Abstract
Background and objective
Pulmonary Daoyin (PD) (evolved from ancient Chinese daoyin skills), is a rehabilitation technology that combines specially designed movements of the arms and body and controlled breathing exercises, to improve the physiological and psychological status of patients with chronic respiratory disease. Pulmonary rehabilitation is effective for patients with chronic obstructive pulmonary disease (COPD), and the efficacy of PD is unknown. The aim of this study is to investigate the effect of a PD program in enhancing activity tolerance, patient-reported outcomes and satisfaction with the effectiveness on patients with COPD.
Materials and methods
The multi-center, randomized controlled trial was conducted from November 2011 to June 2012 in local communities in cities of the 11 research centers in China. It included COPD patients (moderate to very severe) who were recruited from an outpatient clinic. A randomized controlled study included 464 COPD patients who were randomly allocated either to the PD group, participating in a 3-month, ten times-weekly supervised PD-based pulmonary rehabilitation program, or to a control group continuing with regular medical treatment alone. Data were gathered using the 6-minute walking distance (6MWD) test, COPD patient-reported outcomes (COPD-PRO) and Effectiveness Satisfaction Questionnaire for COPD (ESQ-COPD), which was filled out at baseline and 3 months post-intervention. SAS 9.2 was used for statistical analysis.
Results
Of the 464 patients in the study, 461 were included in the full analysis set (FAS); 429 were in the per-protocol analysis set (PPS). After 3-month intervention, there was a significant difference between the two groups in 6MWD (FAS; P=0.049; PPS; P=0.041), total score and all domains of COPD-PRO (FAS; P=0.014; PPS; P=0.003) and ESQ-COPD (FAS; P=0.038; PPS; P<0.001).
Conclusions
The PD program was able to improve the activity tolerance level and satisfaction of COPD patients because of its effectiveness.
Keywords: chronic obstructive pulmonary disease, pulmonary rehabilitation, pulmonary daoyin, patient reported outcomes, effectiveness satisfaction, 6-minute walking distance
Introduction
Chronic obstructive pulmonary disease (COPD) is the forth leading cause of death worldwide, and its prevalence and consequent mortality is expected to increase in the coming decades.1 In China, COPD is the third leading cause of death from Global Burden of Disease Study 2013,2 and an estimated 65 million people will die of COPD between 2003 and 2033.3 COPD is characterized by a progressive deterioration of debilitating symptoms and increasingly frequent exacerbations. Its common symptoms are chronic cough, abnormal sputum, and breathlessness. Initially, patients lose their exercise tolerance due to airflow limitation, and the condition gradually worsens. The goal of treatment in COPD is to reduce symptoms, frequency and severity of exacerbations, and improve exercise tolerance and health status.4
Pulmonary rehabilitation (PR) has been defined as an “evidence-based, multidisciplinary and comprehensive intervention for patients with chronic respiratory disease who are symptomatic and often have decreased daily life activities.”5 PR is a cornerstone of management for patients with COPD, in whom it decreases respiratory rate (by prolonging expiration). The benefits of PR have been extensively reported in COPD, with the assumption that the recommendations are applicable to subjects with other lung diseases.6 In 2006, PR was included in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) for the therapy of stable COPD by the American Thoracic Society (ATS) for the first time, and was the only recommended non-pharmacological treatment.5 The positive effects of PR programs on functional outcome parameters such as dyspnea, exercise capacity, and health-related quality of life have been investigated and proven mostly in patients with COPD,7–9 and are recommended in the recent treatment guideline.4
Traditional Chinese exercise therapy has a long history. Through a combination of mind, breathing and limb movement, it improves body organs function, regulates blood, dredges the meridians, gains the balance between body and mind, and achieves disease prevention, health care and longevity. The commonly used methods are Daoyin, Tai Chi and Qigong.10,11 Daoyin is a form of exercise that is easy to learn and requires no specific training equipment. It should be considered a potential substitute for the PR. Although the effects of daoyin for chronic respiratory disease have been reported in some studies, data supporting it in patients with COPD are scarce and the effects are largely unknown. Therefore, pulmonary daoyin (PD) has been established on the basis of the daoyin skills and theory of Traditional Chinese Medicine (TCM) with the characteristics of COPD. Thus, the aim of our study was to evaluate the benefit of PD program in patients with COPD in terms of activity tolerance, patient-reported outcomes and satisfaction with the effectiveness.
Pulmonary daoyin
Daoyin is a health preservation and therapy method of ancient China that combines specially designed movements of the limbs and trunk and controlled breathing exercises.12 It has the effects of dredging the meridians, promoting the circulation of Qi and blood, cultivating primordial Qi, strengthening the body resistance to eliminate pathogenic factors, stretching the tendons and activating the collaterals, and keeping Yin and Yang in balance.13 Daoyin has some advantages and characteristics in prevention and treatment of diseases, and has irreplaceable advantages in preventive treatment of diseases and the application of kinesiotherapy compared with modern medicine.13 Daoyin is Chinese traditional exercise that includes Tai Chi, Qigong and Baduanjin (eight-section brocades). Studies have shown the benefit of Tai Chi and Qigong in improving lung function, physical performance, activity tolerance level and quality of life in COPD patients.14–16 PD, a TCM PR technology, was established on the basis of the daoyin skills and theory of TCM with the characteristics of COPD. It is a gentle meditative technique that includes a series of physical movements, breathing exercises, and mind regulation. It is based on the principle of integrating and harmonizing one’s mind, breath, posture, and movement. Its effectiveness lies in the element of special breathing and respiratory muscle training, which are important aspects of respiratory management.17 Thus the effects of PD on COPD patients are worthy of further investigation.
Methods
Study design
The protocol of this study has been published in the Journal of Integrative Medicine in 2013.17 This study utilized a multicenter, cluster randomized controlled clinical trial. Subjects were randomly assigned to one of the two groups, namely PD and control group. The random number was generated by SAS 9.2 software. The trial was registered in the ClinicalTrials.gov (NCT01482000) on 29 November 2011, and was conducted from November 2011 (when the first patient was enrolled) to April 2013 (when the last patient completed). All patients signed the informed consent before inclusion, and ethical approval was obtained from the Ethical Research Committee of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine. The batch number is 2011HL-034.
Sample size
According to previous results of a Tai Chi and COPD study,18 forced expiratory volume in 1 second (FEV1) was 0.96±0.39 in the Tai Chi group after 3 months, with 0.11 litre decrease compared with the control group (0.85±0.35). The allowable error (δ) value was 0.11, and the SD value was 0.37, with a power of 0.10 at a 5% significant level (two-sided), and 193 subjects per group were required. In order to cover the potential attrition rate of 20%, 464 subjects (232 per group) were targeted.
Selection of subjects
Subjects clinically diagnosed with COPD according to the Global Strategy for the Diagnosis, Management, and Prevention of COPD, and the Chinese Treatment Guidelines of COPD4,19 were eligible for inclusion in this study. Exclusion criteria can be viewed in the published study protocol.17 Subjects were recruited from the local communities in cities of the 11 research centers.
Intervention protocol
All subjects received patient education. Patients in the PD group were taught the PD technique and continued with their usual therapy; in the control group, patients continued with their usual therapy. The protocol of this study has been published, and more about the patient’s health education and Pulmonary Daoyin actions can be found in the published research program.17
Subjects in the PD group completed a 3-month PD program, which consisted of two sessions per day at least 5 days each week. Along with PD pictures, a DVD was also given to each subject to facilitate daily self-practice. A diary was also provided to each subject for recording the frequency of their self-practice sessions.
Measurement
The 6-min walking distance (6MWD) test, COPD patient-reported outcomes (COPD-PRO) and Effectiveness Satisfaction Questionnaire for COPD (ESQ-COPD) were used because of their simplicity and sensitivity when utilized for health evaluation purposes and their response to the treatment. The 6MWD protocol following ATS guidelines was used.20 The COPD-PRO21 and ESQ-COPD22,23 was developed and validated by our team according to the highest standards and international development specification. Data collection was performed at baseline and 3-months.
Data analysis
Data analyses were conducted using SAS 9.2 software. Descriptive statistics were used to define the demographic characteristics of the sample. The paired-sampled t-test or independent-sampled t-test were used to examine the outcome measures. To account for differences between the two groups at baseline, comparisons between the PD and control groups were conducted using an analysis-of-variance model including the baseline values as covariates (analysis of covariance). A P-value of 0.05 (two-sided) was taken as the level of significance. To preserve the value of randomization, an intention-to-treat (ITT) analysis was applied. Data of last observations were carried forward for withdrawals.
Results
Demographic data
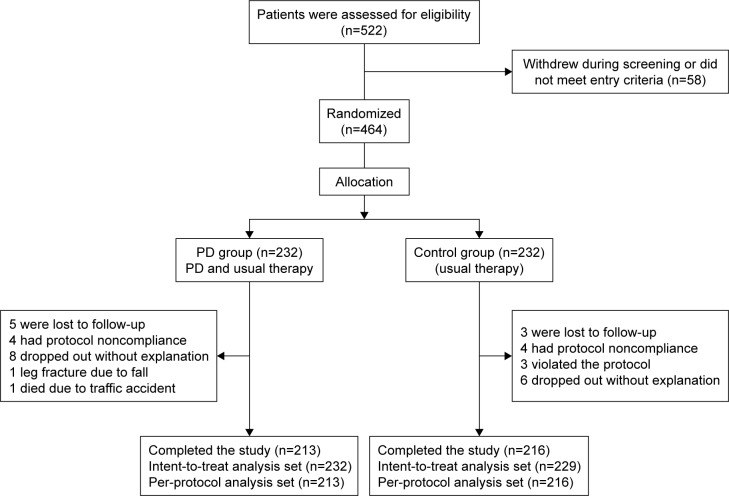
In total, 464 subjects were randomly assigned to each of the following groups: the PD group (n=232) and control group (n=232). A total of 429 patients fully completed the study. Therefore, the per-protocol analysis set (PPS) population was 429 with 213 in the PD group and 216 in the control group. The full analysis set (FAS) population was 461 with 232 in the PD group and 229 in the control group. Patient enrollment and completion values for the study are shown in Figure 1.
Figure 1.
The consort flowchart: to track participants through the randomized controlled trial.
Abbreviation: PD, pulmonary daoyin.
There was no significant difference in gender, age, the course of disease, lung function, and GOLD classification between the two groups (FAS, PPS: P>0.05).
Demographic characteristics by group allocation are shown in Table 1. The conventional Western medication of the two groups before randomization and research period are shown in Tables 2 and 3.
Table 1.
Baseline characteristics of the patients
| Characteristics | Full analysis set
|
Per-protocol analysis set
|
||||||
|---|---|---|---|---|---|---|---|---|
| PD group n=232 | Control group n=229 | t/χ2/Z | P-value | PD group n=213 | Control group n=216 | t/χ2/Z | P-value | |
| Age (years) | 63.38±9.70 | 63.44±11.00 | −0.055 | 0.956 | 63.35±9.75 | 63.35±11.03 | −0.004 | 0.996 |
| Course of diseasea | 147.16±125.86 | 148.71±129.74 | −0.202 | 0.840 | 149.00±127.82 | 147.40±129.37 | −0.083 | 0.934 |
| BMIb | 23.34±3.06 | 23.52±3.62 | −0.573 | 0.567 | 23.42±2.98 | 23.48±3.67 | −0.206 | 0.837 |
| Exacerbationc | ||||||||
| Frequency (times) | 1.2±1.3 | 1.30±1.82 | −0.364 | 0.716 | 1.24±1.29 | 1.33±1.85 | −0.586 | 0.558 |
| Duration (days) | 11.3±5.5 | 12.54±8.85 | −0.818 | 0.413 | 11.34±5.46 | 12.50±9.00 | −0.622 | 0.534 |
| Lung functiond | ||||||||
| FVC (liters) | 2.22±0.69 | 2.30±0.80 | −1.143 | 0.254 | 2.23±0.69 | 2.29±0.77 | −0.868 | 0.386 |
| FEV1 (liters) | 1.20±0.49 | 1.22±0.49 | −0.421 | 0.674 | 1.21±0.50 | 1.21±0.49 | 0.067 | 0.947 |
| FEV1% | 46.57±16.58 | 49.18±16.83 | −1.602 | 0.110 | 47.02±16.48 | 89.89±16.95 | −1.101 | 0.272 |
| Gender | ||||||||
| Male | 152 | 146 | 0.157 | 0.692 | 139 | 136 | 0.246 | 0.620 |
| Female | 80 | 83 | 74 | 80 | ||||
| Smoking status | ||||||||
| Current smoking | 134 | 125 | 0.471 | 0.492 | 122 | 114 | 0.877 | 0.349 |
| Non-smoking | 98 | 104 | 91 | 102 | ||||
| Smoking | 230.43±225.74 | 194.75±209.56 | −1.754 | 0.079 | 230.14±226.99 | 187.31±208.32 | −2.016 | 0.044 |
| Pack-years | ||||||||
| Cigarettes per day | 11.63±12.87 | 10.33±12.50 | −1.195 | 0.232 | 11.11±12.07 | 10.19±12.66 | −1.164 | 0.245 |
| GOLD stagee | ||||||||
| Stage II | 98 | 118 | −1.805 | 0.071 | 93 | 111 | −1.258 | 0.209 |
| Stage III | 96 | 78 | 88 | 72 | ||||
| Stage IV | 38 | 33 | 32 | 33 | ||||
Notes: Data presented as mean ± standard deviation or n.
The course of disease was calculated in months.
The BMI is the weight in kilograms divided by the square of the height in meters.
Exacerbations during the 12 months before screening were self-reported.
Clinical data are from visit 1 (the screening visit).
Severity grades of lung function were determined by guidelines of COPD.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Obstructive Lung Disease.
Table 2.
Baseline of original respiratory medications
| Medications | PD group | Control group | χ2 | P-value |
|---|---|---|---|---|
| GOLD 2 | n=98 | n=118 | ||
| Albuterol sulfate inhalation aerosol | 20 | 18 | 1.149 | 0.765 |
| Formoterol fumarate dehydrate | 10 | 14 | ||
| Albuterol sulfate inhalation aerosol combined with formoterol fumarate dehydrate | 6 | 9 | ||
| Irregular medication | 62 | 77 | ||
| GOLD 3 | n=96 | n=78 | ||
| Albuterol sulfate inhalation aerosol | 2 | 1 | 3.336 | 0.852 |
| Formoterol fumarate dehydrate | 3 | 1 | ||
| Budesonide aerosol | 4 | 1 | ||
| Budesonide/formoterol | 18 | 15 | ||
| Albuterol sulfate inhalation aerosol combined with formoterol fumarate dehydrate | 5 | 3 | ||
| Albuterol sulfate inhalation aerosol combined with fluticasone propionate aerosol | 2 | 2 | ||
| Salmeterol/fluticasone propionate | 23 | 16 | ||
| Irregular medication | 39 | 39 | ||
| GOLD 4 | n=38 | n=33 | ||
| Theophylline | 2 | 1 | 0.718 | 0.982 |
| Carbocysteine | 1 | 1 | ||
| Salmeterol/fluticasone propionate | 8 | 8 | ||
| Budesonide/formoterol | 6 | 4 | ||
| Tiotropium bromide powder for inhalation | 7 | 5 | ||
| Irregular medication | 14 | 14 |
Abbreviations: GOLD, Global Initiative for Obstructive Lung Disease; PD, pulmonary daoyin.
Table 3.
Comparison of the usual therapy in both groups
| Medications | PD group | Control group | Z | P-value |
|---|---|---|---|---|
| GOLD 2 | −0.808 | 0.419 | ||
| Based on therapy of GOLD 1, add one long-acting bronchodilator (when needed): Formoterol fumarate dehydrate | 58 | 72 | ||
| GOLD 3 | ||||
| Based on therapy of GOLD 1, add long-acting bronchodilators and inhaled glucocorticosteroids if repeated exacerbations: Salmeterol/fluticasone propionate | 79 | 70 | ||
| GOLD 4 | ||||
| Inhaled corticosteroid and long-acting beta2–agonist or long-acting anticholinergic | 30 | 33 |
Abbreviations: GOLD, Global Initiative for Obstructive Lung Disease; PD, pulmonary daoyin.
Comparison of 6MWD
There was no significant difference in the mean value of 6MWD between the two groups before treatment (FAS: P=0.899; PPS: P=0.960). After 3 months, the mean value of 6MWD in all the groups was significantly higher than before treatment (P<0.001; Figure 2). At 3 months, the mean value of 6MWD was significantly higher in the PD group compared with the control group (FAS: P=0.049; PPS: P=0.041). The program appeared to have significant effect on the measures of the difference of 6MWD between the two groups, in change from baseline (Table 4).
Figure 2.
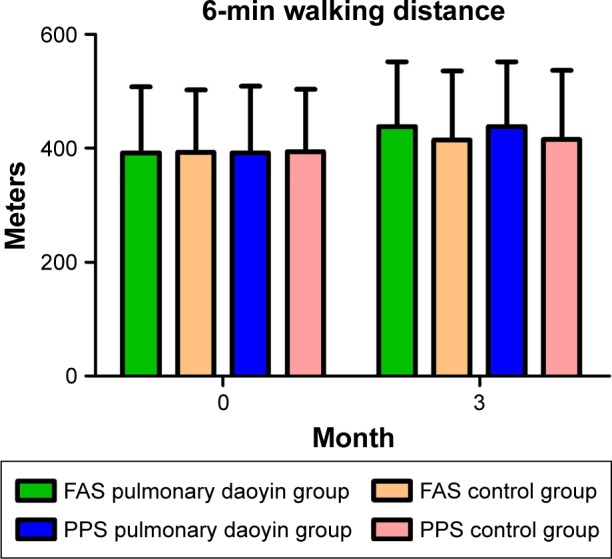
Comparison of the results of 6-minute walking distance.
Abbreviations: FAS, full analysis set; PPS, per-protocol analysis set.
Table 4.
Change in 6MWD, COPD-PRO and ESQ-COPD baseline values 3 months after enrollment, by intervention status
| Variables | Absolute values after program
|
Difference between groups
|
|
|---|---|---|---|
| PD group | Control group | Change from baseline
|
|
|
|
|||
| (n=232) | (n=229) | (95% CI) | |
| 6MWD (m) | 434.40±114.95 | 413.05±116.86* | 38.91 (31.82, 46.00)a |
| COPD-PRO total score | 40.89±7.83 | 42.71±8.02* | −5.46 (−6.31, −4.60)a |
| Clinical symptoms | 31.19±6.57 | 32.40±6.33* | −3.72 (−4.43, −3.01)b |
| Health satisfaction | 5.21±1.42 | 5.45±1.64 | −0.80 (−0.95, −0.64)b |
| Effectiveness | 4.49±1.26 | 4.86±1.37** | −0.94 (−1.10, −0.79)a |
| ESQ-COPD total score | 68.34±7.45 | 65.19±9.41** | 6.46 (5.54, 7.38)a |
| Capacity for life and work | 17.80±3.41 | 16.97±3.27** | 1.30 (0.98, 1.62)a |
| Clinical symptoms | 18.82±2.82 | 18.22±3.32* | 2.66 (2.30, 3.02)a |
| Effect of therapy | 16.13±1.96 | 15.24±2.51** | 1.36 (1.13, 1.59)a |
| Convenience of therapy | 12.28±1.41 | 11.59±1.82** | 0.72 (0.55, 0.89)a |
| Whole effect | 3.31±0.88 | 3.14±0.89* | 0.42 (0.32, 0.51)a |
Notes:
P<0.05,
P<0.01 for comparison with baseline values (unpaired t-test).
P<0.01,
P<0.05, for comparison of difference in change from baseline between groups (analysis of covariance). Values are mean ± SD.
Abbreviations: 6MWD, 6-minute walking distance; COPD, chronic obstructive pulmonary disease; COPD-PRO, COPD patient-related outcomes; ESQ-COPD, Effectiveness Satisfaction Questionnaire for COPD; PD, pulmonary daoyin.
Comparison of COPD-PRO
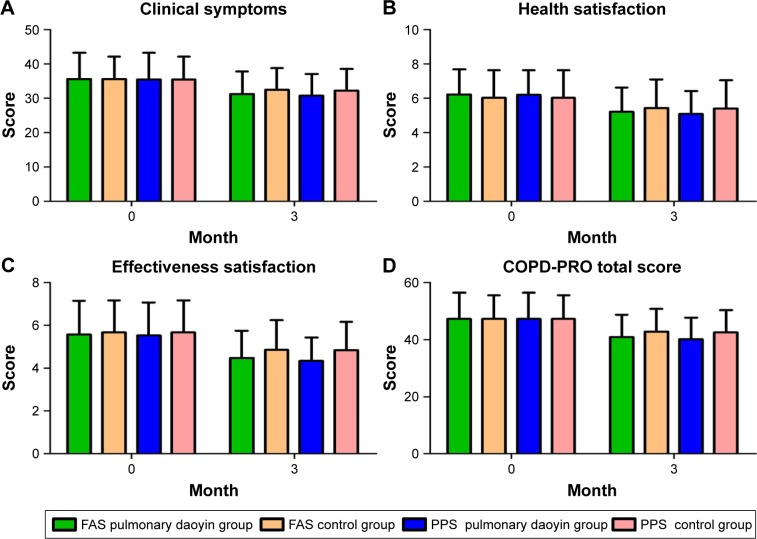
There was no significant difference in the COPD-PRO scores of the two groups before treatment (FAS, PPS: P>0.05). After 3 months, the COPD-PRO scores in all groups were significantly lower than before treatment (P<0.001; Figure 3). At 3 months, the PD group had significantly lower COPD-PRO scores compared with those of the control group (FAS, PPS: P<0.05). The program appeared to have significant effect on measures of the difference in clinical symptoms, effectiveness satisfaction, health satisfaction and the total score between the two groups in change from baseline (Table 4).
Figure 3.
Comparison of the results of COPD-PRO.
Note: The specific changes and comparisons of the results for COPD-PRO in clinical symptom domain (A), health satisfaction domain (B), effectiveness satisfaction domain (C) and total score (D) at baseline and third month between two groups.
Abbreviations: COPD, chronic obstructive pulmonary disease; FAS, full analysis set; COPD-PRO, COPD patient-reported outcomes; PPS, per-protocol analysis set.
Comparison of ESQ-COPD
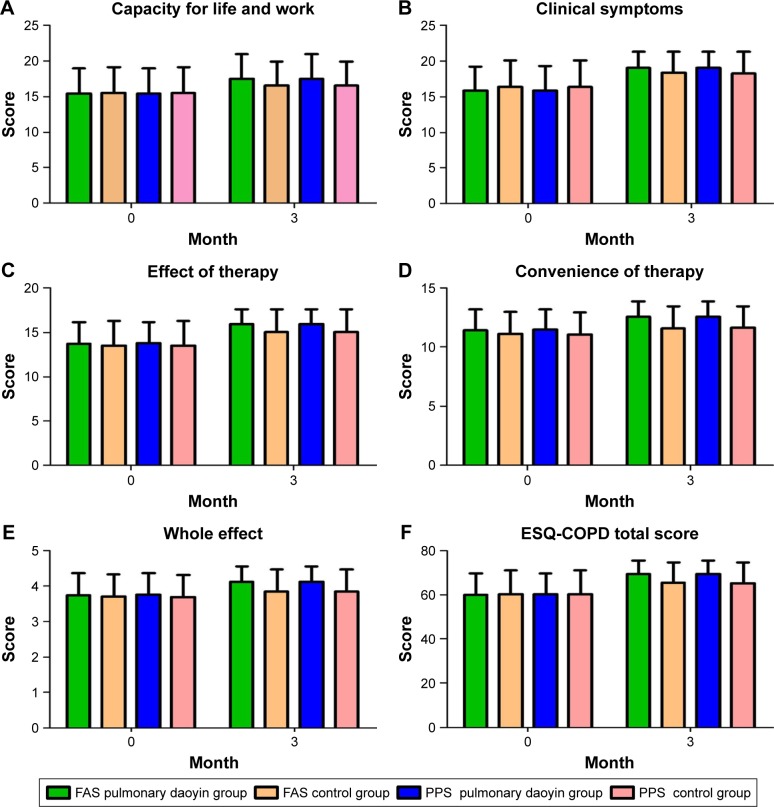
There was no significant difference in the ESQ-COPD scores of the two groups before treatment (FAS, PPS: P>0.05). After 3 months, the ESQ-COPD scores in all groups were significantly higher than before treatment (P<0.001; Figure 4). At 3 months, the PD group had significantly higher ESQ-COPD scores compared with those of the control group (FAS, PPS: P<0.05). The program appeared to have significant effect on measures of the difference of capacity for life and work, clinical symptoms, effect of therapy, convenience of therapy, whole effect domain and the total score between the two groups in change from baseline (Table 4).
Figure 4.
Comparison of the results of ESQ-COPD.
Note: The specific changes and comparisons of the results for ESQ-COPD in capacity for life and work domain (A), clinical symptoms domain (B), effect of therapy domain (C), convenience of therapy domain (D), whole effect domain (E) and total score (F) at baseline and third month between two groups.
Abbreviations: COPD, chronic obstructive pulmonary disease; ESQ-COPD, Effectiveness Satisfaction Questionnaire for COPD; FAS, full analysis set; PPS, per-protocol analysis set.
Discussion
Patients with COPD frequently complain of dyspnea and exercise limitation and become trapped in a vicious cycle of inactivity, initiated by breathlessness.24,25 Exercise training, the important part of PR, has been shown to improve dyspnea and health status and decrease health care use.24 In addition to smoking cessation, PR is the most important method in the management of COPD.26 Although the effectiveness of daoyin in COPD patients has been reported, most studies were not randomized controlled trials. In addition, sample sizes were small, outcome parameters were not accurate and there was no specific rehabilitation technique for COPD. Therefore, a randomized controlled trial was conducted and demonstrated the efficacy of PD in patients with COPD. Our study suggests that the PD program has positive effects on exercise capacity in COPD patients. The results of our study show that the patient-reported outcomes were reduced, while satisfaction with the effectiveness of the patients significantly increased after the 3-month-long PD program. This exercise program can be recommended as an effective alternative training modality in PR programs.
Decreased exercise capacity is one of the main symptoms of COPD patients. Numerous studies have confirmed reduced exercise tolerance of COPD patients with increased airway resistance, ineffective ventilation, hyperinflation and increased elastic load to breathing, gas exchange abnormalities and a mechanical disadvantage of the respiratory muscles;27,28 moreover, reduced exercise capacity further reduces patients’ quality of life.29 In addition, skeletal muscle dysfunction has been reported as an important contributor to exercise limitation in COPD. Therefore, assessment of exercise capacity helps evaluate motor function, quality of life, and prognosis in these patients.
The 6MWD mainly reflects the exercise capacity of patients for comprehensive evaluation of moderate-to-severe disease of systemic functional status. It is a valid indicator for evaluating exercise capacity in patients with clinically stable COPD.30,31 The 6MWD is also considered useful in the determination of the point at which patients should be listed for rehabilitation programs.32 The analysis of 6MWD in this study showed a statistically significant increase in the PD group compared with the control group. The PD program has been established on the basis of the daoyin skills and theory of TCM with the characteristics of COPD. The improvement of exercise capacity is considered to be associated with physical movements and breathing exercises, thereby improving ventilation and exercising skeletal muscle. PD is a form of exercise that requires minimal equipment and no specific training facility, and should be considered a potential substitute for the PR that is currently prescribed.
Symptoms in COPD patients may impair exercise capacity and quality of life. Therefore, an important aim of the assessment and treatment programs in COPD patients is symptom management. In the present study, PR has good advantages in improving clinical symptoms and enhancing the quality of life. The COPD-PRO has inherent correlation with the evaluation of efficacy of Chinese medicine based on clinical symptoms. The COPD-PRO as a valid indicator for evaluating PR can solve the problem of a single evaluation. According to the highest standards and procedures of international scales, the COPD-PRO was developed and validated by our study group.33 The COPD-PRO contains 17 items in three domains: amelioration of clinical symptoms, satisfaction of health condition, and satisfaction of treatment effect. The COPD-PRO has good validity, reliability and responsiveness. The COPD-PRO can provide patients’ responses to the treatments and then evaluate the effect of treatment in a standardized way. Using the COPD-PRO, our results showed that after 3 months of PR, the improvement of effectiveness satisfaction was 13.55% in the PD group and 9.51% in the control group, and there was more improvement of the effectiveness satisfaction scores of the COPD-PRO in the PD group than that in the control group. Dyspnea, cough, and phlegm have been shown to be the main COPD symptoms. The PD program improves the quality of life by reducing symptoms of COPD based on the principle of integrating and harmonizing one’s mind, breath, posture, and movement. Effectiveness satisfaction is defined as how patients evaluate the process of taking the current treatment and their response to the treatment.34 The evaluation of effectiveness satisfaction, although inherently subjective, can reflect the patient’s unique perspective and perceptions on the process, efficiency, and outcomes of the medical care and treatment.35 The ESQ-COPD was developed and examined by our group according to the highest standards and procedures of international scales, and contains 18 items in five domains: capacity for life and work (five items), clinical symptoms (five items), effect of therapy (four items), convenience of therapy (three items), and whole effect domain (one item).36 The ESQ-COPD proved to be a reliable and structurally valid instrument through evaluation,37 and has been used to evaluate the effect of traditional Chinese medicine on satisfaction in COPD patients.38 Using the ESQ-COPD, our results showed that after 3 months of PR, the improvement of effectiveness satisfaction was 13.60% in the PD group and 7.82% in the control group, and there was more improvement of the effectiveness satisfaction scores of the ESQ-COPD in the PD group than in the control group. The PD program improves the COPD patient’s efficacy satisfaction by improving exercise tolerance and reducing clinical symptoms.
Despite the fact that much of the evidence pertaining to the physiological benefit of exercise is based on conventional physical exercise, such as Tai Chi, Baduanjin (eight-section brocades) and Qigong, this study confirms that PD program has a good clinical efficacy. “PD is a gentle meditative technique that applies a series of physical movements, breathing exercises, and mind regulation. It is based on the principle of integrating and harmonizing one’s mind, breath, posture, and movement. Its effectiveness lies in the element of special breathing and respiratory muscle training which are important aspects of COPD management.”17 Thus PD may be a suitable exercise for COPD patients in the community.
Conclusion
This study confirms that the 3-month PD program has beneficial effects on activity capacity and satisfaction of effectiveness of COPD patients. The benefits of practicing PD on COPD clients should be further investigated with a longer follow-up period in order to detect further improvements in physiological and psychosocial status.
Acknowledgments
The authors would like to acknowledge Mr Xue-feng Yu (Department of Respiratory, The Second Hospital of Liaoning University of TCM, Shenyang, People’s Republic of China), Mr Nian-zhi Zhang (Department of Respiratory, The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, People’s Republic of China), Mr Wei Zhang (Department of Respiratory, Shanghai Shuguang Hospital affiliated with Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China), Mr Qing Miao (Department of Respiratory, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China), Mr Gui-ying Liu (Department of Respiratory, The First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China), Mr Zhi-Jia Sun (Department of Respiratory, The First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine, Guangzhou, People’s Republic of China), Mr Zheng-hang Ge (Department of Respiratory, The Second Hospital of Guiyang University of Traditional Chinese Medicine, Guiyang, People’s Republic of China), Mrs Lin Lin (Department of Respiratory, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, People’s Republic of China) and Mrs Yan Dong (Department of Respiratory, The Affiliated Hospital of Chengdu University of Chinese Medicine, Chengdu, People’s Republic of China) for volunteer recruitment, data collection, and providing individualized and continual support for this research.
This work was funded by 2011 Special Fund for TCM-scientific Research in the Public Interest of Ministry of Finance, People’s Republic of China, and State Administration of Traditional Chinese Medicine (No 201107002).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Word Health Organization . The top 10 causes of death. 2000–2015. Geneva: Word Health Organization; 2015. [Accessed January 5, 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/ [Google Scholar]
- 2.Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 3.Lin HH, Murray M, Cohen T, Colijin C, Ezzati M. Effects of smoking and solid-fuel use on COPD, lung cancer, and tuberculosis in China: a time-based, multiple risk factor, modelling study. Lancet. 2008;372(9648):1473–1483. doi: 10.1016/S0140-6736(08)61345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the diagnosis, management, and prevention of COPD. 2016. [Accessed January 5, 2016]. Available from: http://www.goldcopd.org.
- 5.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173(12):1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 6.Lacasse Y, Goldstein R, Lasseron TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;18(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;23(2):CD003793. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan CC, Chu WH, Yang MC, Lee CH, Wu YK, Wu CP. Benefits of pulmonary rehabilitation in patients with COPD and normal exercise capacity. Respir Care. 2013;58(9):1482–1488. doi: 10.4187/respcare.02051. [DOI] [PubMed] [Google Scholar]
- 9.Gloeckl R, Marinov B, Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respir Rev. 2013;22(128):178–186. doi: 10.1183/09059180.00000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu W, Liu X, Wang L, Hu J, Yan J. Effects of Tai Chi on exercise capacity and health-related quality of life in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:1253–1263. doi: 10.2147/COPD.S70862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding M, Zhang W, Li K, Chen X. Effectiveness of t’ai chi and qigong on chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Altern Complement Med. 2014;20(2):79–86. doi: 10.1089/acm.2013.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang MW, Sun XY. Chinese Qiong therapy. Jinan: Shandong Science and Technology Press; 1985. pp. 6–7. [Google Scholar]
- 13.Yu J, Sun Z, Chang W, et al. The mechanism and clinical application of daoyin method. J Shandong University of TCM. 2016;40(2):105–109. [Google Scholar]
- 14.Wu W, Liu X, Wang L, Wang Z, Hu J, Yan J. Effects of Tai Chi on exercise capacity and health-related quality of life in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:1253–1263. doi: 10.2147/COPD.S70862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding M, Zhang W, Li K, Chen X. Effectiveness of t’ai chi and qigong on chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Altern Complement Med. 2014;20(2):79–86. doi: 10.1089/acm.2013.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AW, Lee A, Suen LK, Wam WW. Tai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complement Ther Med. 2011;19(1):3–11. doi: 10.1016/j.ctim.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Yu XQ, Li JS, Li SY, et al. Functional and psychosocial effects of PD on patients with COPD in China: study protocol of a multicenter randomized controlled trial. J Integr Med. 2013;11(2):140–146. doi: 10.3736/jintegrmed2013015. [DOI] [PubMed] [Google Scholar]
- 18.Chan AW, Lee A, Suen LK, Tam WW. Tai chi Qigong improves lung functions and activity tolerance in COPD clients: a single blind, randomized controlled trial. Complement Ther Med. 2011;19(1):3–11. doi: 10.1016/j.ctim.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 19.COPD Study Group of Chinese Society of Respiratory Disease Treatment guidelines of chronic obstructive pulmonary disease (revised 2013) Zhonghua Jie He He Hu Xi Za Zhi. 2013;36(4):255–264. [Google Scholar]
- 20.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Li JS, Wang MH, Yu XQ, et al. Study and preliminary assessment of chronic obstructive pulmonary disease patients report circulating ending scale. China J Chin Med. 2011;26(3):270–274. [Google Scholar]
- 22.Li JS, Wang MH, Yu XQ, et al. The development of the effectiveness satisfaction questionnaire of COPD for chronic obstructive pulmonary disease patient. Liaoning Zhong Yi. 2011;38(7):1251–1253. [Google Scholar]
- 23.Li Jian-sheng, Xie Yang, Yu Xue-qing, et al. An evaluation of self-efficacy and satisfaction with the effectiveness of Bu-Fei Yi-Shen granule combined with acupoint sticking therapy in patients with chronic obstructive pulmonary disease. Eur J Int Med. 2013;5(4):313–325. [Google Scholar]
- 24.Divo M, Pinto-Plata V. Role of exercise in testing and in therapy of COPD. Med Clin North Am. 2012;96(4):753–766. doi: 10.1016/j.mcna.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Singh S, Harrison S, Houchen L, Wagg K. Exercise assessment and training in pulmonary rehabilitation for patients with COPD. Eur J Phys Rehabil Med. 2011;47(3):483–497. [PubMed] [Google Scholar]
- 26.Candemir I, Kaymaz D, Ergun P, Demir N, Egesel N, Sengul F. Assessment of pulmonary rehabilitation efficacy in chronic obstructive pulmonary disease patients using the chronic obstructive pulmonary disease assessment test. Expert Rev Respir Med. 2015;9(4):487–492. doi: 10.1586/17476348.2015.1067608. [DOI] [PubMed] [Google Scholar]
- 27.Lin WC, Yuan SC, Chien JY, Weng SC, Chou MC, Kuo HW. The effects of respiratory training for chronic obstructive pulmonary disease patients: a randomised clinical trial. J Clin Nurs. 2012;21(19–20):2870–2878. doi: 10.1111/j.1365-2702.2012.04124.x. [DOI] [PubMed] [Google Scholar]
- 28.Crisafulli E, Venturelli E, Biscione G, et al. Exercise performance after standard rehabilitation in COPD patients with lung hyperinflation. Intern Emerg Med. 2014;9(1):23–31. doi: 10.1007/s11739-011-0727-z. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z, Zhang SQ. Effects of Qigong on respiratory function and quality of life in patients with stable chronic obstructive pulmonary disease. J Trad Chin Med. 2012;32(8):803–804. [Google Scholar]
- 30.Spruit MA, Polkey MI, Celli B, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2012;13(3):291–297. doi: 10.1016/j.jamda.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6 minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637–643. doi: 10.1183/09031936.00140507. [DOI] [PubMed] [Google Scholar]
- 32.Andrianopoulos V, Holland AE, Singh SJ, et al. Six-minute walk distance in patients with chronic obstructive pulmonary disease: which reference equations should we use? Chron Respir Dis. 2015;12(2):111–119. doi: 10.1177/1479972315575201. [DOI] [PubMed] [Google Scholar]
- 33.Li JS, Wang MH, Yu XQ, Li SY, Xie Y. Development and validation of a patient reported outcome instrument for chronic obstructive pulmonary diseases. Chin J Integr Med. 2015;21(9):667–675. doi: 10.1007/s11655-014-1982-4. [DOI] [PubMed] [Google Scholar]
- 34.Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7(2):204–215. doi: 10.1111/j.1524-4733.2004.72252.x. [DOI] [PubMed] [Google Scholar]
- 35.Rofail D, Taylor F, Regnault A, Filonenko A. Treatment satisfaction instruments for different purposes during a product’s lifecycle: keeping the end in mind. Patient. 2011;4(4):227–240. doi: 10.2165/11595280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Li JS, Wang MH, Yu XQ, et al. Study on the effectiveness satisfaction questionnaire of COPD for chronic obstructive pulmonary disease patient. Zhong Yi Yao Xin Xi. 2010;17(6):8–9. [Google Scholar]
- 37.Li JS, Wang MH, Yu XQ, et al. The development of the Effectiveness Satisfaction Questionnaire of COPD for chronic obstructive pulmonary disease patient. Liaoning Zhong Yi. 2011;38(7):1251–1253. [Google Scholar]
- 38.Li JS, Xie Y, Yu XQ, et al. An evaluation of self-efficacy and satisfaction with the effectiveness of Bu-Fei Yi-Shen granule combined with acupoint sticking therapy in patients with chronic obstructive pulmonary disease. Eur J Int Med. 2013;5(4):313–325. [Google Scholar]