Abstract
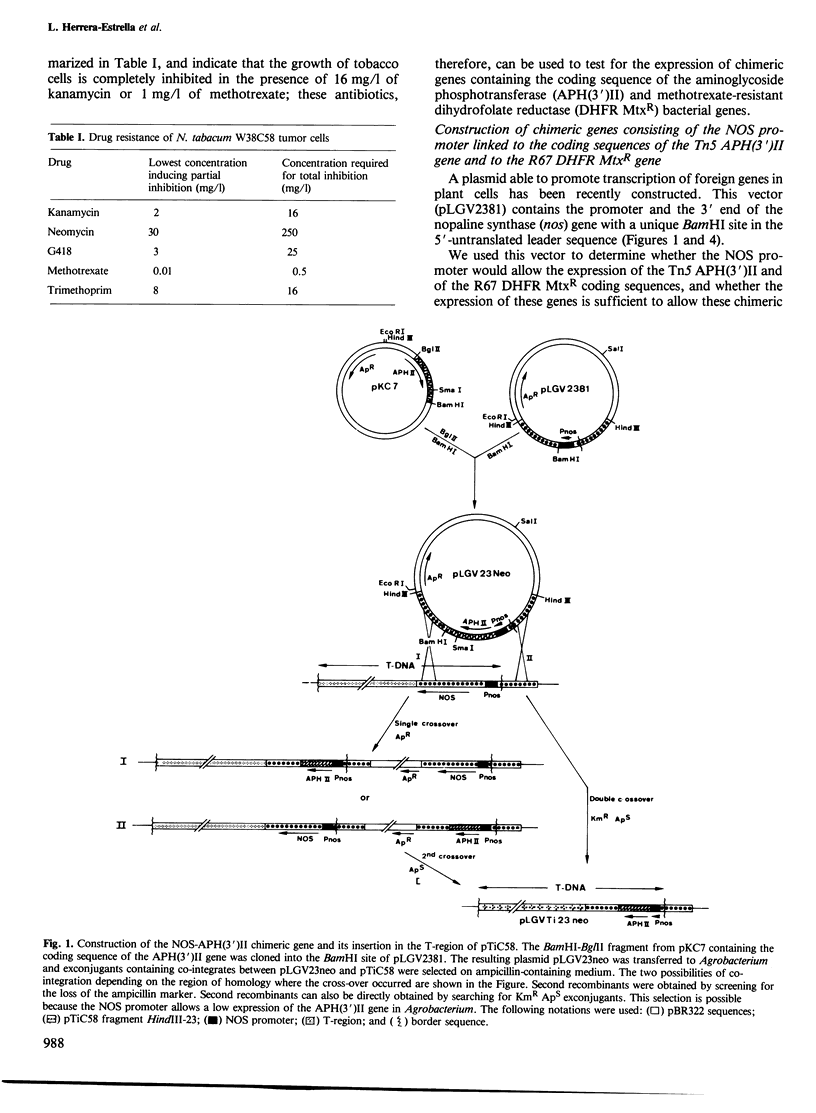
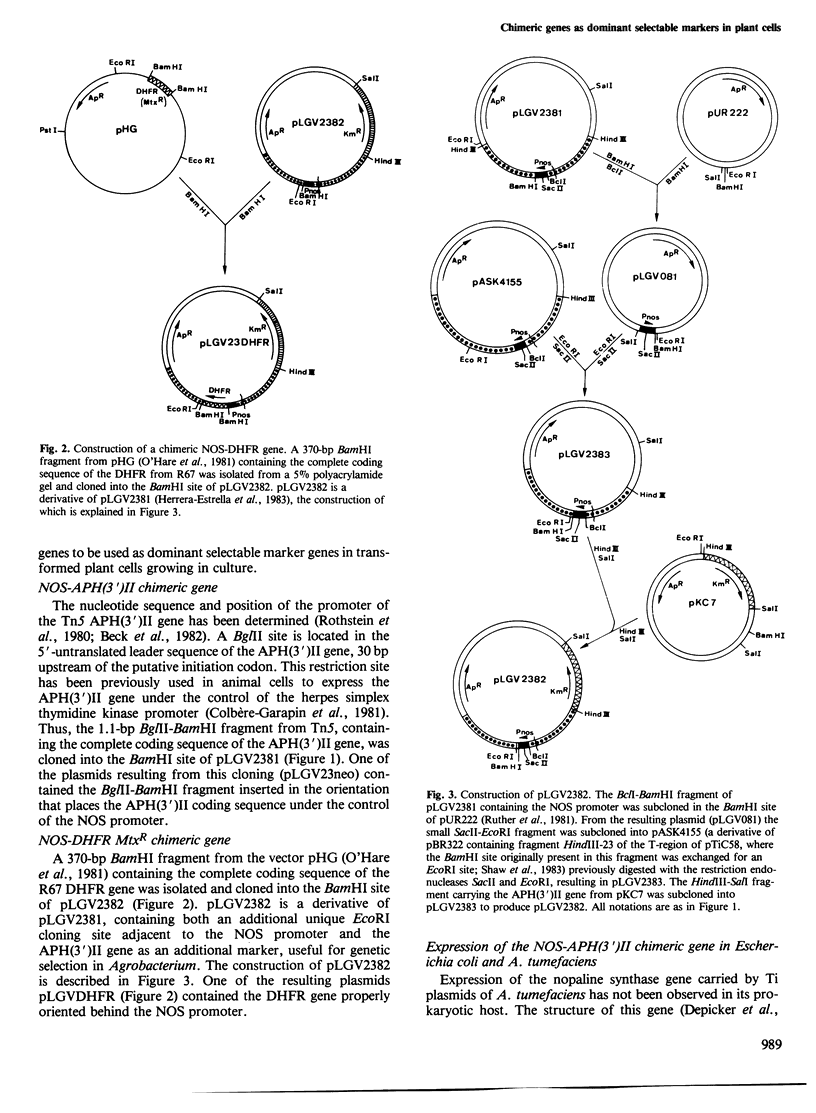
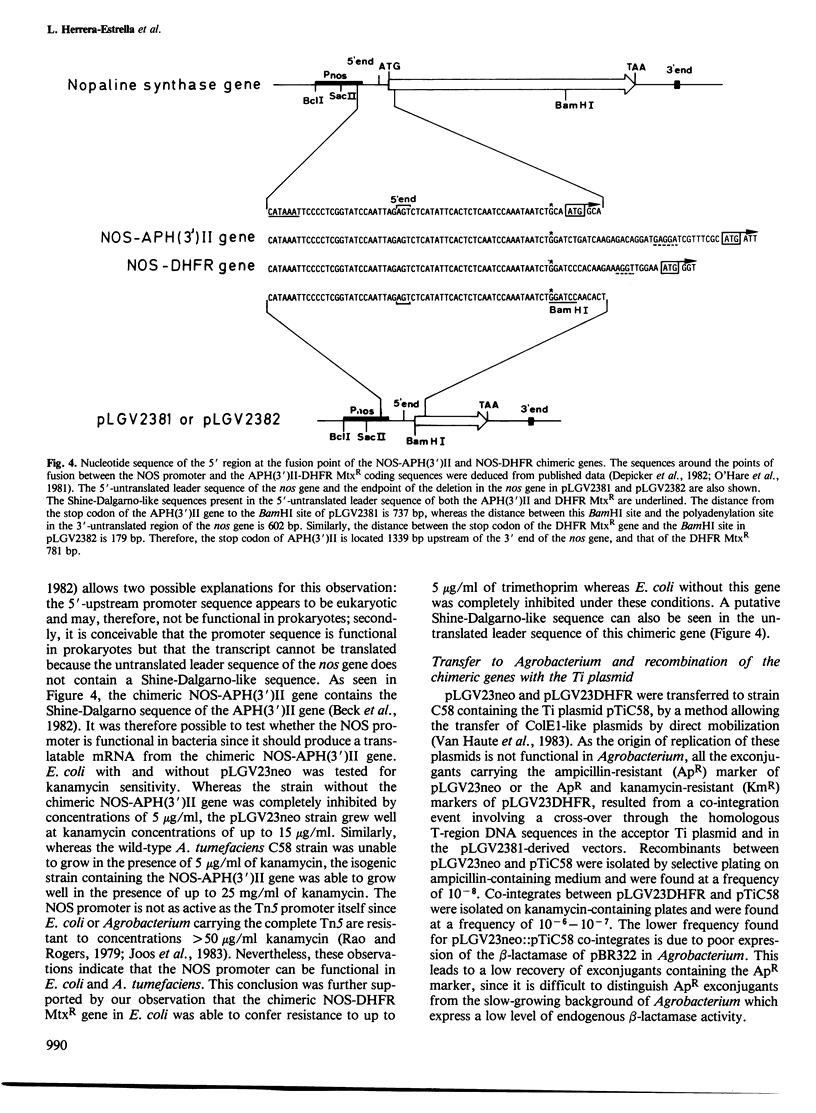
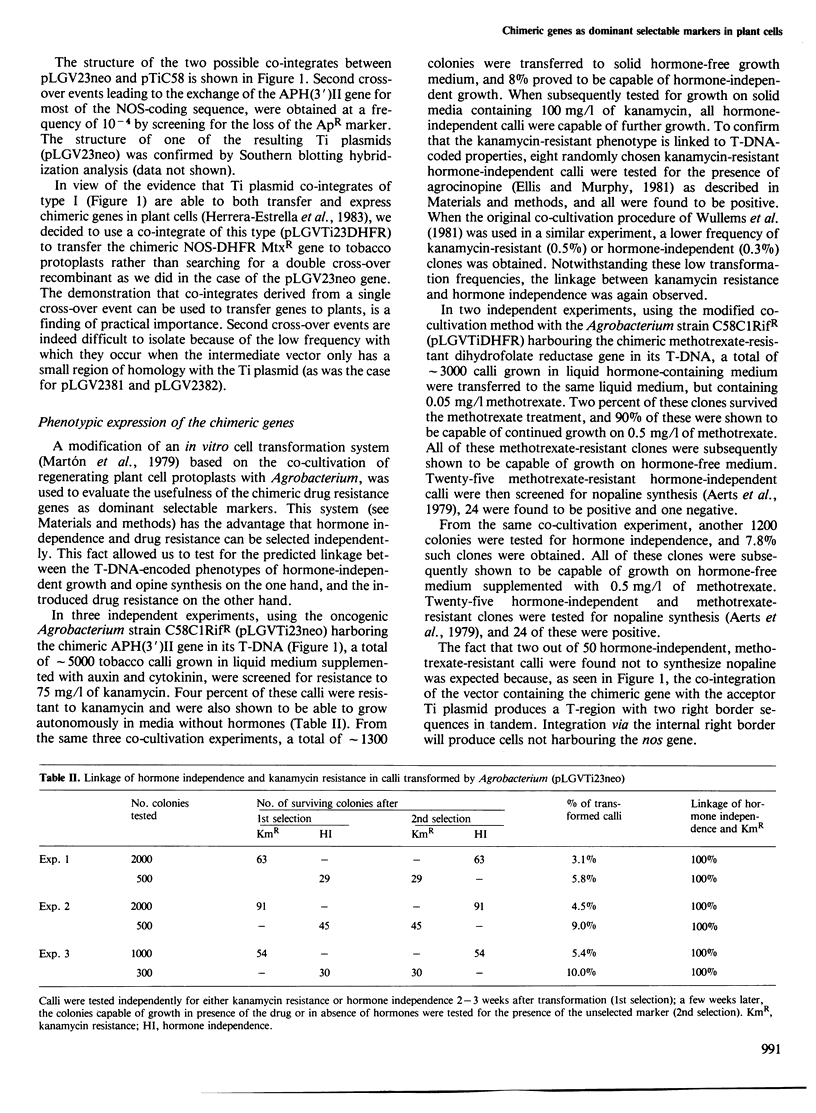
Opine synthases are enzymes produced in dicotyledonous plants as the result of a natural gene transfer phenomenon. Agrobacteria contain Ti plasmids that direct the transfer, stable integration and expression of a number of genes in plants, including the genes coding for octopine or nopaline synthase. This fact was used as the basis for the construction of a number of chimeric genes combining the 5' upstream promoter sequences and most of the untranslated leader sequence of the nopaline synthase (nos) gene with the coding sequence of two bacterial genes: the aminoglycoside phosphotransferase (APH(3')II) gene of Tn5 and the methotrexate-insensitive dihydrofolate reductase (DHFR MtxR) of the R67 plasmid. The APH(3')II enzyme inactivates a number of aminoglycoside antibiotics such as kanamycin, neomycin and G418. Kanamycin, G418 and methotrexate are very toxic to plants. The chimeric NOS-APH(3')II gene, when transferred to tobacco cells using the Ti plasmid as a gene vector, was expressed and conferred resistance to kanamycin to the plant cells. Kanamycin-resistant tobacco cells were shown to contain a typical APH(3')II phosphorylase activity. This chimeric gene can be used as a potent dominant selectable marker in plants. Similar results were also obtained with a NOS-DHFR MtxR gene. Our results demonstrate that foreign genes are not only transferred but are also functionally expressed when the appropriate constructions are made using promoters known to be active in plant cells.
Keywords: selectable marker genes, Ti plasmid, plant-cell transformation, recombinant DNA, antibiotic resistance
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A. C. A Physiological Basis for Autonomous Growth of the Crown-Gall Tumor Cell. Proc Natl Acad Sci U S A. 1958 Apr;44(4):344–349. doi: 10.1073/pnas.44.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- De Greve H., Dhaese P., Seurinck J., Lemmers M., Van Montagu M., Schell J. Nucleotide sequence and transcript map of the Agrobacterium tumefaciens Ti plasmid-encoded octopine synthase gene. J Mol Appl Genet. 1982;1(6):499–511. [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Gielen J., Seurinck L., Van Montagu M., Schell J. Identification of sequences involved in the polyadenylation of higher plant nuclear transcripts using Agrobacterium T-DNA genes as models. EMBO J. 1983;2(3):419–426. doi: 10.1002/j.1460-2075.1983.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling M. E., Elwell L. P. Protein expression in Escherichia coli minicells containing recombinant plasmids specifying trimethoprim-resistant dihydrofolate reductases. J Bacteriol. 1980 Feb;141(2):779–785. doi: 10.1128/jb.141.2.779-785.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Hirth K. P., Edwards C. A., Firtel R. A. A DNA-mediated transformation system for Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7356–7360. doi: 10.1073/pnas.79.23.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A., Davies J. Expression of a transposable antibiotic resistance element in Saccharomyces. Nature. 1980 Oct 30;287(5785):869–871. doi: 10.1038/287869a0. [DOI] [PubMed] [Google Scholar]
- Joos H., Inzé D., Caplan A., Sormann M., Van Montagu M., Schell J. Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell. 1983 Apr;32(4):1057–1067. doi: 10.1016/0092-8674(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Leemans J., Deblaere R., Willmitzer L., De Greve H., Hernalsteens J. P., Van Montagu M., Schell J. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J. 1982;1(1):147–152. doi: 10.1002/j.1460-2075.1982.tb01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans J., Shaw C., Deblaere R., De Greve H., Hernalsteens J. P., Maes M., Van Montagu M., Schell J. Site-specific mutagenesis of Agrobacterium Ti plasmids and transfer of genes to plant cells. J Mol Appl Genet. 1981;1(2):149–164. [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A., Márton L. Streptomycin-resistant plants from callus culture of haploid tobacco. Nat New Biol. 1973 Jul 4;244(131):29–30. doi: 10.1038/newbio244029a0. [DOI] [PubMed] [Google Scholar]
- Morejohn L. C., Fosket D. E. Higher plant tubulin identified by self-assembly into microtubules in vitro. Nature. 1982 Jun 3;297(5865):426–428. doi: 10.1038/297426a0. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Benoist C., Breathnach R. Transformation of mouse fibroblasts to methotrexate resistance by a recombinant plasmid expressing a prokaryotic dihydrofolate reductase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1527–1531. doi: 10.1073/pnas.78.3.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauncz J. K. Thin-layer chromatography of basic water-soluble antibiotics on resin-coated chromatoplates. J Antibiot (Tokyo) 1972 Nov;25(11):677–678. doi: 10.7164/antibiotics.25.677. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Yin J. C., Yong-di Z., Johnson R. C., Reznikoff W. S. Genetic organization of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):99–105. doi: 10.1101/sqb.1981.045.01.018. [DOI] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G., Klipp W., Hillebrand A., Ehring R., Koncz C., Schröder J. The conserved part of the T-region in Ti-plasmids expresses four proteins in bacteria. EMBO J. 1983;2(3):403–409. doi: 10.1002/j.1460-2075.1983.tb01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Perucho M., Kurtz D., Dana S., Pellicer A., Axel R., Silverstein S. Transformation of mammalian cells with an amplifiable dominant-acting gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3567–3570. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wullems G. J., Molendijk L., Ooms G., Schilperoort R. A. Differential expression of crown gall tumor markers in transformants obtained after in vitro Agrobacterium tumefaciens-induced transformation of cell wall regenerating protoplasts derived from Nicotiana tabacum. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4344–4348. doi: 10.1073/pnas.78.7.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]



