Abstract
Inhibition of the WEE1 tyrosine kinase enhances anticancer chemotherapy efficacy. Accordingly, the WEE1 inhibitor AZD1775 (previously MK-1775) is currently under evaluation in clinical trials for cancer in combination with chemotherapy. AZD1775 has been reported to display high selectivity and is therefore used in many studies as a probe to interrogate WEE1 biology. However, AZD1775 also exhibits anticancer activity as a single agent although the underlying mechanism is not fully understood. Using a chemical proteomics approach, we here describe a proteome-wide survey of AZD1775 targets in lung cancer cells and identify several previously unknown targets in addition to WEE1. In particular, we observed polo-like kinase 1 (PLK1) as a new target of AZD1775. Importantly, in vitro kinase assays showed PLK1 and WEE1 to be inhibited by AZD1775 with similar potency. Subsequent loss-of-function experiments using RNAi for WEE1 and PLK1 suggested that targeting PLK1 enhances the pro-apoptotic and antiproliferative effects observed with WEE1 knockdown. Combination of RNAi with AZD1775 treatment suggested WEE1 and PLK1 to be the most relevant targets for mediating AZD1775’s anticancer effects. Furthermore, disruption of WEE1 by CRISPR-Cas9 sensitized H322 lung cancer cells to AZD1775 to similar extent as the potent PLK1 inhibitor BI-2536 suggesting a complex crosstalk between PLK1 by WEE1. In summary, we show that AZD1775 is a potent dual WEE1 and PLK1 inhibitor, which limits its use as a specific molecular probe for WEE1. However, PLK1 inhibition makes important contributions to the single agent mechanism of action of AZD1775 and enhances its anticancer effects.
Introduction
The WEE1 tyrosine kinase is a critical regulator of the G2/M cell cycle checkpoint via phosphorylation of CDK1 (aka Cdc2) at Tyr15, which inhibits CDK1/cyclin B kinase activity.1, 2 Inhibition of WEE1 overrides DNA damage-induced cell cycle arrest in cells with a dysfunctional p53-enforced G1 checkpoint and drives TP53-mutant cancer cells into mitotic catastrophe. It is therefore an attractive target for enhancing the effects of chemotherapeutic anticancer drugs that generate DNA damage, such as platinum drugs.3, 4 AZD1775 (previously MK-1775) is a potent small molecule inhibitor of WEE1 kinase and to the best of our knowledge is currently the only WEE1 inhibitor in clinical trials for cancer.5 AZD1775 is also used in many studies as a probe to interrogate WEE1 biology as it has been described to display high target selectivity, although details on kinome-wide target inhibition have not been reported. We and others have previously observed that AZD1775 does not just exhibit anticancer activity in combination with radiation or chemotherapy,5–8 but also as a single agent in various tumor types.9–12 Such single agent activity has been confirmed in early phase clinical trials and appears to be accompanied by a better safety profile than when AZD1775 is administered in combination with cytotoxic chemotherapy drugs or CHK1 kinase inhibitors.13, 14 However, the underlying mechanism for this is not fully understood considering that single agent AZD1775 activity was found to be independent of TP53 mutational status.8–10 In addition, a recent medicinal chemistry study reported superior antiproliferative single agent activity of AZD1775 compared to other similarly potent WEE1 inhibitors.15 We hypothesized that these differences could be the result of differential cellular target profiles. Employing chemical proteomics, we describe here the proteome-wide characterization of the AZD1775 target profile in lung cancer cells and, in addition to WEE1, identify several new kinase targets. In particular, we observed polo-like kinase 1 (PLK1), which performs several important mitotic functions and is a bona fide anticancer target in its own right,16–18 to be a new target of AZD1775. PLK1 is also known to directly regulate WEE1 activity by phosphorylation of Ser53, which leads to ubiquitination and subsequent proteasomal degradation of WEE1.19, 20 Importantly, PLK1 and WEE1 were inhibited by AZD1775 with similar nanomolar potency and subsequent loss-of-function experiments using RNA interference and CRISPR-Cas9 suggested that this dual targeting makes important contributions to AZD1775’s single agent anticancer activity. These findings furthermore indicate that use of AZD1775 as a molecular probe for WEE1 warrants caution.
Results
Single agent AZD1775 induces apoptosis independently of WEE1 and pCDK1 levels
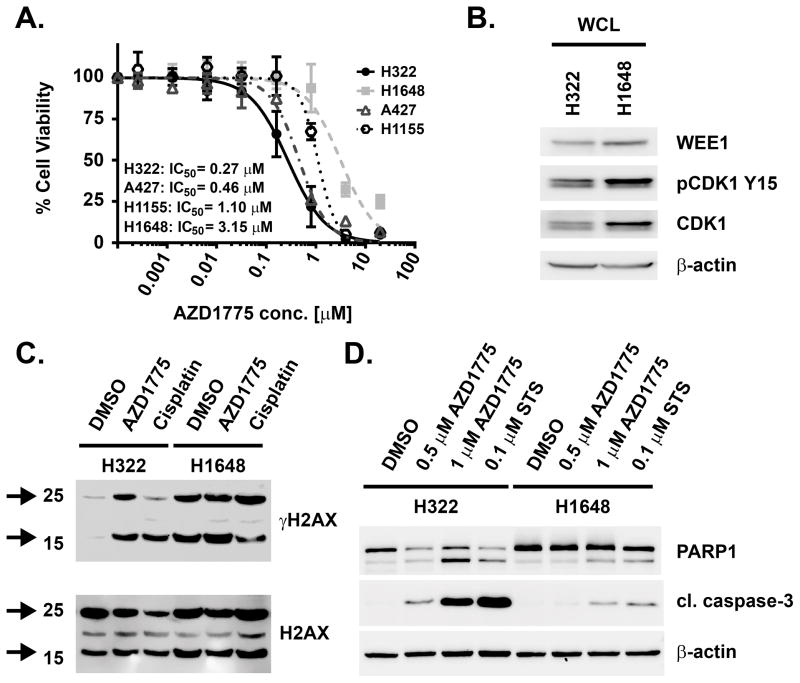
AZD1775 has been described previously to exhibit single agent anticancer activity in various tumor types,9–11 including non-small cell lung cancer (NSCLC).7, 9 We observed that AZD1775 inhibited viability of several NSCLC cell lines with sub- to low micromolar potency (Figure 1A). The most sensitive cell line in this panel, H322, was inhibited at AZD1775 concentrations that were well below the observed mean patient plasma levels of 1.65 μM.13 However, another NSCLC cell line, H1648, was approximately 10-fold less sensitive to AZD1775 than H322 although both cell lines exhibited similar levels of WEE1 protein expression and activity, as indicated by phospho-Tyr15 CDK1 (Figure 1B). Both cell lines feature TP53 mutations according to the catalogue of somatic mutations in cancer (COSMIC).21 In H322 cells, AZD1775 furthermore potently increased phosphorylation of Serine 139 in histone H2AX (γH2AX) (Figure 1C), as well as PARP1 and caspase-3 cleavage (Figure 1D), which are indicative of DNA damage and induction of apoptosis, respectively. Apoptosis induction was markedly more pronounced in H322 cells than in H1648 (Figure 1D). Together, these results suggest that AZD1775 displays potent cellular anticancer effects in NSCLC cells as a single agent irrespective of relative WEE1 or pCDK1 levels.
Figure 1. Single agent cellular anticancer activity of AZD1775 in NSCLC cells.
(A) Dose-response curves for cell viability effects of 72 h AZD1775 treatment on H322, A427, H1155 and H1648 NSCLC cells and IC50 values for inhibition of viability. (B) Immunoblot analysis of untreated H322 and H1648 cells for WEE1, CDK1 and pY15 CDK1. (C) Immunoblot analysis of γH2AX and total H2AX in H322 and H1648 cells upon 4 h AZD1775 (1 μM) or cisplatin (14 μM) treatment. Arrows indicate un-ubiquitinated (~16 kDa) and mono-ubiquitinated (~25 kDa) γH2AX. (D) Immunoblot analysis of PARP1 and caspase 3 cleavage in H322 and H1648 cells treated with indicated concentrations of AZD1775 or 100 nM staurosporine (STS) for 48 h.
A proteome-wide target survey reveals PLK1 as a potent AZD1775 target
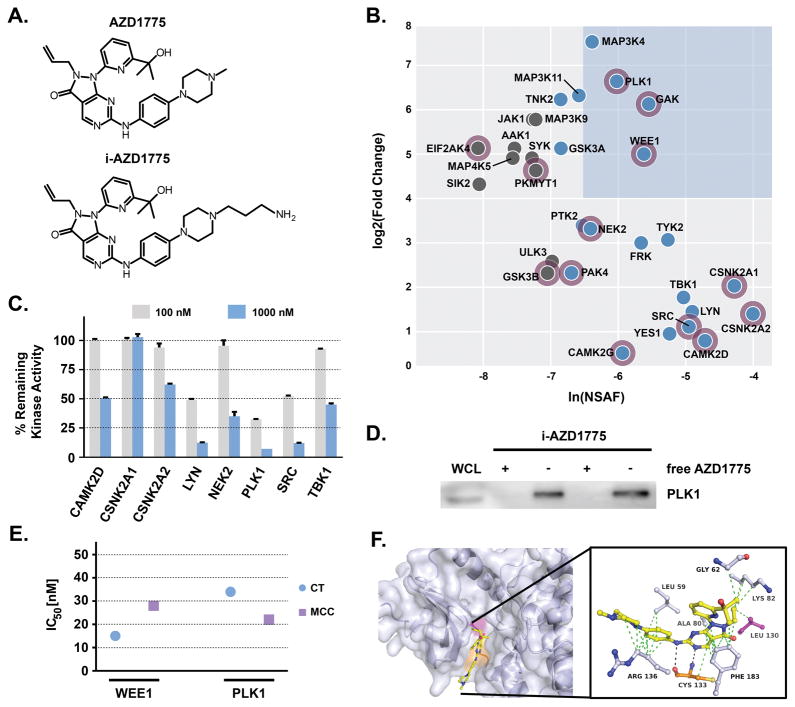
We have previously observed that kinase inhibitors can have broad ranges of target selectivity.22, 23 Considering the unexplained single agent effects of AZD1775 on cancer cells, we hypothesized that AZD1775 may have so far unknown cellular targets in addition to WEE1, which could contribute to its overall cellular activity. To identify additional targets of AZD1775, we deployed an unbiased mass spectrometry (MS)-based chemical proteomics approach, which is a postgenomic adaptation of classical drug affinity purification.24 In order to generate an AZD1775 affinity matrix, an immobilizable analogue of AZD1775 (i-AZD1775, Figure 2A, Figure S1A) was designed based on structural comparison with publically available co-crystal structures of WEE1 with other inhibitors, such as PD331618 and PD352396 (PDB: 3BIZ and PDB: 3BI6). These co-crystal structure analyses suggested that the N-methyl-piperazine moiety of AZD1775 would be solvent exposed upon kinase binding. An in vitro kinase assay confirmed that i-AZD1775 retained the ability to inhibit WEE1 to similar extent as unmodified AZD1775 (Figure S1B). Using i-AZD1775 for drug affinity purifications of protein from H322 total cell lysates, subsequent LC-MS/MS analysis and protein database search identified multiple kinases as target candidates of AZD1775 (Figure 2B, Table S1), including the known targets WEE1 and YES1, as well as the weaker target PKMYT1.5 Several observed kinases were annotated by gene ontology as affecting the cell cycle. Prioritization of kinase hits was done by determining the normalized spectral abundance factor (NSAF)25 and comparison with pulldowns from samples that have been treated with unmodified AZD1775 (Table S2). The NSAF is a measure of relative protein abundance in the pulldown eluate, which is affected by drug affinity and protein expression levels and reflects the relative contribution of each protein to the cellular target profile. Competition with unmodified AZD1775 is a measure of target specificity as the free drug will bind its targets in solution and reduce their ability to bind to the affinity matrix whereas background proteins are not affected. Among those kinases with high NSAF values and fold changes, WEE1, GAK and PLK1 were most prominent (Figure 2B). Evaluation of target inhibition across a panel of the most prominent kinases using in vitro kinase assays (GAK assay not commercially available) validated several of these kinases, such as LYN, SRC and PLK1, as potent AZD1775 targets (Figure 2C). The most potently inhibited of these, PLK1, constituted a particularly intriguing target as it is well-known to be a direct upstream kinase and negative regulator of WEE1 activity and stability. Immunoblot analysis of i-AZD1775 pulldowns confirmed the specificity of the interaction with AZD1775 as competition with AZD1775 completely abrogated its enrichment (Figure 2D and Figure S2). Importantly, the detailed comparison by in vitro kinase assays showed that PLK1 and WEE1 were inhibited by AZD1775 with similarly potent IC50 values ranging from 15–28 nM and 22–34 nM for WEE1 and PLK1, respectively (Figure 2E). This was independent of the source of AZD1775, as both commercially available AZD1775 as well as in-house synthesized behaved similarly. The interaction of AZD1775 with PLK1 was also supported by in silico docking experiments using the publically available X-ray co-crystal structure of PLK1 with the known PLK1 inhibitor BI-2536 (PDB: 2RKU). The resulting model suggests that AZD1775 binds to the ATP binding site of PLK1 through hydrogen bonding with the hinge region (Cys133), stabilized by multiple Van-der-Waals interactions with several amino acid residues, including the gatekeeper residue (Leu130) (Figure 2F). In summary, chemical proteomics experiments identified several new AZD1775 kinase targets, of which PLK1 was inhibited with a low double digit nanomolar IC50 similar to WEE1.
Figure 2. Target profile of AZD1775 in H322 NSCLC cells.
(A) Chemical structures of AZD1775 and its immobilizable analogue i-AZD1775. (B) Protein kinase interaction profile of AZD1775 in H322 cells. Blue box highlights prominent kinase target candidates with high spectral abundance (based on NSAF: normalized spectral abundance factor) and specificity (based on fold change of spectral counts between i-AZD1775 affinity pulldowns and competition experiments with unmodified AZD1775). Blue nodes indicate kinases with high NSAF (> −7), grey nodes with low NSAF (< −7). Purple halo indicates kinases with gene ontology annotation for cell cycle. (C) Inhibition of identified kinases by 100 nM or 1000 nM AZD1775 in in vitro kinase assays (Reaction Biology). (D) PLK1 immunoblot of i-AZD1775 affinity pulldowns (−) and AZD1775 competition pulldowns (+) from H322 whole cell lysates (WCL). (E) Comparison of WEE1 and PLK1 inhibition by AZD1775 in in vitro kinase assays (Eurofins). Displayed are the IC50 values observed for both kinases using commercial (CT: Chemietek) and in-house synthesized (MCC: Moffitt Cancer Center) AZD1775. (F) In silico docking of AZD1775 to PLK1 based on the X-ray co-crystal structure of PLK1 with BI-2536, PDB: 2RKU.
PLK1 is a functionally relevant target in lung cancer cells
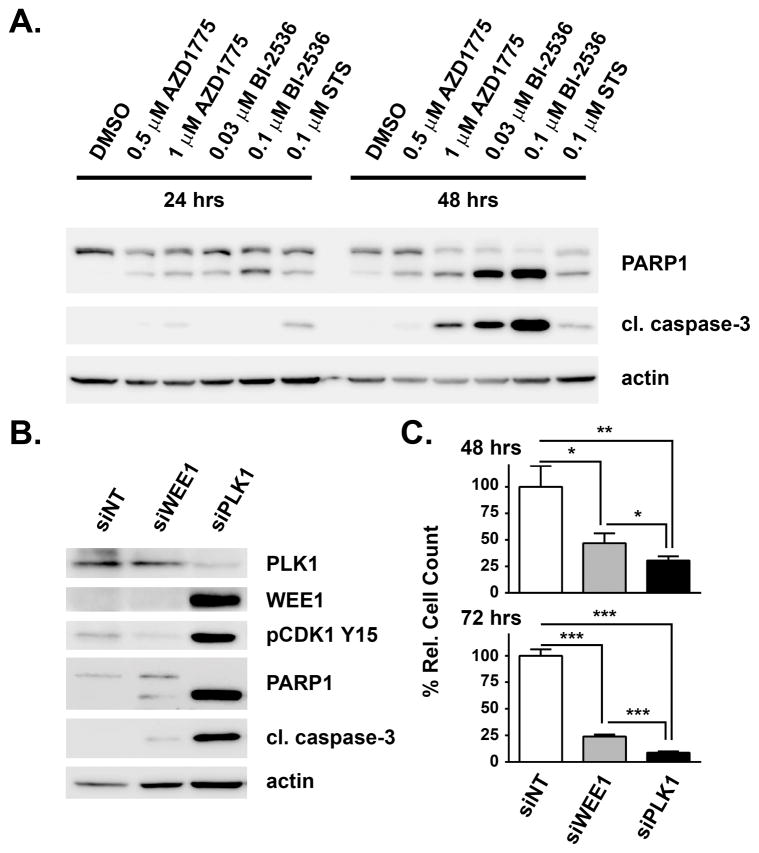
PLK1 is a validated anticancer target in its own right and several small molecule PLK1 inhibitors are being evaluated in the clinic for various types of cancer.17 However, not all cancer cells are equally sensitive to PLK1 inhibition. To determine the sensitivity of H322 cells to inhibition of PLK1 relative to WEE1, cells were treated with different concentrations of BI-2536 and AZD1775. BI-2536 treatment strongly induced apoptosis as determined by PARP1 and caspase 3 cleavage (Figure 3A). Notably, 0.03 μM BI-2536 caused a higher degree of apoptosis than 1 μM AZD1775, which is bioequivalent to this BI-2536 dose with regard to PLK1 inhibition based on their in vitro IC50 values. Consistently, loss of PLK1 function through siRNA-mediated silencing resulted in apoptosis, which judging by PARP1 and caspase 3 cleavage, was much more pronounced than upon silencing of WEE1 (Figure 3B). In addition, both WEE1 protein levels and CDK1 Tyr15 phosphorylation were increased upon PLK1 knockdown, whereas pCDK1 was as expected reduced upon loss of its upstream kinase WEE1. Induction of apoptosis was furthermore accompanied by a significant reduction of cell number, which was observed both with WEE1 and PLK1 knockdown (Figure 3C). Again, the effects elicited by PLK1 silencing were stronger than those resulting from WEE1 loss. In contrast, silencing of another prominent AZD1775 target candidate, GAK, had no effect on apoptosis or cell viability (Figure S3). In summary, loss of function of PLK1 caused a prominent induction of apoptosis reflected as increased cleavage of PARP1 and caspase 3 and as reduction of cell number, which are more pronounced than upon loss of WEE1 function. These findings suggest that WEE1 and PLK1 are both functionally relevant targets of AZD1775 in these cells.
Figure 3. Validation of functional relevance of WEE1 and PLK1 in H322 cells.
(A) Immunoblot analysis of the effects of AZD1775 treatment in comparison with the PLK1 inhibitor BI-2536 on PARP1 and caspase 3 cleavage in H322 cells at the indicated time points and concentrations. Treatment with 100 nM staurosporine (STS) served as positive control. (B) Immunoblot analysis for indicated proteins of siRNA-mediated knockdown of WEE1 and PLK1 in H322 cells after 48 h. (C) Effects of siRNA-mediated knockdown of WEE1 and PLK1 on H322 cell number after 48 h and 72 h. Asterisks indicate p-value ≤ 0.05 (*), 0.01 (**) and 0.001 (***) as determined by t-test.
Dual inhibition of WEE1 and PLK1 resembles AZD1775 activity
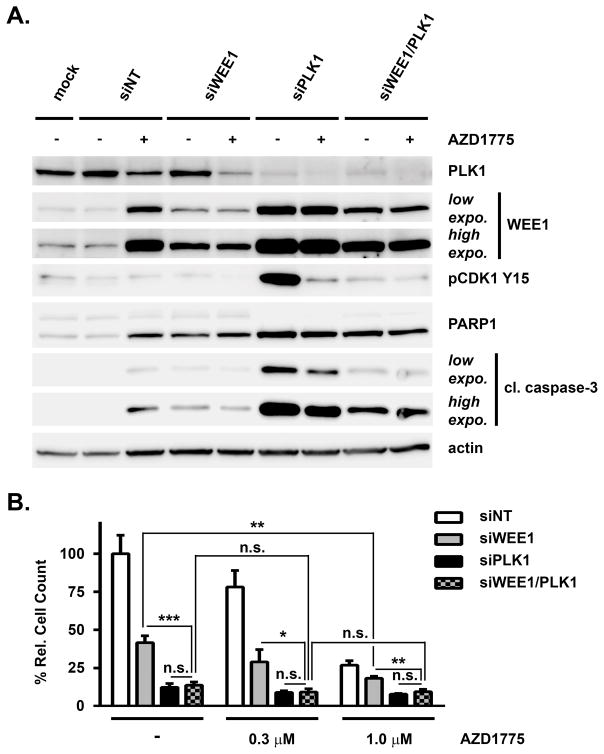
To determine, if the dual inhibition of WEE1 and PLK1 is sufficient to explain the single agent activity of AZD1775, single and double siRNA-mediated knockdowns of WEE1 and PLK1 were combined with AZD1775 treatment. Consistent with our previous observations, WEE1 silencing or AZD1775 treatment elicited PARP1 and caspase 3 cleavage compared to DMSO/siNT treatment in H322 cells (Figure 4A). As before, PLK1 knockdown showed yet stronger induction of apoptosis. Interestingly, double knockdown of WEE1 and PLK1 caused enhanced apoptosis compared to WEE1 silencing alone, whereas compared to PLK1 silencing alone it was markedly reduced. Furthermore, AZD1775 treatment in combination with WEE1 knockdown caused a slight increase in PARP1 cleavage as compared to WEE1 knockdown alone, whereas AZD1775 combination with PLK1 knockdown decreased PARP1 and caspase 3 cleavage suggesting that neither WEE1 nor PLK1 single knockdown entirely recapitulate AZD1775 drug action. In contrast, AZD1775 treatment in addition to WEE1/PLK1 double knockdown did not alter PARP1 and caspase 3 cleavage compared to double knockdown treated with DMSO. Similar results were also obtained using another AZD1775-sensitive NSCLC cell line, A427 (Figure S4A). This suggests that dual inhibition of WEE1 and PLK1 can fully explain the effects of AZD1775 on cell apoptosis and that other targets are unlikely to make additional contributions to this effect. Double knockdown of WEE1 and PLK1 caused a significantly stronger reduction of cell growth than WEE1 knockdown alone in H322 cells (Figure 4B). However, this was limited to the same level achieved with PLK1 knockdown. Moreover, combination of siWEE1 with 1 μM AZD1775 further reduced cell growth compared to siWEE1/DMSO suggesting contributions by additional AZD1775 targets. In contrast, addition of increasing concentrations of AZD1775 to WEE1/PLK1 double knockdown did not cause any significant further reduction of H322 cell number suggesting that combined inhibition of both targets recapitulates AZD1775 drug action in these cells. In A427 cells, AZD1775 treatment further reduced cell growth although also in these cells double knockdown was more effective than single WEE1 silencing (Figure S4B). In summary, these results suggest that dual inhibition of WEE1 and PLK1 is largely responsible for the cellular anticancer effects of AZD1775.
Figure 4. Effects of dual targeting of WEE1 and PLK1 on apoptosis and proliferation of H322 cells.
(A) Immunoblot analysis for indicated proteins of siRNA-mediated single and double knockdown of WEE1 and PLK1 in H322 cells 72 h after transfection and 48 h of 1 μM AZD1775 (+) or DMSO (−) treatment. Mock: DMSO and lipofectamine only. Low expo.: low exposure; high expo.: high exposure. (B) Effects of siRNA-mediated single and double knockdown of WEE1 and PLK1 on H322 cell number 72 h after transfection and 48 h of DMSO (−), 0.3 μM AZD1775 or 1 μM AZD1775 treatment. Asterisks indicate p-value ≤ 0.05 (*), 0.01 (**) and 0.001 (***) as determined by t-test; n.s.: not significant.
Loss of WEE1 hypersensitizes cells to AZD1775
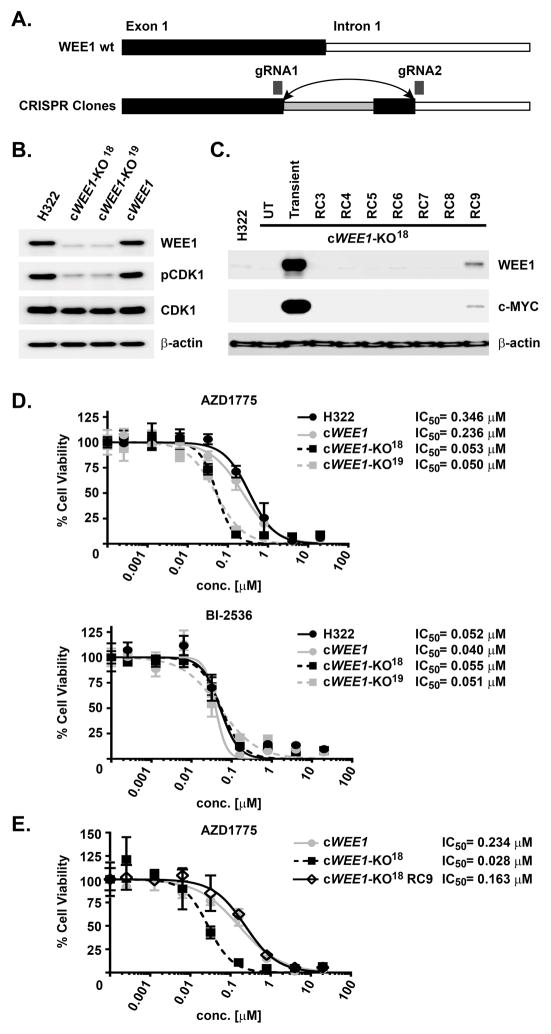
To corroborate the mechanism of AZD1775 in lung cancer cells, we used a CRISPR-Cas9 genome editing technique to stably knock out WEE1 in H322 cells. Stable WEE1-knockout clones were generated by targeting a gene segment spanning the coding exon 1/intron 1 splice site, which led to a sequence reversion and truncation as confirmed by DNA sequencing (Figure 5A). Immunoblot analysis confirmed loss of WEE1 and downstream CDK1 phosphorylation of the two selected CRISPR-Cas9 clones cWEE1-KO18 and cWEE1-KO19 (Figure 5B). No viable clone with a confirmed CRISPR-Cas9-mediated deletion of PLK1 was obtained consistent with an essential role for PLK1.26, 27 A separate CRISPR-Cas9 clone, cWEE1, retained WEE1 expression and CDK1 phosphorylation and thus constituted a suitable control. In order to evaluate specificity of the WEE1 knockout, clone cWEE1-KO18 was stably reconstituted by expression of c-myc-tagged WEE1, which was most pronounced in reconstitution clone RC9 (Figure 5C). Determining cell viability of parental H322 and the generated CRISPR-Cas9 clones upon treatment with AZD1775 interestingly showed a 5–6-fold increased sensitivity of the knockout clones cWEE1-KO18 and cWEE1-KO19 compared to parental H322 or to cWEE1 control cells with excellent agreement between both knockout and control cell lines, respectively (Figure 5D). In contrast, such a shift was not observed upon treatment with the PLK1 inhibitor BI-2536. However, treatment of the WEE1-reconstituted clone cWEE1-KO18(RC9) with AZD1775 completely reversed the sensitizing effect indicating that the observed effect is WEE1-specific (Figure 5E). Together, these results corroborate that WEE1 and PLK1 are the most relevant AZD1775 targets in these cells and suggest a complex crosstalk between PLK1 by WEE1, disruption of which hypersensitizes cells to AZD1775 treatment due to PLK1 inhibition.
Figure 5. Effects of AZD1775 and BI-2536 on viability of WEE1 CRISPR-Cas9 knockout cells.
(A) Schematic for genetic knockout strategy of WEE1 by CRISPR-Cas9 targeting the WEE1 exon 1/intron 1 splice site. Wt: wild-type; gRNA: guide RNA. (B) Immunoblot analysis for indicated proteins of different H322-based CRISPR-Cas9 clones resulting in identification of the knockout clones cWEE1-KO18 and cWEE1-KO19, as well as the negative control clone cWEE1 with intact WEE1 expression. (C) Immunoblot analysis for indicated proteins of cWEE1-KO18 clones reconstituted by ectopic expression of myc-tagged WEE1 (RC#). UT: untransfected; Transient: transient transfection. (D) Dose-response curves for inhibition of viability of parental H322 cells and indicated CRISPR-Cas9 clones upon 72 h treatment with AZD1775 or BI-2536, as determined by the CellTiterGlo assay, with corresponding IC50 values. (E) Dose-response curves for inhibition of viability of parental H322 cells, WEE1 knockout clone cWEE1-KO18 and WEE1 reconstitution clone RC9 upon 72 h treatment with AZD1775, as determined by the CellTiterGlo assay, with corresponding IC50 values.
WEE1 and PLK1 inhibition both contribute to induction of DNA damage
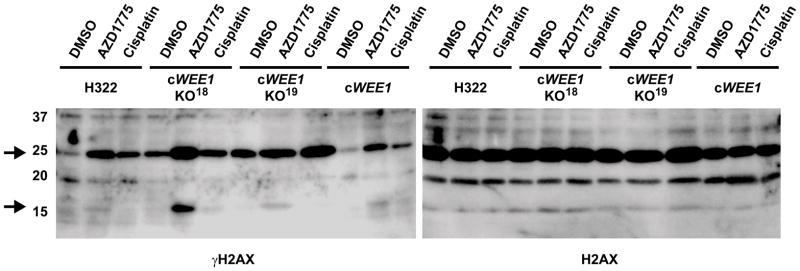
In order to determine the relevance of dual targeting of WEE1 and PLK1 by AZD1775 on its ability to induce DNA damage, γH2AX levels of parental H322 cells were compared to the WEE1 knockout and control clones. Consistent with our previous observations, this analysis revealed that AZD1775 and cisplatin can induce DNA damage in parental H322 and cWEE1 control cells, which both showed similarly low basal levels of γH2AX (Figure 6). In comparison, basal γH2AX levels were markedly elevated upon loss of WEE1 in both knockout clones, cWEE1-KO18 and cWEE1-KO19. Notably, treatment with AZD1775 further increased γH2AX demonstrating that the overall effect of AZD1775 on the induction of DNA damage cannot be attributed to inhibition of WEE1 alone. These results support the notion that the induction of DNA damage by AZD1775 is the combined effect of inhibition of both targets, WEE1 and PLK1.
Figure 6. Effects of AZD1775 on γH2AX levels in WEE1 CRISPR-Cas9 knockout cells.
Immunoblot analysis of γH2AX and total H2AX in parental H322 cells and indicated CRISPR-Cas9 knockout (cWEE1-KO18, cWEE1-KO19) and negative control (cWEE1) clones upon 4 h AZD1775 (1 μM) or cisplatin (17 μM) treatment. Arrows indicate un-ubiquitinated (~16 kDa) and mono-ubiquitinated (~25 kDa) γH2AX.
Discussion
Kinase inhibitors, while being valuable drugs and powerful molecular probes, have been recognized to display widely varying degrees of target selectivity,23, 28–31 which may lead to toxicity or conversely enable repurposing of clinical compounds.32, 33 Understanding a kinase inhibitor’s cellular target profile also has important implications for the correct evaluation of its biological effects, as non-canonical targets can either decrease or enhance cellular efficacy,34 and assist in dissecting the wiring maps of the targeted signaling networks.
In this study, we have applied a chemical proteomics approach in order to elucidate the single agent anticancer mechanism of action (MoA) of the widely used clinical WEE1 kinase inhibitor AZD1775. Whereas WEE1 biology is sufficiently well characterized to explain the synergy of AZD1775 with cytotoxic chemotherapy drugs, such as cisplatin, which has been demonstrated across multiple studies and cancer types, its single agent activity was not fully understood. Drawing on the unbiased nature of our approach, we identified and validated several unknown targets of AZD1775 in addition to the previously described WEE1, YES1 and PKMYT1 kinases.5 Being the most potent among the new targets, PLK1 was inhibited by AZD1775 with similar potency as the intended target WEE1 itself. This is potentially important as PLK1 is well documented to be a negative regulator of WEE1 activity by acting immediately upstream and directly phosphorylating WEE1, which results in ubiquitination and proteasomal degradation of WEE1.17, 19, 20, 35, 36 Inhibition of PLK1 has been shown to release this negative regulation of WEE1, which is consistent with our findings that PLK1 knockdown increases WEE1 protein levels as well as CDK1 phosphorylation. Thus, it could be argued that it may be of no overall consequence for AZD1775’s MoA as AZD1775, being a potent WEE1 inhibitor, subsequently counteracts this effect through downstream inhibition of WEE1 enzymatic activity. However, our observations suggest otherwise as dual inhibition/silencing of WEE1 and PLK1 consistently leads to more pronounced effects with regard to reduction of cell viability and induction of apoptosis and DNA damage as compared to knockdown/knockout of WEE1 alone. This can be largely explained by the fact that WEE1 is not the only substrate of PLK1, but that PLK1 displays a wide range of cellular substrates and is involved in multiple cellular processes, particularly during mitosis.37, 38 For instance, PLK1 has been recently shown to exist in discrete functional pools along the kinetochore-centromere axis.39 Thus, AZD1775 can be reasonably expected to elicit a number of WEE1 pathway-independent effects mediated through PLK1 inhibition. In this context it is important to note that PLK1 depletion has been demonstrated to potently induce apoptosis, which is consistent with our observations in lung cancer cells.26, 40 Furthermore, whereas the regulatory mechanism and signaling consequences of PLK1 on WEE1 are well characterized, our CRISPR-Cas9 knockout experiments suggest that there may also exist a previously unrecognized reciprocal control mechanism of PLK1 by WEE1 as knockout of WEE1 surprisingly enhanced AZD1775 cellular activity. Since AZD1775 potently inhibits activity of both targets, it is not clear, if this mechanism involves WEE1 kinase activity or allosteric protein binding. More detailed structure-function studies would be required to elucidate the mechanism of this interaction.
While dual inhibition of WEE1 and PLK1 appears to be superior to inhibition of WEE1 alone, future comparison with more selective inhibitors of WEE1, which have no or only little potency against PLK1, is necessary to fully answer this question. Notably, in preclinical studies many cancer cells are sensitive to single agent AZD1775 and single agent efficacy has also been observed in the clinic.13 Another important aspect to consider is the effect on in vivo toxicity, which may be different between dual WEE1/PLK1 and selective WEE1 inhibitors. In this context it is important to note, that PLK1 depletion in general has been shown to be more tolerable to normal than to cancer cells.26 Consistently, a recent phase I study found AZD1775 monotherapy to be well tolerated in spite of approximately 6-fold higher AZD1775 doses, which did not reach the maximum tolerated dose (MTD) in this patient cohort, as compared to the MTD in combination with platinum chemotherapy.14
In summary, we here show that the clinical drug candidate AZD1775 is a potent inhibitor not just of its intended target WEE1, but also of its upstream regulator and independent anticancer target PLK1. We therefore propose that AZD1775 should be regarded as a dual inhibitor of WEE1 and PLK1 instead of a selective, or even highly selective, WEE1 inhibitor and suggest that previous mechanistic studies that use AZD1775 be interpreted in this context. Thus, whereas AZD1775 has the potential to be a drug that may produce significant clinical benefit to cancer patients, also or maybe particularly as a single agent, it should only be used with caution as a probe molecule to interrogate WEE1 biology.
Methods
For a full description of utilized methods and reagents please see Supporting Methods.
Chemical Proteomics and Data Analysis
Cells were harvested, pelleted by centrifugation and lysed with an equal volume lysis buffer (50 mM Tris, 5% glycerol, 1.5 mM MgCl2, 100 mM NaCl, 0.2% NP-40, 25 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 1 mM DTT, 30 μM TLCK, 30 μM TPCK, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 10 μg/mL trypsin inhibitor, pH 7.5) as described previously.41 The lysis mixture was centrifuged twice at 27,000 × g and 4 °C (10 min, 20 min). Protein concentration was determined using a Bradford assay.
Affinity pulldown experiments were performed essentially as described before.22, 42 All enrichments were performed in duplicate. i-AZD1775 was immobilized on NHS-activated beads and blocked overnight. Affinity pulldown experiments were performed by incubating lysate (5 mg protein per sample) and competition compound (20 μM free AZD1775) or DMSO for 2 h at 4 °C with drug-modified beads. After washing beads with lysis buffer, bound proteins were eluted by heating to 100 °C in Laemmli buffer. A portion of each eluate was set aside for analysis by immunoblotting. Samples were separated by SDS-PAGE, digested in-gel with trypsin and analyzed by LC-MS/MS using a QExactive mass spectrometer (Thermo Fisher). Data was searched against the SwissProt 2014_06 human protein database using the Mascot search engine (Matrix Science). Up to two missed cleavages by trypsin were allowed and carbamidomethylation of cysteine and methionine oxidation were selected as variable modifications. Results were visualized in Scaffold (www.proteomesoftware.com), using a protein threshold of 99%, minimum of 2 peptides, and a peptide threshold of 95%. Peptide counts were analyzed as Exclusive Unique Spectrum Count (EUSC), except where otherwise specified. The total spectrum count (TSC) for all proteins in each sample was exported and bait/inter/prey input files were prepared. For analysis, the i-AZD1775 affinity purifications were compared against the competition purifications with free AZD1775. The input file was processed using SAINTexpress,43 and the fold-change and Normalized Spectral Abundance Factors (NSAF)25 were plotted on a bubble graph using the freely available online analysis tool APOSTL.44
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute R01 CA181746 (to U.R.), by the H. Lee Moffitt Cancer Center and Research Institute as a Team Science Grant and the Moffitt Lung Cancer Center of Excellence. We furthermore wish to acknowledge the Moffitt Chemical Biology (Chemistry Unit), Proteomics and Molecular Genomics Core Facilities, which are supported by the National Cancer Institute (Award No. P30-CA076292) as a Cancer Center Support Grant.
Footnotes
Competing financial interests
A.N.A.M. and U.R. are inventors on U.S. Patent Application No. 15/125,629 (“PAXIP1 as a biomarker for WEE1 inhibitor therapy”). The other authors declare no competing financial interests.
Additional data and methods. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–22. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- 2.Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–7. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 3.Do K, Doroshow JH, Kummar S. Wee1 kinase as a target for cancer therapy. Cell Cycle. 2013;12:3159–64. doi: 10.4161/cc.26062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N, Imagaki K, Ohtani J, Sakai T, Yoshizumi T, Mizuarai S, Iwasawa Y, Kotani H. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther. 2010;9:514–22. doi: 10.4161/cbt.9.7.11115. [DOI] [PubMed] [Google Scholar]
- 5.Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M, Kimura T, Kaneko N, Ohtani J, Yamanaka K, Itadani H, Takahashi-Suzuki I, Fukasawa K, Oki H, Nambu T, Jiang J, Sakai T, Arakawa H, Sakamoto T, Sagara T, Yoshizumi T, Mizuarai S, Kotani H. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8:2992–3000. doi: 10.1158/1535-7163.MCT-09-0463. [DOI] [PubMed] [Google Scholar]
- 6.Bridges KA, Chen X, Liu H, Rock C, Buchholz TA, Shumway SD, Skinner HD, Meyn RE. MK-8776, a novel chk1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Oncotarget. 2016;7:71660–71672. doi: 10.18632/oncotarget.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jhuraney A, Woods NT, Wright G, Rix L, Kinose F, Kroeger JL, Remily-Wood E, Cress WD, Koomen JM, Brantley SG, Gray JE, Haura EB, Rix U, Monteiro AN. PAXIP1 Potentiates the Combination of WEE1 Inhibitor AZD1775 and Platinum Agents in Lung Cancer. Mol Cancer Ther. 2016;15:1669–81. doi: 10.1158/1535-7163.MCT-15-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajeshkumar NV, De Oliveira E, Ottenhof N, Watters J, Brooks D, Demuth T, Shumway SD, Mizuarai S, Hirai H, Maitra A, Hidalgo M. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17:2799–806. doi: 10.1158/1078-0432.CCR-10-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guertin AD, Li J, Liu Y, Hurd MS, Schuller AG, Long B, Hirsch HA, Feldman I, Benita Y, Toniatti C, Zawel L, Fawell SE, Gilliland DG, Shumway SD. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol Cancer Ther. 2013;12:1442–52. doi: 10.1158/1535-7163.MCT-13-0025. [DOI] [PubMed] [Google Scholar]
- 10.Kreahling JM, Gemmer JY, Reed D, Letson D, Bui M, Altiok S. MK1775, a selective Wee1 inhibitor, shows single-agent antitumor activity against sarcoma cells. Mol Cancer Ther. 2012;11:174–82. doi: 10.1158/1535-7163.MCT-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moser R, Xu C, Kao M, Annis J, Lerma LA, Schaupp CM, Gurley KE, Jang IS, Biktasova A, Yarbrough WG, Margolin AA, Grandori C, Kemp CJ, Mendez E. Functional kinomics identifies candidate therapeutic targets in head and neck cancer. Clin Cancer Res. 2014;20:4274–88. doi: 10.1158/1078-0432.CCR-13-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lescarbeau RS, Lei L, Bakken KK, Sims PA, Sarkaria JN, Canoll P, White FM. Quantitative Phosphoproteomics Reveals Wee1 Kinase as a Therapeutic Target in a Model of Proneural Glioblastoma. Mol Cancer Ther. 2016;15:1332–43. doi: 10.1158/1535-7163.MCT-15-0692-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Do K, Wilsker D, Ji J, Zlott J, Freshwater T, Kinders RJ, Collins J, Chen AP, Doroshow JH, Kummar S. Phase I Study of Single-Agent AZD1775 (MK-1775), a Wee1 Kinase Inhibitor, in Patients With Refractory Solid Tumors. J Clin Oncol. 2015;33:3409–15. doi: 10.1200/JCO.2014.60.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leijen S, van Geel RM, Pavlick AC, Tibes R, Rosen L, Razak AR, Lam R, Demuth T, Rose S, Lee MA, Freshwater T, Shumway S, Liang LW, Oza AM, Schellens JH, Shapiro GI. Phase I Study Evaluating WEE1 Inhibitor AZD1775 As Monotherapy and in Combination With Gemcitabine, Cisplatin, or Carboplatin in Patients With Advanced Solid Tumors. J Clin Oncol. 2016;34:4371–4380. doi: 10.1200/JCO.2016.67.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matheson CJ, Venkataraman S, Amani V, Harris PS, Backos DS, Donson AM, Wempe MF, Foreman NK, Vibhakar R, Reigan P. A WEE1 Inhibitor Analog of AZD1775 Maintains Synergy with Cisplatin and Demonstrates Reduced Single-Agent Cytotoxicity in Medulloblastoma Cells. ACS Chem Biol. 2016;11:921–30. doi: 10.1021/acschembio.5b00725. [DOI] [PubMed] [Google Scholar]
- 16.Gutteridge RE, Ndiaye MA, Liu X, Ahmad N. Plk1 Inhibitors in Cancer Therapy: From Laboratory to Clinics. Mol Cancer Ther. 2016;15:1427–35. doi: 10.1158/1535-7163.MCT-15-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–41. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 18.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9:643–60. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 19.van Vugt MA, Bras A, Medema RH. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, Hunter T, Osada H. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc Natl Acad Sci U S A. 2004;101:4419–24. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, Kok CY, Jia M, De T, Teague JW, Stratton MR, McDermott U, Campbell PJ. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–11. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumi NJ, Kuenzi BM, Knezevic CE, Remsing Rix LL, Rix U. Chemoproteomics Reveals Novel Protein and Lipid Kinase Targets of Clinical CDK4/6 Inhibitors in Lung Cancer. ACS Chem Biol. 2015;10:2680–6. doi: 10.1021/acschembio.5b00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter GE, Rix U, Carlson SM, Gleixner KV, Grebien F, Gridling M, Muller AC, Breitwieser FP, Bilban M, Colinge J, Valent P, Bennett KL, White FM, Superti-Furga G. Systems-pharmacology dissection of a drug synergy in imatinib-resistant CML. Nat Chem Biol. 2012;8:905–12. doi: 10.1038/nchembio.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rix U, Superti-Furga G. Target profiling of small molecules by chemical proteomics. Nat Chem Biol. 2009;5:616–24. doi: 10.1038/nchembio.216. [DOI] [PubMed] [Google Scholar]
- 25.Zybailov BL, Florens L, Washburn MP. Quantitative shotgun proteomics using a protease with broad specificity and normalized spectral abundance factors. Mol Biosyst. 2007;3:354–60. doi: 10.1039/b701483j. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Lei M, Erikson RL. Normal cells, but not cancer cells, survive severe Plk1 depletion. Mol Cell Biol. 2006;26:2093–108. doi: 10.1128/MCB.26.6.2093-2108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wachowicz P, Fernandez-Miranda G, Marugan C, Escobar B, de Carcer G. Genetic depletion of Polo-like kinase 1 leads to embryonic lethality due to mitotic aberrancies. Bioessays. 2016;38(Suppl 1):S96–S106. doi: 10.1002/bies.201670908. [DOI] [PubMed] [Google Scholar]
- 28.Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, Reader V, Sweetman G, Bauer A, Bouwmeester T, Hopf C, Kruse U, Neubauer G, Ramsden N, Rick J, Kuster B, Drewes G. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol. 2007;25:1035–44. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- 29.Brehmer D, Greff Z, Godl K, Blencke S, Kurtenbach A, Weber M, Muller S, Klebl B, Cotten M, Keri G, Wissing J, Daub H. Cellular targets of gefitinib. Cancer Res. 2005;65:379–82. [PubMed] [Google Scholar]
- 30.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046–51. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 31.Winter GE, Rix U, Lissat A, Stukalov A, Mullner MK, Bennett KL, Colinge J, Nijman SM, Kubicek S, Kovar H, Kontny U, Superti-Furga G. An integrated chemical biology approach identifies specific vulnerability of Ewing’s sarcoma to combined inhibition of Aurora kinases A and B. Mol Cancer Ther. 2011;10:1846–56. doi: 10.1158/1535-7163.MCT-11-0100. [DOI] [PubMed] [Google Scholar]
- 32.Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R, Mark EJ, Batten JM, Chen H, Wilner KD, Kwak EL, Clark JW, Carbone DP, Ji H, Engelman JA, Mino-Kenudson M, Pao W, Iafrate AJ. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–70. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sillaber C, Herrmann H, Bennett K, Rix U, Baumgartner C, Bohm A, Herndlhofer S, Tschachler E, Superti-Furga G, Jager U, Valent P. Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur J Clin Invest. 2009;39:1098–109. doi: 10.1111/j.1365-2362.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 34.Knight ZA, Lin H, Shokat KM. Targeting the cancer kinome through polypharmacology. Nat Rev Cancer. 2010;10:130–7. doi: 10.1038/nrc2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe N, Arai H, Iwasaki J, Shiina M, Ogata K, Hunter T, Osada H. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc Natl Acad Sci U S A. 2005;102:11663–8. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayad NG, Rankin S, Murakami M, Jebanathirajah J, Gygi S, Kirschner MW. Tome-1, a trigger of mitotic entry, is degraded during G1 via the APC. Cell. 2003;113:101–13. doi: 10.1016/s0092-8674(03)00232-0. [DOI] [PubMed] [Google Scholar]
- 37.Kettenbach AN, Schweppe DK, Faherty BK, Pechenick D, Pletnev AA, Gerber SA. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Science Signal. 2011;4:rs5. doi: 10.1126/scisignal.2001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oppermann FS, Grundner-Culemann K, Kumar C, Gruss OJ, Jallepalli PV, Daub H. Combination of chemical genetics and phosphoproteomics for kinase signaling analysis enables confident identification of cellular downstream targets. Mol Cell Proteomics. 2012;11:O111 012351. doi: 10.1074/mcp.O111.012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lera RF, Potts GK, Suzuki A, Johnson JM, Salmon ED, Coon JJ, Burkard ME. Decoding Polo-like kinase 1 signaling along the kinetochore-centromere axis. Nat Chem Biol. 2016;12:411–8. doi: 10.1038/nchembio.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci U S A. 2003;100:5789–94. doi: 10.1073/pnas.1031523100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Kocher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–63. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 42.Knezevic CE, Wright G, Remsing Rix LL, Kim W, Kuenzi BM, Luo Y, Watters JM, Koomen JM, Haura EB, Monteiro AN, Radu C, Lawrence HR, Rix U. Proteome-wide Profiling of Clinical PARP Inhibitors Reveals Compound-Specific Secondary Targets. Cell Chem Biol. 2016;23:1490–1503. doi: 10.1016/j.chembiol.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teo G, Liu G, Zhang J, Nesvizhskii AI, Gingras AC, Choi H. SAINTexpress: improvements and additional features in Significance Analysis of INTeractome software. J Proteomics. 2014;100:37–43. doi: 10.1016/j.jprot.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuenzi BM, Borne AL, Li J, Haura EB, Eschrich SA, Koomen JM, Rix U, Stewart PA. APOSTL: An Interactive Galaxy Pipeline for Reproducible Analysis of Affinity Proteomics Data. J Proteome Res. 2016;15:4747–4754. doi: 10.1021/acs.jproteome.6b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.