (t21s > 3.4, ps < .003). However, for most of the night (except ZTs 15, 21, 22, and 23), the activity counts were higher for the OPT lesion group with no overlap of the SEMs (effect sizes (ds) ranged from .89 to 2.8), and the opposite was true for group comparisons during the day at ZTs 1, 6, 10, and 11 (ds ranged from .35 to .74).
DD Condition
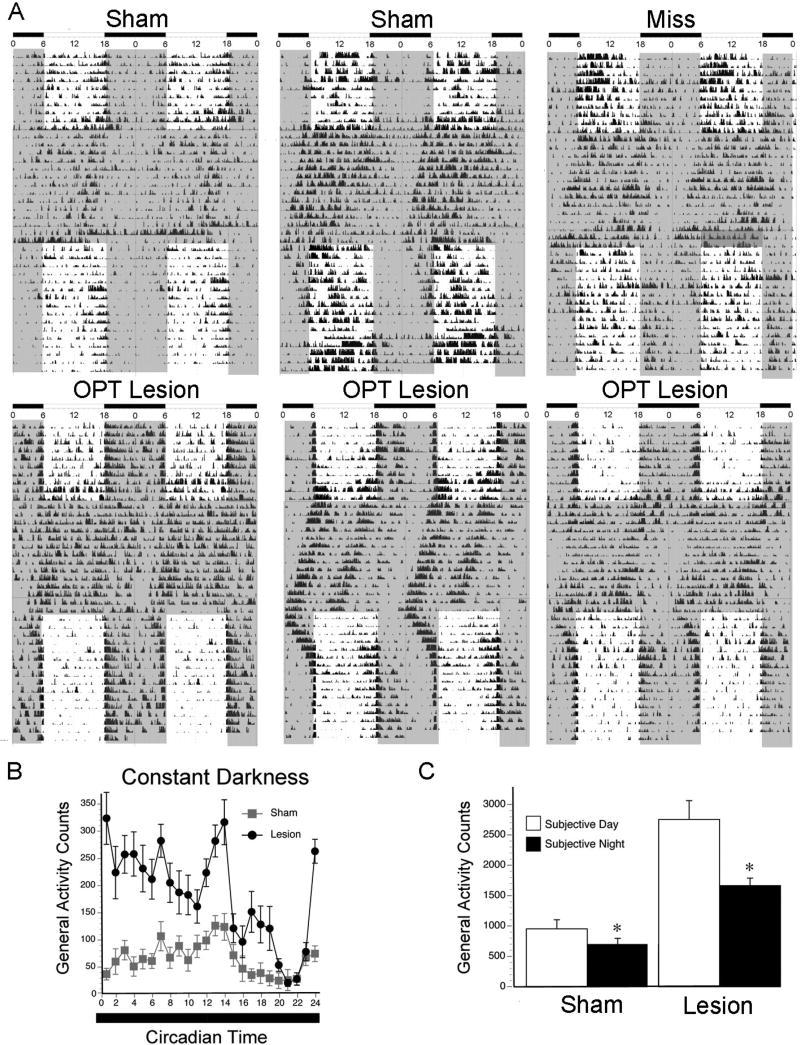
Figure 4A depicts representative actograms of 2 shams, 1 miss, and 3 grass rats with bilateral OPT lesions in 12:12 LD, 2 weeks of DD, and reentrainment to 12:12 LD. All animals showed free running rhythms in DD. As illustrated by the top panels of Figure 4A, during the free-run of animals without OPT lesions the majority of the activity occurred during the subjective day, which was a continuation of the pattern of the entrained rhythm. In sharp contrast, and as illustrated by the lower panels of Figure 4A, when placed in DD, the animals with OPT lesions showed an immediate increase in activity during the subjective day. When transferred back to LD, all animals returned to their pre-DD profiles, with OPT lesioned animals showing again a preferential display of activity at night.
Figure 4. Effects of lesions on behavior in constant darkness.
(A) Double-plotted actograms from 2 representative shams, 1 miss with damage to the hippocampus, and 3 grass rats with bilateral damage to the OPT in 12:12 LD conditions, 2 weeks of constant darkness, and finally 12:12 LD conditions (same animals as presented in Figure 2). Gray shaded areas indicate darkness, and white indicates period in which light is present. Note that in DD all animals showed free running rhythms, but that different from the other animals those with OPT lesions showed enhanced activity during the subjective day as defined by the previous light phase under LD.(B) Patterns of activity in DD are depicted for shams (in gray) and grass rats with complete OPT lesions (in black). (C) Average total activity counts during the day and night are depicted for shams and lesions during the subjective day and subjective night. Means are presented along with standard error of the mean (SEM) for each value. All animals were significantly more active during the subjective day as compared to the subjective night. * = significant difference between day and night (p < .05).
For both the shams and the OPT lesioned animals, analyses of the group data (Figure 4B) revealed a significant main effect of circadian phase (i.e., subjective day vs subjective night) (F1,21 = 10.2, p = .004) and a main effect of surgical condition (F1,21 = 28.2, p < .001), but no significant interaction (p > .05). Thus, both groups were significantly more active during their subjective days (Figure 4C) than their subjective nights in DD [shams: (t7 = 3.2, p = .014, d = .74); OPT group: (t14 = 3.6, p = .003, d = .90)]. A comparison of the postsurgical patterns shown by the OPT lesion group in LD (Figure 3, right panel) and DD (Figure 4B) indicates that for this group, the primary effect of DD was to preferentially increase activity during the natural active phase of this diurnal species. Therefore, activity was increased after removal of the light during the subjective day, suggesting that after OPT lesions the animals suppress activity in response to light. That hypothesis was directly tested by exposing the animals to light and dark pulses and by using an ultradian illumination paradigm.
Masking: Light and Dark Pulses in a LD Cycle
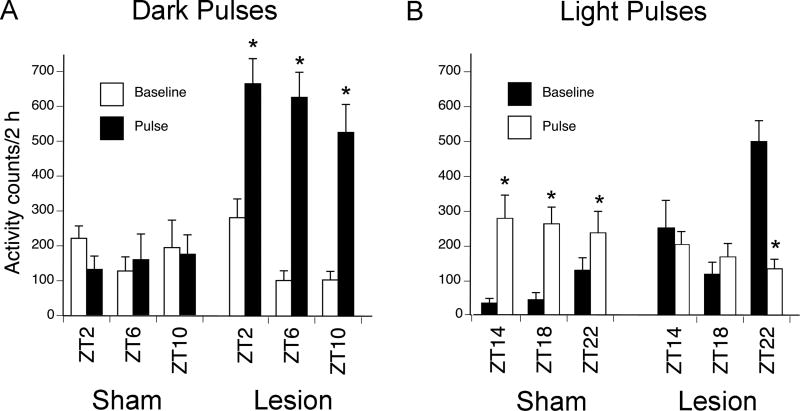
Figure 5 illustrates the effects on general activity of dark pulses presented during the light phase (A) and of light pulses presented during the dark phase (B) to shams and OPT lesioned animals. For shams given dark pulses (Figure 5A), an ANOVA revealed no main effect of time, no main effect of pulse and no interaction between time of day (i.e., ZT 2, 6, and 10) and lighting condition (i.e., baseline vs. dark pulse) (ps > .05). For grass rats with OPT lesions given dark pulses (Figure 5A), an ANOVA revealed a significant main effect of time (F2,28 = 6.8, p = .004), and a significant main effect of pulse (F1,14 = 55.1, p < .001), but no interaction between the two variables (p > .05). Thus, dark pulses elevated activity to a level about three times higher than baseline in the OPT animals at all ZTs tested; the same pulses had no effects on the activity of the shams.
Figure 5. Masking responses to dark and light pulses in shams and grass rats with OPT lesions.
Masking response to dark pulses at ZTs 2, 6, and 10 (A) and light pulses at ZTs 14, 18, and 22 (B) in shams and grass rats with OPT lesions. Means +/−SEM. Grass rats with OPT lesions exhibited significantly more activity in response to dark pulses at all ZTs, whereas shams did not respond to dark pulses at any ZT. Grass rats with OPT lesions exhibited significantly less activity in response to light pulse at ZT22, whereas shams exhibited significantly more activity to light pulses at all ZTs. * = significant difference in activity counts between baseline and 2-h pulse (p < .05).
For shams given light pulses (Figure 5B), an ANOVA revealed a significant main effect of pulse (F1,7 = 22.6, p = .002), but no significant main effect of time and no interaction between the two variables (ps > .05); light pulses resulted in increased activity at all ZTs tested. For grass rats with OPT lesions given light pulses (Figure 5B), a significant interaction between time of day and lighting condition was found (F2,28 = 17.6, p = .000). Paired t-tests revealed that behavior was significantly decreased to about one third of baseline following a light pulse, but only at ZT22 (t14 = 7.5, p < .001; d = 1.9) a sampling time associated with a high baseline of activity. Thus, light pulses delivered at night reliably increased activity in the shams, but had either no effect (ZTs 14 and 18) or an effect in the opposite direction (ZT 22) in the OPT animals.
Masking in an Ultradian Cycle
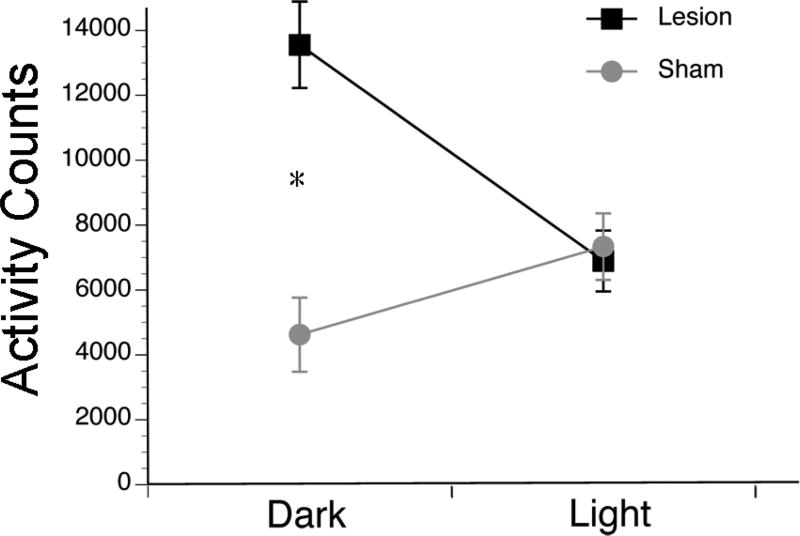
The effects of a 7-h ultradian light-dark cycle (3.5 h of light followed by 3.5 h of darkness repeated for 9 days) were assessed using a 2-way mixed ANOVA for lighting condition (within-subjects; light vs. dark) and surgical condition (between-subjects; sham vs. OPT lesion). The ANOVA revealed a significant interaction between lighting condition and surgical condition (F1,21 = 35.1, p < .001; Figure 6). As shown in Figure 6, compared to activity during the dark intervals, light stimulated activity in the shams and had the opposite effect for OPT lesioned animals; an independent-samples t-test revealed that OPT lesioned animals were more active in the darkness as compared to the light (t15 = 6.2, p < .001, d = 1.6), whereas shams were more active in the light as compared to the dark (t7 = 3.7, p = .008, d = 1.3). The two groups differed significantly in the dark (t21 = 4.4, p < .001, d = 2.1), but not in the light (p > .05).
Figure 6. An ultradian cycle of light and darkness affected behavior differentially in shams and grass rats with OPT lesions.
Shams (gray lines) exhibited significantly more activity in light as compared to darkness, whereas OPT lesioned grass rats (black lines) exhibited significantly more activity in darkness as compared to light. * = significant difference between shams and lesioned grass rats in the darkness (p < .05).
Pupillary Reflexes
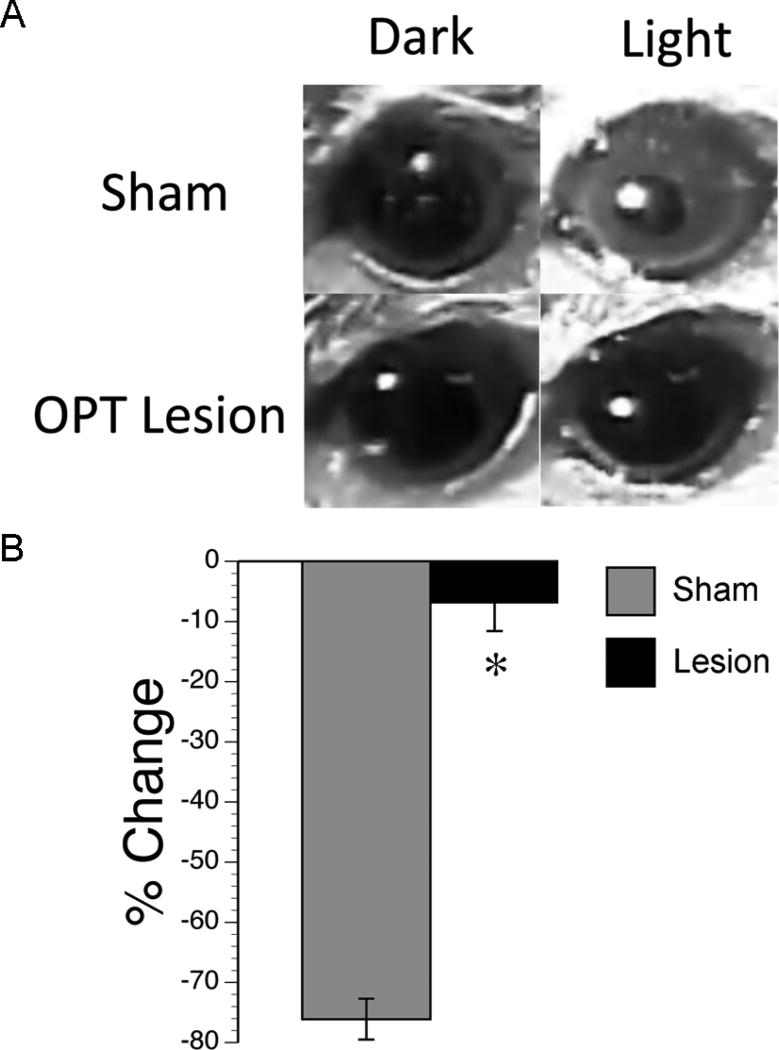
A one-way ANOVA revealed a significant difference in pupillary reflex between shams and grass rats with OPT lesions (F1,21 = 493.3, p < .001). Specifically, whereas the pupils of shams reduced their size by over 70% following a pulse of light, the pupils of grass rats with OPT lesions only reduced their size by less than 10% following a pulse of light (Figure 7). Importantly, for OPT lesioned animals, this reduction in size was not significantly different from zero (t14 = 1.9, p > .05), but it was for shams (t7 = 67.1, p < .0001). Finally, no difference was found for pupillary size in the darkness between shams and grass rats with OPT lesions (F1,21 = .756, p > .05). Therefore, light stimulation caused the pupils to constrict in shams, but not in OPT lesioned grass rats.
Figure 7. Pupillary reflex was abolished following OPT lesions.
(A) Photomicrographs of a representative sham (top row) and grass rat with bilateral OPT lesions (bottom row) in darkness (left column) and following a 3-s pulse of light (right column). Note the constriction of the pupil in light in shams, and the lack of change in pupil size in grass rats with OPT lesions following a pulse of light. (B) Percent change in size of the pupil in shams (gray bars) and grass rats with OPT lesions (black bars). Means +/− SEM. * = significant difference between shams and lesions (p < .05).
Behavior in Open Field and Light-dark Box
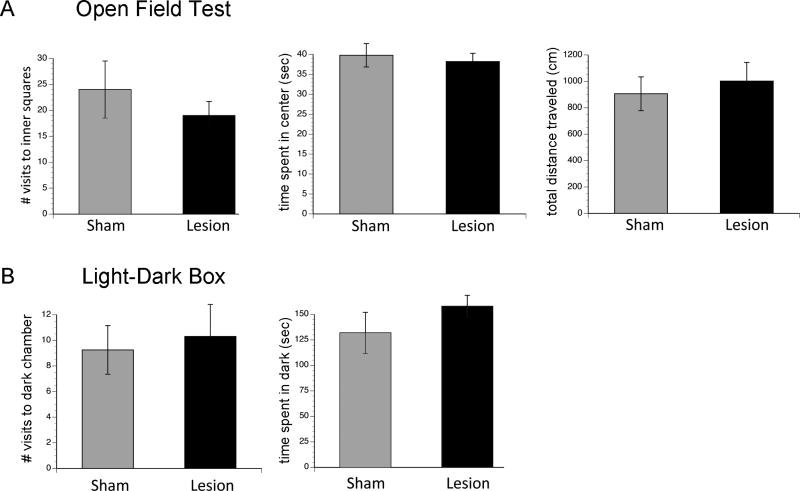
To test the hypothesis that after OPT lesions light becomes aversive, we compared animals with OPT lesions and shams in two tasks: bright open field and light-dark boxes. As shown in Figure 8, a one-way ANOVA revealed no significant difference between shams and lesioned animals in terms of the number of visits to the inner squares of the open field test the total amount of time spent in the center of the OFT, or for total distance traveled in the OFT (ps > .05). In addition, a one-way ANOVA revealed no significant difference between shams and lesioned animals in terms of the amount of time spent in the darkness in a light-dark box (ps > .05).
Figure 8. Behavior in an open field test and light-dark box was not affected by OPT lesions.
For the open field test (A), no significant difference was found in the number of visits to the inner squares, time spent in the center (sec), or total distance traveled (cm) in shams as compared to grass rats with OPT lesions. For a light-dark box (B), no significant difference was found in the number of visits to the dark chamber or time spent in darkness (seconds) in shams as compared to grass rats with OPT lesions. Means +/− SEM. Gray bars represent shams; black bars represent grass rats with OPT lesions.
Fos-ir
Nuclear staining for Fos (Figure 9) was evident in all the areas examined as seen previously in grass rats (Gall et al., 2014). Figure 9 depicts the distribution and number of Fos-positive cells in the SCN (Figure 9A), IGL (Figure 9B), and VLG (Figure 9C) in shams and grass rats with bilateral OPT lesions that were either sacrificed following a 2-h pulse of light or in complete darkness.
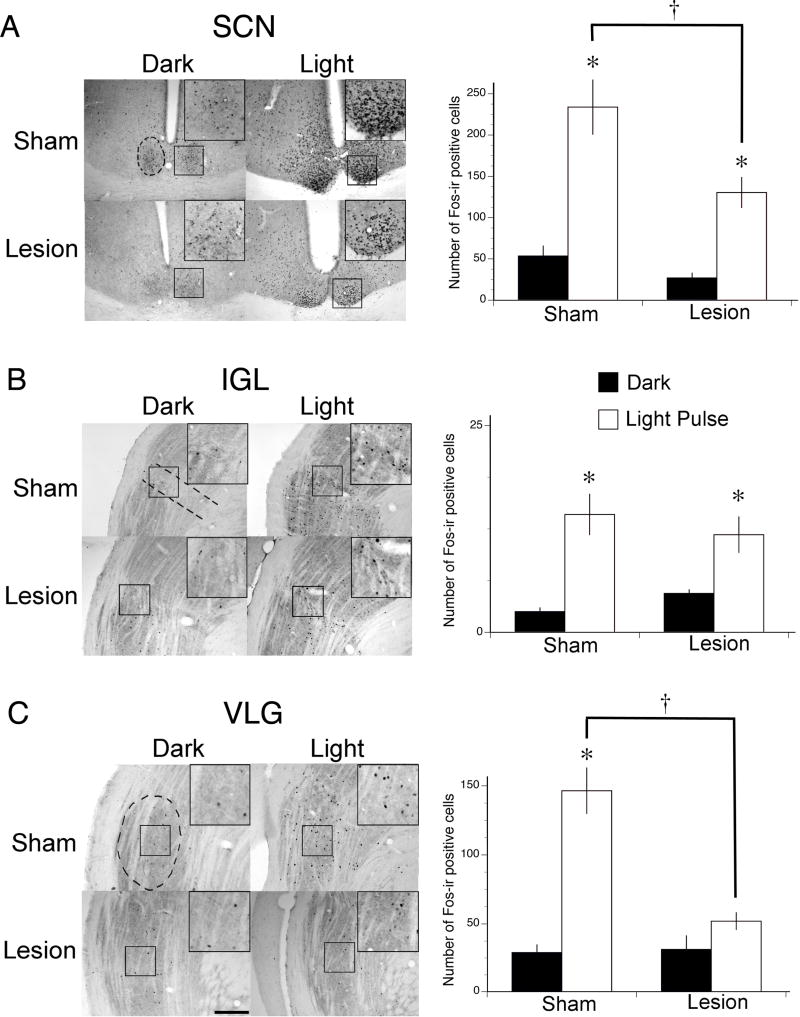
Figure 9. OPT lesions did not affect the Fos response to light in the SCN or IGL, but resulted in a lack of light responsiveness in the VLG.
(Left column) Representative photomicrographs of Fos induction in the (A) SCN, (B) IGL, and (C) VLG in shams and OPT lesioned grass rats on a control night vs. following a LP. Fos staining was intense following a light pulse in all 3 brain regions for shams, but only for the SCN and IGL in OPT lesioned grass rats. Dotted lines indicate the borders of each respective nucleus. The insets represent magnified views of Fos cells within the borders of each nucleus. (Right column) Mean number of Fos-ir positive cells in the (A) SCN, (B) IGL, and (C) VLG in shams and OPT lesioned grass rats sacrificed in the dark (black bars) as compared to those sacrificed following a LP (white bars). * = significantly different from control night. † = significant difference between sham and lesion. Abbreviations: SCN: suprachiasmatic nucleus; IGL: intergeniculate nucleus; VLG: ventrolateral geniculate nucleus. Scale bar represents 400 µm.
Fos-ir in the SCN
As shown in Figure 9A, for the SCN, the ANOVA revealed a significant main effect of lighting condition (F1,19 = 54.1, p < .0001), and a significant main effect of surgical condition (F1,19 = 11.4, p < .005), but no interaction between the conditions (p > .05). For shams, there was significantly higher Fos expression in the SCN (F1,6 = 25.5, p = .002, d = 3.6) in animals sacrificed following a light pulse as compared to those sacrificed in complete darkness. For grass rats with OPT lesions, there was also significantly higher Fos expression in the SCN (F1,13 = 24.2, p < .001, d = 2.6) in those animals sacrificed following a light pulse as compared to those sacrificed in complete darkness.
Fos-ir in the IGL
As shown in Figure 9B, for the IGL, the ANOVA revealed a significant main effect of lighting condition (F1,19 = 59.0, p < .0001), but no significant effect of surgical condition (p = .949) and no significant interaction (p > .05). For shams there was significantly higher Fos expression in the IGL (F1,6 = 21.9, p = .003, d = 3.3) in animals sacrificed following a light pulse as compared to those sacrificed in complete darkness. For grass rats with OPT lesions there was also significantly higher Fos expression in the IGL (F1,13 = 33.5, p < .001, d = 3.0) in those animals sacrificed following a light pulse as compared to those sacrificed in complete darkness.
Fos-ir in the VLG
As shown in Figure 9C, for the VLG, the ANOVA revealed a significant interaction between lighting condition and surgical condition (F1,19 = 21.7, p < .001). For shams, the ANOVA revealed significantly higher Fos in the VLG in animals sacrificed following a light pulse as compared to those sacrificed in complete darkness (F1,6 = 43.2, p = .001, d = 4.6). For grass rats with OPT lesions, there was no significant difference between those sacrificed following a light pulse as compared to those sacrificed in complete darkness (p > .05). Therefore, the VLG was light responsive in shams, but not significantly so in grass rats with bilateral OPT lesions.
Fos-ir for shams vs. OPT lesioned animals
Comparisons between surgical condition revealed no significant differences between shams and OPT lesioned animals in the darkness for any brain region analyzed (i.e., SCN, IGL, OPT; ps > .05), whereas the SCN and VLG exhibited higher levels of Fos following a light pulse in shams as compared to OPT lesioned animals (ps < .05). Therefore, the SCN and VLG respond differently to light following OPT lesions as compared to shams.
Discussion
Masking and General Activity
Bilateral lesions of the OPT resulted in a significant change in masking responses to light and darkness. Specifically, in grass rats with OPT lesions, light pulses significantly reduced general activity at ZT22, while dark pulses significantly increased general activity at all time points. In contrast, and as expected (Shuboni et al., 2012), light pulses increased general activity at all time points in controls, whereas dark pulses had no effect. In addition, light (as compared to darkness) presented to grass rats with OPT lesions in the context of both a 12:12 LD cycle and a 7-h ultradian cycle (3.5:3.5 LD) suppressed general activity, whereas controls exhibited increased general activity during the light phases (as compared to the dark phases) of these cycles. Finally, darkness presented as acute pulses during the 12:12 LD cycle, in constant darkness, or during the recurrent 3.5 hr dark interval of an ultradian LD cycle significantly increased general activity of lesioned animals, but did not affect it in control animals. Altogether, our results suggest that normal masking responses to light and darkness are dependent upon the OPT in grass rats.
In addition to the clear effects on masking, the lesions increased baseline general activity under some conditions (e.g., in DD), an effect that may be related to damage beyond the OPT. Specifically, most of our lesioned animals had significant damage to the hippocampus, which is associated with increased activity in grass rats (Gall et al., 2013). Here, lesions that destroyed all or part of the hippocampus and spared the OPT resulted in increased general activity without affecting masking (Figure 2, “miss”). Therefore, it is likely that the OPT is critical for the masking effects we observed, whereas damage to the hippocampus increases activity, as others have shown in laboratory rats (Good and Honey, 1997, Bannerman et al., 1999, Godsil et al., 2005). Outside the hippocampus, other areas that received incidental damage, such as the anterior and posterior pretectum, may have also contributed to changes in general activity that we are not able to evaluate from the available data.
Circadian rhythms
Although masking was significantly affected by OPT lesions, circadian control of activity was apparently intact. Importantly, when grass rats with OPT lesions were placed in constant darkness, “diurnality” was restored, as indicated by the increase in activity during the subjective day, thus, revealing the presence of a circadian signal for enhanced display of activity at the appropriate phase for this diurnal species. These results are consistent with those of OPT lesions in nocturnal rats, which produce significant changes in how REM sleep is controlled by changes in illumination, without inducing circadian deficits (Miller et al., 1998, Miller et al., 1999).
OPT lesioned grass rats vs. IGL lesioned grass rats
Interestingly, for masking, the effects of OPT lesions were similar in some ways to those of IGL lesions. Specifically, grass rats with either OPT or IGL lesions exhibited increased general activity in response to dark pulses, and reduced general activity in response to light pulses (Gall et al., 2013). After IGL lesions, Fos expression in the OPT of grass rats is reduced by light, which is the opposite of what is seen in intact controls (Gall et al., 2014). Thus reversing how the OPT responds to light by removing IGL influences, as well as direct damage to the OPT produce similar behavioral effects; both manipulations interfere with the normal masking responses of diurnal grass rats. This suggests that the normal flow of information about light via a pathway that includes the IGL and the OPT is necessary for the maintenance of appropriate masking responses to light in these diurnal grass rats.
Grass rats with OPT or IGL lesions have similar patterns of general activity in 12:12 LD conditions. However, whereas grass rats with IGL lesions maintain their nocturnal pattern in constant darkness, grass rats with OPT lesions exhibit a robust diurnal pattern in constant darkness. Therefore, we suggest that the IGL is critical for normal masking responses to light and darkness, as well as for maintaining the day-active circadian profile of grass rats. The involvement of the IGL in several aspects of circadian regulation has been well documented (Harrington, 1997). In contrast, the OPT appears to be critical for masking responses to light and darkness typical of this diurnal species, but is not involved in circadian regulation. We showed recently that grass rats with SCN lesions become arrhythmic, but can still respond normally to pulses of light (i.e., masking is not affected) (Gall et al., 2016). Therefore, the neural mechanisms underlying masking and circadian effects can be disentangled in these animals, as it is possible to affect one system without affecting the other.
The pupillary light reflex and anxiety-like behavior
OPT lesions in grass rats abolished the pupillary light reflex, as they do in nocturnal rats (Trejo and Cicerone, 1984, Clarke and Ikeda, 1985a, b, Young and Lund, 1994). This raised the possibility that an increase in light traversing the dilated pupils caused the lesioned grass rats to be more anxious, leading to a reduction in activity in the light. We examined this possibility using standard tests of anxiety, which revealed no differences between lesioned and control animals in an open field test and in light-dark box test. Therefore, our data suggest that the observed effects of lesions on masking were not due to heightened anxiety in the presence of light. Furthermore, other studies have shown that abolishing the pupillary light reflex does not affect anxiety-like responses in nocturnal rodents (Kozicz et al., 2011). It is possible that the changes in masking responses, although not mediated by enhanced light-induced anxiety, may nevertheless stem from the loss of the pupillary reflex, rather than by the interruption of a brain circuit that includes the OPT. This possibility could be tested by evaluating masking responses in animals with dilated pupils, but without neural damage.
Light-induced Fos expression
Our data on light-induced Fos expression suggest that the SCN remains light-responsive following lesions of the OPT, however the overall Fos counts are significantly reduced following these lesions. These data suggest that the OPT is likely to connect with the SCN in grass rats, as it does in rats (Mikkelsen and Vrang, 1994). In contrast to the SCN, light-induced Fos expression in the IGL was completely unaffected by OPT lesions. Taken together with earlier observations (Gall et al., 2014), it is evident that the reciprocal anatomical connections that exist between the IGL and the OPT (Morin, 2013) do not result in reciprocal functional effects of lesions to these two regions; IGL lesions reverse the effects of light exposure (i.e., Fos expression) in the OPT (Gall et al., 2014), but light activation of the IGL is not apparently affected by lesions of the OPT (present results). In contrast to the SCN and IGL, the VLG was light-responsive in controls, but not in OPT lesioned animals. The role of the VLG in masking is currently unknown. This nucleus has extensive connections with visual and non-visual brain regions, including the OPT (Conley and Friederich-Ecsy, 1993; Morin and Blanchard, 1998; Livingston and Mustari, 2000) and participates in the control of visuomotor responses (Harrington, 1997). In laboratory rats the VLG is involved in eye blink conditioning using light as the conditioned stimulus, suggesting its importance for receiving light input to mediate associative cerebellar learning (Halverson and Freeman, 2010). In rats, the OPT and VLG show preferential Fos activation after acute light pulses as opposed to chronic exposure to light, a feature consistent with the suggestion that these two brain areas are involved in masking response to light pulses (Prichard et al., 2002). With all of these data taken together, we hypothesize the OPT lesions affect the responsiveness of the VLG, which affects the way grass rats respond to light. Clearly, more work needs to be done to elucidate the mechanisms by which the OPT has profound effects on masking in grass rats.
It is important to note that our effect sizes, especially for Fos in retinorecipient brain regions (e.g., SCN, IGL, and VLG), were quite large, indicating that our findings were very robust. Given the opposing behavioral changes that occur in light and darkness in grass rats, along with the stark behavioral differences between shams and OPT lesioned animals, it is not surprising that our effect sizes were so large.
In nocturnal rodents, ipRGCs send extensive projections to the OPT and VLG (Hattar et al., 2006, Brown et al., 2010, Ecker et al., 2010), whereas in grass rats, these projections are abundant in the OPT, but relatively sparse in the VLG (Langel et al., 2015). Thus, a switch to a diurnal masking profile may be reflected in a more important role for the OPT in providing to the VLG information transduced by the ipRGCs. Based upon these anatomical differences and our present results, we suggest that in grass rats, the VLG receives input from the OPT, and when the OPT is lesioned, the VLG is at least partially deprived of information about light signals that have been transduced by ipRGCs. Therefore, it is possible that the VLG and OPT work together to produce the normal masking response to light in the grass rat. These observations raise the possibility that the effects on masking responses to light seen after IGL lesions in grass rats (Gall et al., 2013) may result from changes in how the OPT responds to light after IGL lesions (Gall et al., 2014), and how that change affects the VLG.
Conclusions
In summary, we have shown that OPT lesions produce an increase in night time activity in grass rats kept in 12:12 LD conditions, and reverse the masking responses to light and darkness at specific time points, but do not affect the circadian pattern of activity when animals are kept in constant conditions. In grass rats, whereas the IGL is involved in both masking and circadian rhythms, the SCN appears to only be involved in circadian rhythmicity (Gall et al., 2016), while the OPT contributes to masking, but not to the circadian regulation of activity. These data raise important questions regarding the relationships between the IGL, SCN, VLG, and OPT, all of which are retinorecipient brain areas (Shuboni et al., 2012). Interconnections the nuclei of the subcortical visual shell may be critical for modulating masking effects of light in grass rats, and perhaps in other diurnal species.
Highlights.
The olivary pretectal nucleus (OPT) receives direct retinal input and is involved in the pupillary light reflex.
We lesioned the OPT in diurnal grass rats and evaluated behavioral responses in grass rats to various lighting conditions.
Controls increased activity to light pulses, whereas OPT lesioned grass rats increased activity to dark pulses.
In grass rats, the OPT and its thalamic connections mediate masking responses to light and darkness.
Acknowledgments
We thank Dorela Shuboni, Jennifer Langel, and Thomas Groves for technical assistance. Supported by a National Science Foundation (NSF) grant (IOS-1051919, to L.S., A.A.N., and L.Y.) and A.J.G. was supported by a National Institutes of Health (NIH) Ruth L. Kirschstein National Research Service Award (NRSA) from the National Institute of Neurological Disorders and Stroke (NINDS) (F32 NS083360-01). We also thank the Hope College Psychology Department for their support of this project, and the Hope College Division of Social Sciences for providing startup funds to AG.
Abbreviations
- IGL
intergeniculate leaflet
- OPT
olivary pretectal nucleus
- CT
circadian time
- ZT
zeitgeber time
- APT
anterior pretectal nucleus
- PPT
posterior pretectal nucleus
- DLG
dorsolateral geniculate nucleus
- VLG
ventrolateral geniculate nucleus;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Allen AE, Storchi R, Martial FP, Petersen RS, Montemurro MA, Brown TM, Lucas RJ. Melanopsin-driven light adaptation in mouse vision. Curr Biol. 2014;24:2481–2490. doi: 10.1016/j.cub.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo MA, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Ruiz A, Nunez AA. Fos expression in arousal and reward area s of the brain in grass rats following induced wakefulness. Physiol Behav. 2011;103:384–392. doi: 10.1016/j.physbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RJ, Ikeda H. Luminance and darkness detectors in the olivary and posterior pretectal nuclei and their relationship to the pupillary light reflex in the rat. I. Studies with steady luminance levels. Exp Brain Res. 1985a;57:224–232. doi: 10.1007/BF00236527. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Ikeda H. Luminance detectors in the olivary pretectal nucleus and their relationship to the pupillary light reflex in the rat. II. Studies using sinusoidal light. Exp Brain Res. 1985b;59:83–90. doi: 10.1007/BF00237669. [DOI] [PubMed] [Google Scholar]
- Conley M, Friederich-Ecsy B. Functional organization of the ventral lateral geniculate complex of the tree shrew (Tupaia belangeri): II. Connections with the cortex, thalamus, and brainstem. J Comp Neurol. 1993;328(1):21–42. doi: 10.1002/cne.903280103. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents: II. The variety of phase response curves. J Comp Physiol. 1976a;106:253–266. [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents: IV. Entrainment: Pacemaker as Clock. J Comp Physiol. 1976b;106:291–331. [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglionc cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Shuboni DD, Yan L, Nunez AA, Smale L. Suprachiasmatic nucleus and subparaventricular zone lesions disrupt circadian rhythmicity but not light-induced masking behavior in Nile grass rats. J Biol Rhythm. 2016;31:170–181. doi: 10.1177/0748730415626251. [DOI] [PubMed] [Google Scholar]
- Gall AJ, Smale L, Yan L, Nunez AA. Lesions of the Intergeniculate Leaflet Lead to a Reorganization in Circadian Regulation and a Reversal in Masking Responses to Photic Stimuli in the Nile Grass Rat. PloS One. 2013;8:e67387. doi: 10.1371/journal.pone.0067387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Yan L, Smale L, Nunez AA. Intergeniculate leaflet lesions result in differential activation of brain regions following the presentation of photic stimuli in Nile grass rats. Neurosci Lett. 2014;579:101–105. doi: 10.1016/j.neulet.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, Clarke RJ. The pupillary light reflex pathway of the primate. J Am Optom Assoc. 1995;66:415–418. [PubMed] [Google Scholar]
- Gamlin PD, Zhang H, Clarke RJ. Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Exp Brain Res. 1995;106(1):169–176. doi: 10.1007/BF00241367. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Stefanacci L, Fanselow MS. Bright light suppresses hyperactivity induced by excitotoxic dorsal hippocampus lesions in the rat. Behav Neurosci. 2005;119:1339–1352. doi: 10.1037/0735-7044.119.5.1339. [DOI] [PubMed] [Google Scholar]
- Good M, Honey RC. Dissociable effects of selective lesions to hippocampal subsystems on exploratory behavior, contextual learning, and spatial learning. Behav Neurosci. 1997;111:487–493. doi: 10.1037//0735-7044.111.3.487. [DOI] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Ventral lateral geniculate input to the me d ial pons in necessary for visual eyeblink conditioning in rats. Learn Memory. 2010;17:80–85. doi: 10.1101/lm.1572710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav R. 1997;21(5):705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Rusak B. Luminance coding properties of intergeniculate leaflet neurons in the golden hamster and the effects of chronic clorgyline. Brain Res. 1991;554(1–2):95–104. doi: 10.1016/0006-8993(91)90176-v. [DOI] [PubMed] [Google Scholar]
- Klooster J, Vrensen GF, Muller LJ, van der Want JJ. Efferent projections of the olivary pretectal nucleus in the albino rat subserving the pupillary light reflex and related reflexes. A light microscopic tracing study. Brain Res. 1995;688:34–46. doi: 10.1016/0006-8993(95)00497-e. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PDR, Palkovits M, Horn AKE, Toledo CAB, Ryabinin AE. The Edinger-Westphal nucleus: a historical, structural and functional perspective on a dichotomous terminology. J Comp Neurol. 2011;519:1413–1434. doi: 10.1002/cne.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langel JL, Smale L, Esquiva G, Hannibal J. Central melanopsin projections in the diurnal rodent, Arvicanthis niloticus. Front Neuroanat. 2015;9:93. doi: 10.3389/fnana.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston CA, Mustari MJ. The anatomical organization of the macaque pregeniculate complex. Brain Res. 2000;876(1–2):166–179. doi: 10.1016/s0006-8993(00)02647-0. [DOI] [PubMed] [Google Scholar]
- McElhinny T. Masters Dissertation Masters Dissertation. East Lansing: Michigan State University; 1996. Reproductive Biology and Biological Rhythms in Arvicanthis niloticus. [Google Scholar]
- Mikkelsen JD, Vrang N. A direct pretectosuprachiasmatic projection in the rat. Neuroscience. 1994;62(2):497–505. doi: 10.1016/0306-4522(94)90382-4. [DOI] [PubMed] [Google Scholar]
- Miller AM, Miller RB, Obermeyer WH, Behan M, Benca RM. The pretectum mediates rapid eye movement sleep regulation by light. Behav Neurosci. 1999;113:755–765. doi: 10.1037//0735-7044.113.4.755. [DOI] [PubMed] [Google Scholar]
- Miller AM, Obermeyer WH, Behan M, Benca RM. The superior colliculus-pretectum mediates the direct effects of light on sleep. P Natl Acad Sci USA. 1998;95:8957–8962. doi: 10.1073/pnas.95.15.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Weis R, Moga MM. Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J Comp Neurol. 2000;420:398–418. doi: 10.1002/(sici)1096-9861(20000508)420:3<398::aid-cne9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2013;243:4–20. doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH. Interconnections among nuclei of the subcortical visual shell: the intergeniculate leaflet is a major constituent of the hamster subcortical visual system. J Comp Neurol. 1998;396:288–309. [PubMed] [Google Scholar]
- Prichard JR, Stoffel RT, Quimby DL, Obermeyer WH, Benca RM, Behan M. Fos immunoreactivity in rat subcortical visual shell in response to illuminance changes. Neuroscience. 2002;114:781–793. doi: 10.1016/s0306-4522(02)00293-2. [DOI] [PubMed] [Google Scholar]
- Redlin U. Neural basis and biological function of masking by light in mammals: suppression of melatonin and locomotor activity. Chronobiol Int. 2001;18:737–758. doi: 10.1081/cbi-100107511. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Urbanski HF, Nunez AA, Smale L. Projections of the suprachiasmatic nucleus and ventral subparaventricular zone in the Nile grass rat (Arvicanthis niloticus) Brain Res. 2011;1367:146–161. doi: 10.1016/j.brainres.2010.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuboni DD, Cramm S, Yan L, Nunez AA, Smale L. Acute behavioral responses to light and darkness in nocturnal Mus musculus and diurnal Arvicanthis niloticus. J Biol Rhythm. 2012;27:299–307. doi: 10.1177/0748730412449723. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythm. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Szkudlarek HJ, Orlowska P, Lewandowski MH. Light-induced responses of slow oscillatory neurons of the rat olivary pretectal nucleus. PloS One. 2012;7:e33083. doi: 10.1371/journal.pone.0033083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo LJ, Cicerone CM. Cells in the pretectal olivary nucleus are in the pathway for the direct light reflex of the pupil in the rat. Brain Res. 1984;300:49–62. doi: 10.1016/0006-8993(84)91340-4. [DOI] [PubMed] [Google Scholar]
- Young MJ, Lund RD. The anatomical substrates subserving the pupillary light relfex in rats: origin of the consensual pupillary response. Neuroscience. 1994;62:481–496. doi: 10.1016/0306-4522(94)90381-6. [DOI] [PubMed] [Google Scholar]