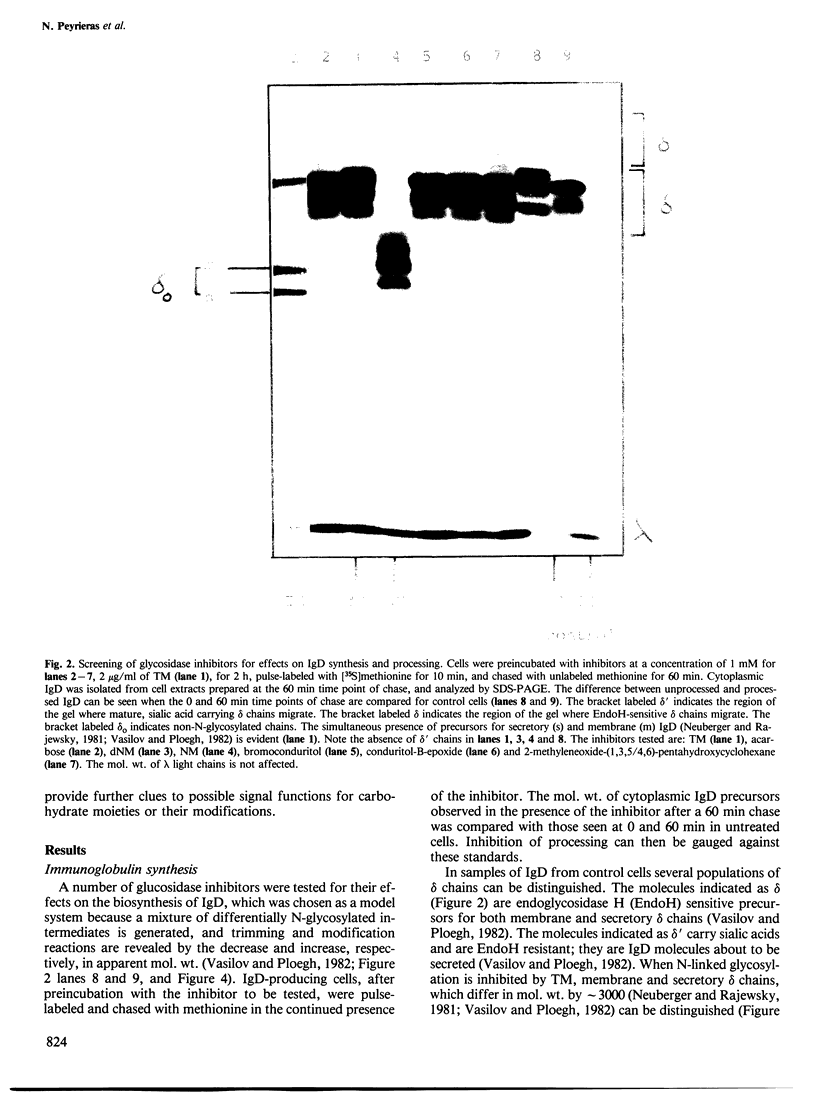
Abstract
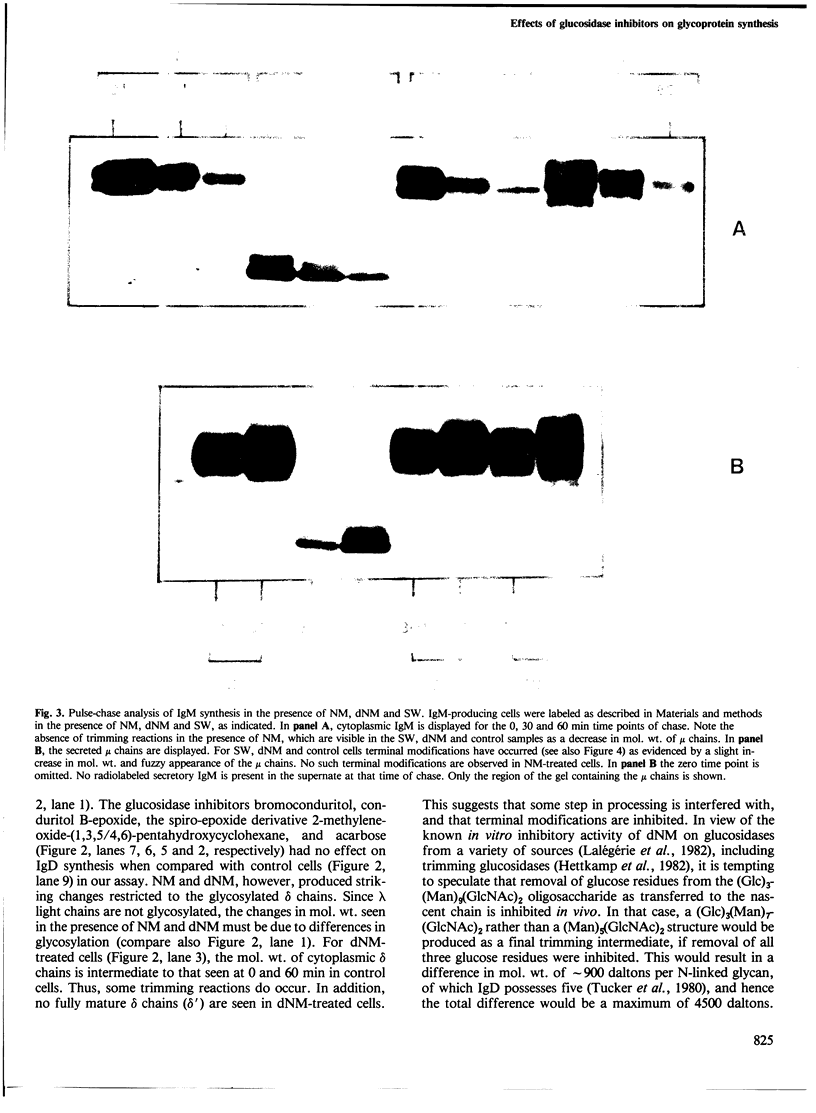
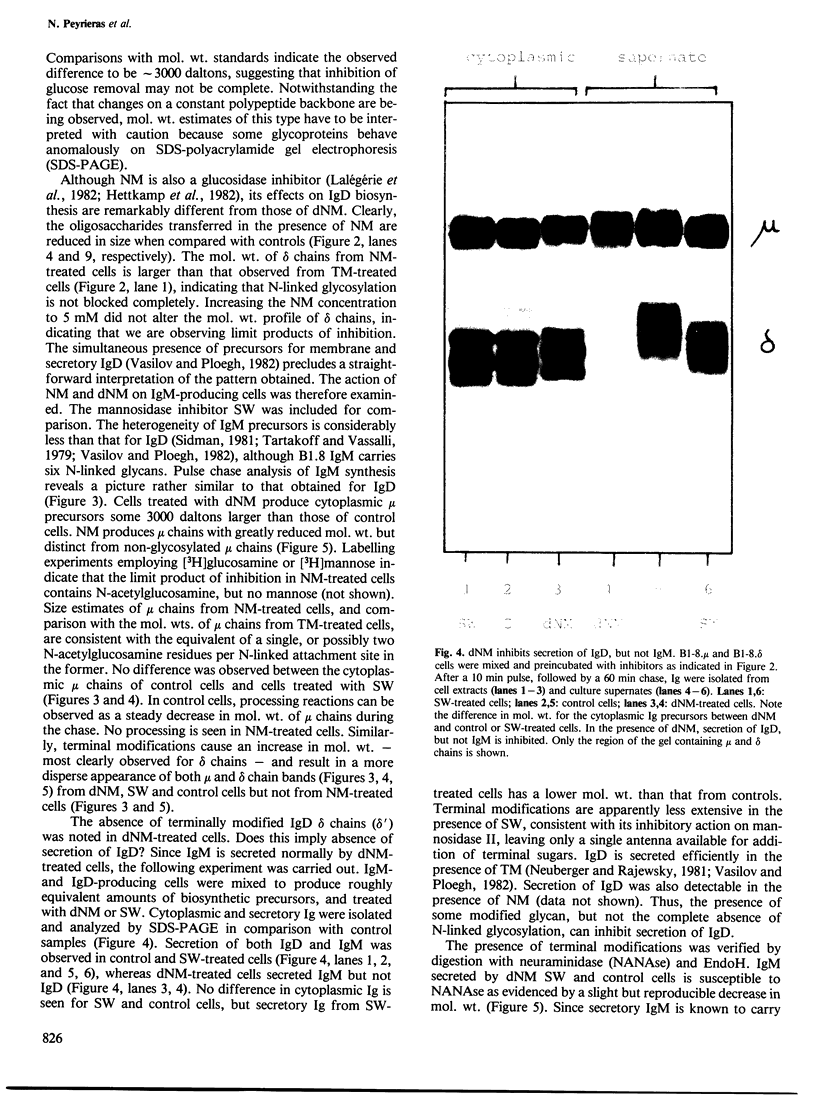
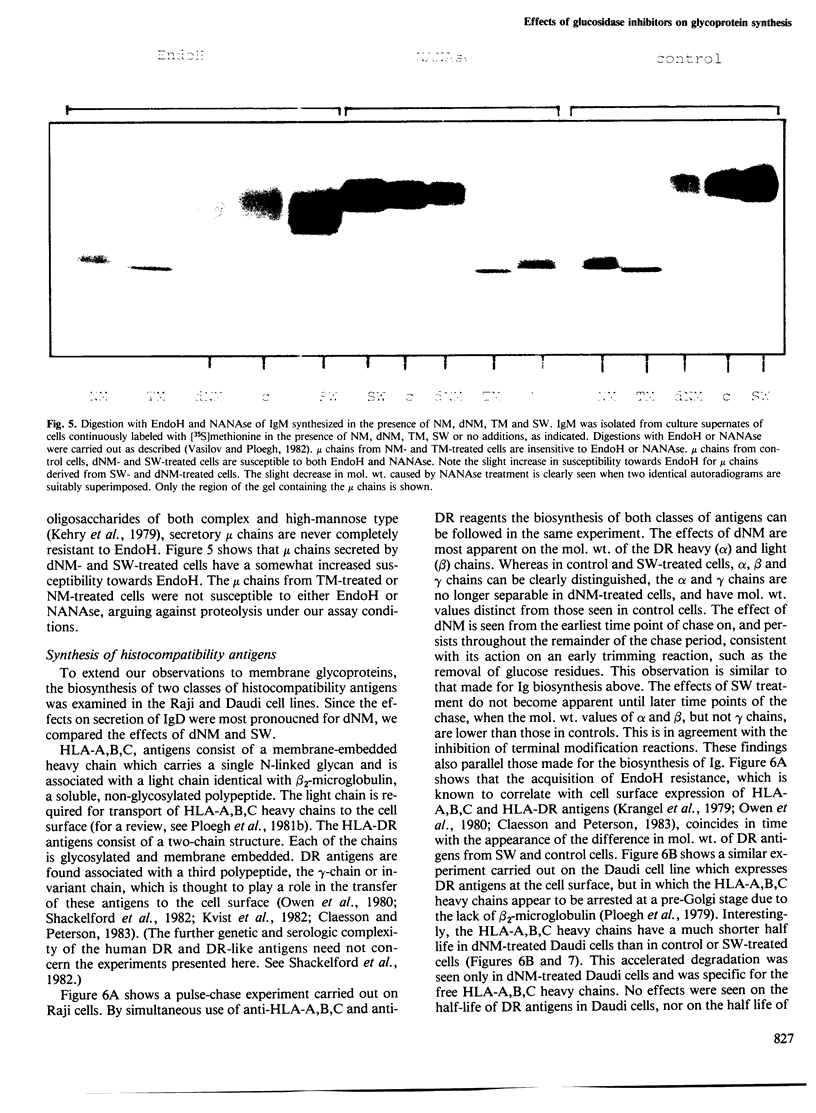
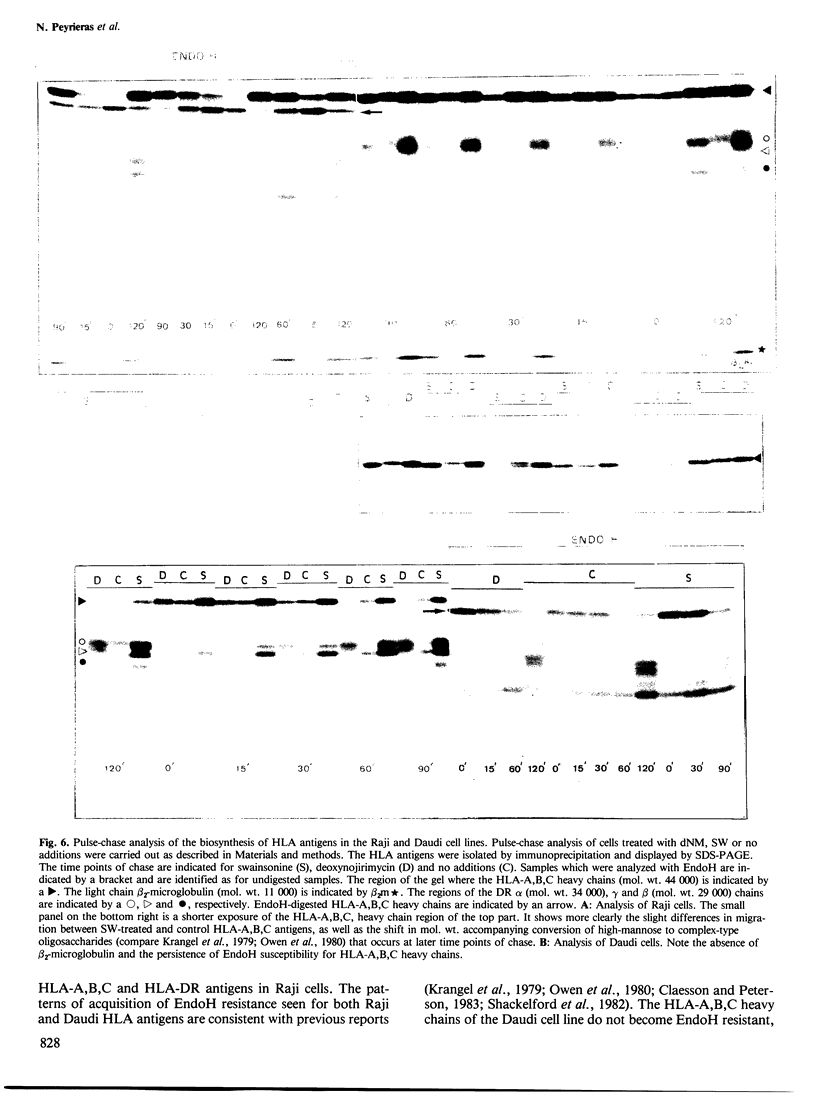
The glucosidase inhibitors nojirimycin (NM) and 1-deoxynojirimycin (dNM) interfere with N-linked glycosylation. The effects of NM and dNM on the biosynthesis of secretory glycoproteins (IgD and IgM) and membrane glycoproteins (HLA-A, B, C and -DR antigens) have been examined. Whereas treatment of IgD- and IgM-producing cells with NM results in the transfer of drastically shortened oligosaccharide side chains, treatment with dNM inhibits trimming, most probably through interaction with glucosidase I and/or II. A comparison of NM and dNM with tunicamycin and the mannosidase inhibitor swainsonine (SW) show that each of the inhibitors interferes with N-linked glycosylation in a distinct manner. For both Ig and HLA antigens, the effects of SW are discernible at the final stages of glycan maturation only, whereas the effects of dNM are observed quite early in the biosynthetic process. The secretion of IgD, but not IgM, was blocked in dNM-treated cells. The HLA-A, B, C heavy chains synthesized by the Daudi cell line were degraded in an accelerated fashion in dNM-treated cells, but no effects were seen on the HLA-DR antigens in these cells. Although both SW and dNM interfere with trimming, further modifications of the oligosaccharide side chains occur, and show that the two processes are not obligately coupled. Glucosidase inhibitors such as NM and dNM, as well as the mannosidase inhibitor SW, allow modification of glycan structure, and may be used to study the biological role of glycoprotein oligosaccharides and their modifications.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chapman A., Trowbridge I. S., Hyman R., Kornfeld S. Structure of the lipid-linked oligosaccharides that accumulate in class E Thy-1-negative mutant lymphomas. Cell. 1979 Jul;17(3):509–515. doi: 10.1016/0092-8674(79)90259-9. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel C. A., Goldberg D. E., Kornfeld S. Identification and characterization of cells deficient in the mannose 6-phosphate receptor: evidence for an alternate pathway for lysosomal enzyme targeting. Proc Natl Acad Sci U S A. 1983 Feb;80(3):775–779. doi: 10.1073/pnas.80.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone P., Fueresz L., Pious D. Gene dosage and gene expression in the HLA region: evidence from deletion variants. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1235–1239. doi: 10.1073/pnas.79.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hettkamp H., Bause E., Legler G. Inhibition by nojirimycin and 1-deoxynojirimycin of microsomal glucosidases from calf liver acting on the glycoprotein oligosaccharides Glc1-3Man9GlcNAc2. Biosci Rep. 1982 Nov;2(11):899–906. doi: 10.1007/BF01114896. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kehry M., Sibley C., Fuhrman J., Schilling J., Hood L. E. Amino acid sequence of a mouse immunoglobulin mu chain. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2932–2936. doi: 10.1073/pnas.76.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag S. S., Robbins A. R. A Chinese hamster ovary cell mutant deficient in glucosylation of lipid-linked oligosaccharide synthesizes lysosomal enzymes of altered structure and function. J Biol Chem. 1982 Jul 25;257(14):8424–8431. [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lalégerie P., Legler G., Yon J. M. The use of inhibitors in the study of glycosidases. Biochimie. 1982 Nov-Dec;64(11-12):977–1000. doi: 10.1016/s0300-9084(82)80379-9. [DOI] [PubMed] [Google Scholar]
- Legler G. Glucosidases. Methods Enzymol. 1977;46:368–381. doi: 10.1016/s0076-6879(77)46044-0. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S., Rajewsky K. Switch from hapten-specific immunoglobulin M to immunoglobulin D secretion in a hybrid mouse cell line. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1138–1142. doi: 10.1073/pnas.78.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Stominger J. L. Biosynthesis and cell surface localization of nonglycosylated human histocompatibility antigens. J Immunol. 1981 Jan;126(1):270–275. [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Kaufman J. F., Korman A. J., Strominger J. L. HLA-DR antigens: structure, separation of subpopulations, gene cloning and function. Immunol Rev. 1982;66:133–187. doi: 10.1111/j.1600-065x.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Sidman C. B lymphocyte differentiation and the control of IgM mu chain expression. Cell. 1981 Feb;23(2):379–389. doi: 10.1016/0092-8674(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P. Plasma cell immunoglobulin M molecules. Their biosynthesis, assembly, and intracellular transport. J Cell Biol. 1979 Nov;83(2 Pt 1):284–299. doi: 10.1083/jcb.83.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. W., Liu C. P., Mushinski J. F., Blattner F. R. Mouse immunoglobulin D: messenger RNA and genomic DNA sequences. Science. 1980 Sep 19;209(4463):1353–1360. doi: 10.1126/science.6968091. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Vasilov R. G., Ploegh H. L. Biosynthesis of murine immunoglobulin D: heterogeneity of glycosylation. Eur J Immunol. 1982 Oct;12(10):804–813. doi: 10.1002/eji.1830121003. [DOI] [PubMed] [Google Scholar]