Abstract
Mycobacterium tuberculosis (Mtb) and helminth infections elicit antagonistic immune effector functions and are co-endemic in several regions of the world. We therefore hypothesized that helminth infection may influence Mtb-specific T-cell immune responses. We evaluated the cytokine profile of Mtb-specific T cells in 72 individuals with pulmonary TB disease recruited from two Sub-Saharan regions with high and moderate helminth burden i.e. 55 from Tanzania (TZ) and 17 from South Africa (SA), respectively. We showed that Mtb-specific CD4 T-cell functional profile of TB patients from Tanzania are primarily composed of polyfunctional Th1 and Th2 cells, associated with increased expression of Gata-3 and reduced expression of T-bet in memory CD4 T cells. In contrast, the cytokine profile of Mtb-specific CD4 T cells of TB patients from SA was dominated by single IFN-γ and dual IFN-γ/TNF-α and associated with TB-induced systemic inflammation and elevated serum levels of type I IFNs. Of note, the proportion of patients with Mtb-specific CD8 T cells was significantly reduced in Mtb/helminth co-infected patients from TZ. It is likely that the underlying helminth infection and possibly genetic and other unknown environmental factors may have caused the induction of mixed Th1/Th2 Mtb-specific CD4 T cell responses in patients from TZ. Taken together, these results indicate that the generation of Mtb-specific CD4 and CD8 T cell responses may be substantially influenced by environmental factors in vivo. These observations may have major impact in the identification of immune biomarkers of disease status and correlates of protection.
Author summary
Mycobacterium tuberculosis (Mtb) and helminth infections are co-endemic in several regions of the world and their immune responses may be mutually antagonistic. We therefore hypothesized that helminth infection would impact and potentially shape Mtb-specific T-cell responses and systemic inflammation in patients suffering from active pulmonary tuberculosis (TB) enrolled from two helminth endemic regions i.e. Tanzania (TZ) and South Africa (SA). In this study, we demonstrate for the first time that TB patients from SA and TZ harbor distinct immune responses to Mtb antigens. Indeed, we showed that Mtb-specific CD4 T-cell responses of TB patients from TZ were composed by a mixed T helper type 1 (Th1) and Th2 responses. In contrast, the cytokine profile of Mtb-specific CD4 T cells of TB patients from SA was dominated by Th1 cells and associated with TB-induced systemic inflammation and elevated serum levels of type I IFN. Taken together, these data indicate that Mtb-specific T-cell responses are diverse in human populations and can be strongly influenced by host and pathogen genetic background, co-infections and yet unknown environmental factors. Identification of correlates of risk and protection from TB disease will help in the rational development of protective T-cell based vaccines against TB, early monitoring TB treatment outcomes and focused follow up of high risk populations.
Introduction
Helminth infections are endemic in many African countries with different prevalence depending on the geographic region helminth species and age of population [1]. Soil transmitted helminth infections are among the most common infections, transmitted via soil contaminated by eggs excreted from human faeces [2]. Of note, helminth infections are co-endemic in many geographic areas endemic for Mycobacterium tuberculosis (Mtb), HIV-1 and Plasmodium falciparum. Therefore, co-infections of helminths with Mtb, HIV-1 and/or Plasmodium falciparum occur in a large proportion of the subjects [3].
Mycobacterium tuberculosis (Mtb) is a facultative intracellular organism, obligate aerobe, infecting primarily lungs via the aerogenic route [4]. It has been recently estimated that 1.7 billion people are infected with Mtb among which 5–15% will develop tuberculosis disease (TB) [5]. To date, the only vaccine available to prevent TB disease consist of an attenuate strain of M. bovis, the Bacillus of Calmette et Guérin (BCG). While BCG immunization protects from life-threatening disseminated forms of TB disease in children, its efficacy in adults is inconsistent [6]. The protective components of Mtb-specific immunity are partially defined. Several studies have underscored the essential role of IL-12/IFN-γ axis in the protection against Mtb infection [7–10]. In addition, an efficient CD4 T-cell response probably associated with type 1 cytokine secretion is associated with the control of Mtb infection, since a severe reduction in the CD4 T cell number during HIV infection or the suppression of their function following anti-TNF-α therapy are associated with increased risk of TB reactivation [11, 12].
The current paradigm of human cellular immunity indicates that functionally-distinct CD4 T-cell populations are specifically involved against a variety of pathogens depending on their size and their intra- or extra-cellular localization. In this model, type 1 helper CD4 T cells (Th1 cells) intervene against viruses and intracellular pathogens, Th2 cells against parasites such as worms and Th17 cells against extracellular pathogens [13, 14] including bacteria and fungi [14, 15]. Consistent with this paradigm, the protective Mtb-specific T-cell response is usually ascribed to typical Th1 response with CD4 T cells producing cytokines such as IFN-γ or TNF-α that contribute to the recruitment of monocytes and granulocytes and activate the anti-microbial activity of macrophages [16, 17]. By contrast, helminth infections induce IL-4/IL-5 and IL-13 producing CD4 T cells and regulatory T cells [18–21] associated with the alternative activation of macrophages for repair of tissues injured upon migration of worms across different body compartments [1]. With regard to the generation of the functionally distinct T helper CD4 T cell populations, the pioneering studies from Romagnani and others [22, 23], clearly demonstrated the critical importance of the cytokine environment, rather than the specific antigen in the development of distinct T helper antigen-specific CD4 T cells [24, 25]. In this regard, the presence of an IL-4 cytokine background favored the development of Th2 specific to pathogens that usually induce the classical Th1 responses [23, 26].
Multiple studies performed in Mtb/helminth co-infected individuals have focused on the impact of helminth infection on 1) TB diagnosis [27–35], 2) reactivation of TB from latently infected individuals (LTBI) [36, 37] and 3) BCG vaccine immunogenicity [38–40]. In addition, it was recently shown that helminth infection may interfere and/or influence innate [41], cellular [42–44] and humoral [45] immune responses to Mtb. However limited information is available on the cytokine profile of Mtb-specific T-cell immune responses in subjects with Mtb/helminth co-infection.
In the present study, we investigated the cytokine profile of Mtb-specific immune response in patients with clinically active TB from two countries i.e. Tanzania (TZ) and South Africa (SA) in the presence or absence of active helminth infections. We provide evidence that the functional profile of Mtb-specific CD4 T cells of TB patients from TZ was characterized by a mixed Th1/Th2 cytokine profile, while that from SA was associated with a typical single IFN-γ and dual IFN-γ/TNF-α Th1 profile.
These results demonstrate that distinct functional profiles of CD4 T-cell responses can be directed against the same pathogen, i.e. Mtb, in human populations from different geographic areas. Ad hoc designed studies will be needed to define the factors driving the distinct functional profiles as well as to determine whether the distinct functional profiles are associated with variation of the TB pathology and/or response to drug therapy.
Results
The aims of the present study were i) to delineate Mtb-specific T-cell responses in patients with pulmonary tuberculosis (TB) and helminth co-infection from two Sub-Saharan countries, e.g. Tanzania (TZ) and South Africa (SA) and ii) to determine the influence of ongoing or past helminth infections on Mtb-specific T-cells responses. Therefore, we analyzed the cytokine profile and cell lineage T cell transcription factor expression in Mtb-specific CD4 T cells and serum cytokine levels in 72 individuals (Table 1). The patients were screened for active helminth infections and/or previous exposure to helminths and the cohort stratified into three groups: 1) active TB patients from SA with no sign of active helminth infections, 2) active TB patients from TZ with no sign of active helminth infections and 3) active TB patients co-infected with helminths from TZ (see flow chart, S1 Fig).
Table 1. Demographic and clinical data.
| ID | Origin | Number | Mean Age | Gender | Helminth infection | BCG vaccination status |
|---|---|---|---|---|---|---|
| TB SA | South Africa | 17 | 36 | 3F/12M | 11% past exposure | 17/17 (100%) |
| TB TZ | Tanzania | 25 | 29 | 9F/16M | 28% past exposure | 25/25 (100%) |
| Mtb/helminthTZ | Tanzania | 30 | 30 | 6F/24M | Ongoing | 30/30 (100%) |
Mtb-specific CD4 T cells from TB patients from TZ have mixed Th1/Th2 cytokine profile
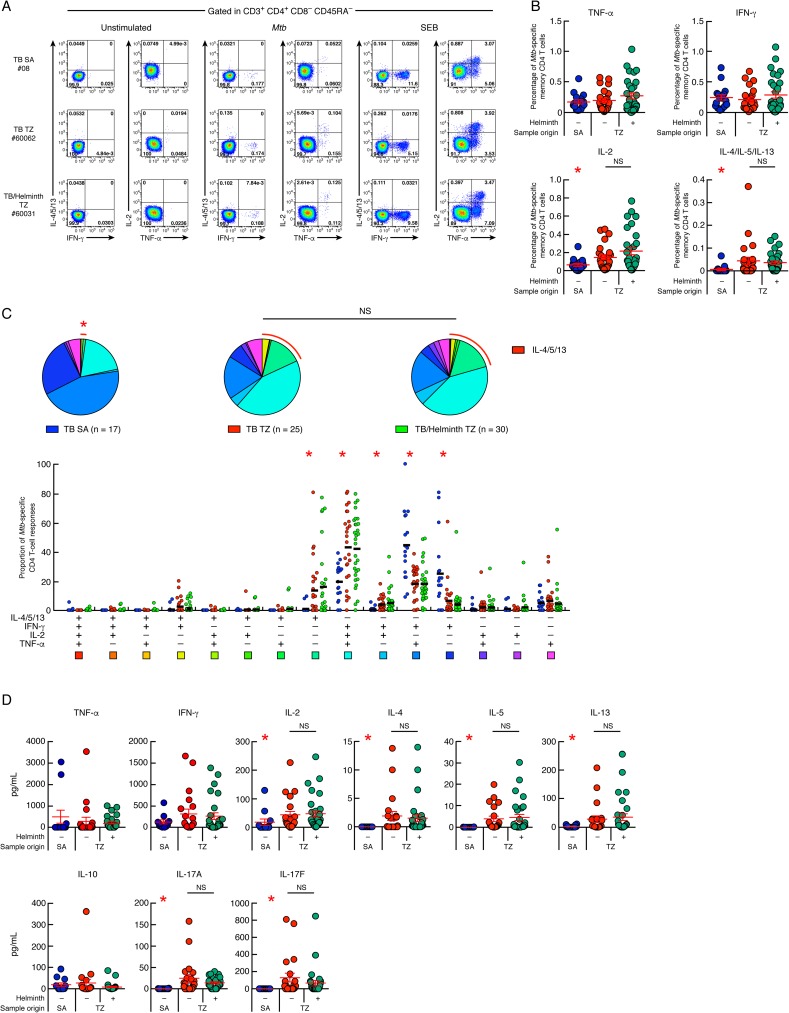
The functional profiles of Mtb-specific CD4 T-cell responses were assessed by intracellular cytokine staining (ICS) according to the gating strategy shown in S2 Fig. In particular, the ability of Mtb-specific CD4 T cells to produce IFN-γ, TNF-α, IL-2, IL-4, IL-5 and/or IL-13 in response to ESAT-6 and CFP-10 peptide pools stimulation was assessed by multi-parametric flow cytometry in 25 TB patients and 30 Mtb/helminth co-infected patients from TZ and compared to 17 TB patients from SA. Of note, Th2 cytokines i.e. IL-4, IL-5 and IL-13 were all assessed in the same flow cytometry fluorescence channel, which allowed the assessment of total Th2 cytokine production but prevented direct identification of individual IL-4, IL-5 or IL-13 Mtb-specific CD4 T-cell responses. Cytokine profiles of Mtb-specific CD4 T cells from three representative TB patients from SA (#08) and TZ (TB (#60062) and Mtb/helminth co-infected patient (#60031) are shown in Fig 1A. We first compared the frequencies of cytokine-producing Mtb-specific memory CD4 T cells from TB patients from SA versus TB patients from TZ (Fig 1B). The cumulative data showed a significantly higher IL-2+ and IL-4/IL-5/IL-13+ Mtb-specific memory CD4 T-cell frequencies in TB patients from TZ compared with TB patients from SA (P<0.05), while the frequencies of IFN-γ and TNF-α producing Mtb-specific memory CD4 T cells were not significantly different between TB patients from TZ and from SA (P>0.05; Fig 1B). Interestingly, no significant differences were observed for Th1 and Th2 cytokine producing CD4 T cells between TB patients from TZ with and without ongoing helminth co-infections (P>0.05) (Fig 1B).
Fig 1. Mtb-specific CD4 T cells from TB patients from TZ have mixed Th1/Th2 cytokine profile.
(A) Representative flow cytometry profile of Mtb-specific CD4 T cells producing IFN-γ, IL-4/5/13, TNF-α and/or IL-2 of one TB patient from SA (#08), one TB patients from TZ (#60062) and one Mtb/helminth co-infected patient from TZ (#60031). Cytokine profiles of CD4 T cells stimulated with SEB (positive control) or left unstimulated (negative control) are also shown. (B) Percentage of Mtb-specific CD4 T cells producing TNF-α, IFN-γ, IL-2 or IL-4/5/13 of TB patients from SA (n = 17), TB patients from TZ (n = 25) and Mtb/helminth co-infected patients from TZ (n = 30). (C) Proportion of Mtb-specific CD4 T-cell responses producing IFN-γ, IL-4/5/13, TNF-α and/or IL-2 of TB patients from SA (n = 17), TB patients from TZ (n = 25) and Mtb/helminth co-infected patients from TZ (n = 30). All the possible combinations of the responses are shown on the x axis and the percentage of the functionally distinct cell populations within the Mtb-specific CD4 T-cell populations are shown on the y axis. Responses are grouped and color-coded on the basis of the number of functions. The pie chart summarizes the data, and each slice corresponds to the fraction of Mtb-specific CD4 T cell response with a given number of functions within the responding CD4 T-cell population. Bars correspond to the fractions of different functionally distinct CD4 T-cell populations within the total CD4 T cells. Red arcs correspond to IL-4/5/13-producing CD4 T-cell populations. (D) Levels of IFN-γ, TNF-α, IL-10, IL-2, IL-4, IL-5, IL-13, IL-17A and IL-17F produced in Mtb-stimulated culture supernatants of TB patients from SA (n = 12), TB patients from TZ (n = 21) and Mtb/helminth co-infected patients from TZ (n = 29) assessed by luminex assay. TB patients were color coded (B and D); TB patients from SA, blue; TB patients from TZ, red and Mtb/helminth co-infected patients, green. Red stars indicate statistical significance. Statistical significance (* = P<0.05) was calculated using one way Anova Kruskal-Wallis test followed by a Mann-Whitney test (B and D). Statistical analyses of the global cytokine profiles (pie charts, C) were performed by partial permutation tests using the SPICE software as described [97].
We next analyzed the cytokine profile of Mtb-specific memory CD4 T cells of TB patients from SA and TZ (Fig 1C, pie charts). The cytokine profile of Mtb-specific memory CD4 T cells of TB patients from SA was significantly different from of TB patients from TZ (P<0.05; Fig 1C, pie charts). Again, no significant differences were observed between TB patients and Mtb/helminth co-infected patients from TZ (P>0.05) (Fig 1C, pie charts). In depth analysis showed that Mtb-specific CD4 T-cell responses of TB patients from TZ were significantly enriched in polyfunctional IFN-γ+IL-2+TNF-α+IL-4/5/13- CD4 T cells (triple IFN-γ/IL-2/TNF-α Mtb-specific CD4 T cells) and in IFN-γ-IL-2-TNF-α-IL-4/5/13+ CD4 T-cell populations (single IL-4/5/13 Mtb-specific CD4 T cells) as compared to TB patients from SA (43–42% versus 20% for triple IFN-γ/IL-2/TNF-α and 14–16% versus 1% for single IL-4/5/13; P<0.05) (Fig 1C). In contrast, Mtb-specific CD4 T-cell responses of TB patients from SA were significantly enriched in IFN-γ+IL-2-TNF-α+IL-4/5/13- (dual IFN-γ/TNF-α Mtb-specific CD4 T cells) and in IFN-γ+IL-2-TNF-α-IL-4/5/13- CD4 T-cell populations (single IFN-γ Mtb-specific CD4 T cells) as compared to TB patients from TZ (about 45% versus 20–18% for dual IFN-γ/TNF-α and 25% versus 7–4% for single IFN-γ+; P<0.05) (Fig 1C). No significant differences were observed between the Mtb-specific CD4 T-cell cytokine profile of TB patients and Mtb/helminth co-infected patients from TZ (P>0.05) (Fig 1C). Of note, Schistosoma mansoni-specific CD4 T-cell responses were evaluated on Mtb/helminth co-infected individuals from TZ using S. mansoni soluble egg antigens (SEA) by polychromatic flow cytometry (n = 7). The results obtained showed that SEA-specific CD4 T-cell responses were dominated by single TNF-α and single IL-4/IL-5/IL-13-producing CD4 T cells, while polyfunctional IFN-γ+IL-2+TNF-α+ CD4 T cells represented less than 5% of total SEA-specific CD4 T-cell responses confirming previous observations [46, 47] (S3 Fig).
To better estimate the influence of 1) different helminth species, 2) infection caused by more than one helminth (polyparasitism), 3) differences in helminth lung migration capacity and 4) past helminth exposure on the generation of Th2 Mtb-specific CD4 T-cells, the proportion of IL-4/5/13-producing Mtb-specific CD4 T cells was compared between TB patients from TZ with helminth infection caused by one or more helminth species (S4A Fig), or between TB patients from TZ coinfected with helminth species exhibiting (hookworms and Strongyloides stercoralis) or not (S. mansoni, Schistosoma haematobium and Wuchereria bancrofti) lung migration capacity (S4B Fig). Similarly, the proportion of IL-4/5/13-producing Mtb-specific CD4 T cells was compared between TB patients from SA or TZ with evidence of past exposure to helminths versus TB patients with no sign of ongoing or past exposure to helminths (S4C and S4D Fig). The cumulative data showed that the proportion of single IL-4/5/13-producing Mtb-specific CD4 T cells from active TB cases was not influenced by 1) polyparasitism (including W. bancrofti, hookworm, S. mansoni, S. haematobium or S. stercoralis) (P>0.05) (S4A Fig), 2) by helminth infections caused by helminth species exhibiting lung migration capacity, or 3) by past exposure to helminths (S4B and S4C Fig).
To further characterize the cytokine profile of Mtb-specific T-cells, multiplex bead array analyses (luminex) were performed on supernatants of ESAT-6/CFP-10 peptide pool (Mtb)-stimulated cell cultures. The cumulative data showed that Mtb-stimulated cell culture supernatants of TB patients from TZ secreted similar levels of IFN-γ, TNF-α and IL-10 (P>0.05), but significantly higher levels of IL-2, IL-4, IL-5, IL-13, IL-17A and IL-17F than Mtb-stimulated cell culture supernatants of TB patients from SA (P<0.05; Fig 1D). However, no significant differences were observed between TB patients and Mtb/helminth co-infected patients from TZ (P>0.05; Fig 1D), confirming our flow cytometry analyses. In addition, the levels of Th2 cytokines secreted in Mtb-stimulated cell culture supernatants of Mtb/helminth patients from TZ was not influenced by individual species of helminth infections, by polyparasitism (P>0.05) (S4E Fig), by helminth species exhibiting lung migration capacity (S4F Fig), or by past exposure to helminths (S4G and S4H Fig).
Taken together, our data indicate that a high proportion of Mtb-specific CD4 T cells from Tanzanian TB patients have a mixed Th1/Th2 cytokine profile which is observed either in patients with active helminth infection or in a large proportion of patients with positive helminth serology. In contrast, Mtb-specific CD4 T cells from South African TB cases have a classical, previously described Th1 cytokine profile.
Memory CD4 T cells of TB patients from TZ harbor increased Gata-3 and reduced T-bet expression
We then determined whether the development of Mtb-specific Th2 CD4 T cells was associated with changes in the expression of Th1 and Th2 cell lineage transcription factors, T-bet and Gata-3 respectively [48, 49].
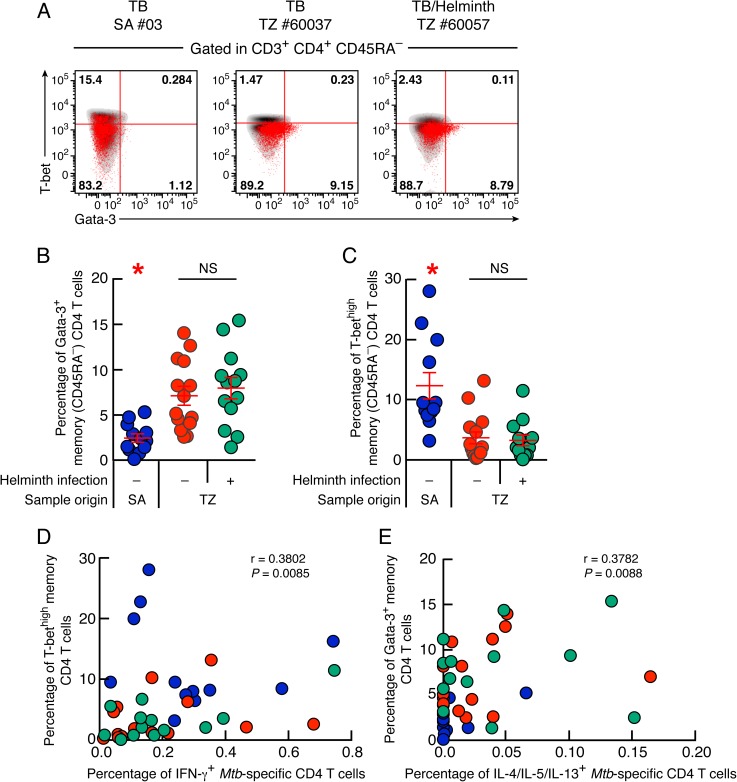
Representative examples and cumulative data showed that the percentage of memory CD4 T cells expressing Gata-3 was significantly increased in TB and Mtb/helminth co-infected patients from TZ (7% and 7.9%, respectively) compared with TB patients from SA (2.4%; P<0.05) (Fig 2A and 2B). In contrast, the percentage of memory CD4 T cells expressing high levels of T-bet (T-bethigh) was significantly lower in TB and Mtb/helminth patients from TZ (3.6% and 3.2%, respectively) as compared to TB patients from SA (12.3%; P<0.05) (Fig 2A and 2C). However, the frequencies of memory CD4 T cells expressing Gata-3 or T-bethigh did not differ between TB patients and Mtb/helminth co-infected patients from TZ (P>0.05) (Fig 2B and 2C). In addition, the frequency of memory CD4 T cells expressing Gata-3 of Mtb/helminth co-infected patients from TZ was not influenced by the helminth species and by polyparasitism (P>0.05) (S4I Fig), by helminth species exhibiting lung migration capacity (S4J Fig), or by past exposure to helminths (S4K and S4L Fig). Interestingly, the percentage of T-bethigh memory CD4 T cells negatively correlated with the percentage of memory CD4 T cells expressing Gata-3 (r = -0.6745; P<0.0001) (S5 Fig) thus supporting previous observations [50].
Fig 2. Memory CD4 T cells of TB patients from TZ harbor increased Gata-3 and reduced T-bet expression.
(A) Representative flow cytometry profile of memory (CD45RA-) CD4 T cells (red dots) isolated from one TB patient from SA (#03), one TB patient (#60037) and one Mtb/helminth co-infected patient (#60057) from TZ expressing Gata-3 and/or T-bet. Gates were set using naïve (CD45RA+) CD4 T cells and CD8 T cells from each individual. Gata-3 and T-bet expression of individual CD8 T-cell are also shown (black dots). Percentage of memory (CD45RA-) CD4 T cells isolated from TB patients from SA (n = 12), TB patients (n = 14) and Mtb/helminth co-infected patients (n = 13) from TZ expressing Gata-3 (B) or T-bethigh (C). (D) Correlation between the percentage of IFN-γ-producing Mtb-specific CD4 T cells and the percentage of memory CD4 T cells expressing T-bethigh of TB patients from SA (n = 12), TB (n = 14) and Mtb/helminth co-infected patients from TZ (n = 13). (E) Correlation between the percentage of IL-4/5/13-producing Mtb-specific CD4 T cells and the percentage of memory CD4 T cells expressing Gata-3 of TB patients from SA (n = 12), TB (n = 14) and Mtb/helminth co-infected patients from TZ (n = 13). TB patients were color coded (B-E); TB patients from SA, blue; TB patients from TZ, red and Mtb/helminth co-infected patients, green. Red stars indicate statistical significance. Statistical significance (* = P<0.05) was calculated using one way Anova Kruskal-Wallis test followed by a Mann-Whitney test (B and C) or Spearman rank test for correlation (D and E).
We then determined whether the expression of T-bet or Gata-3 by memory CD4 T cells was associated with Mtb-specific CD4 T-cell cytokine profile of TB patients and Mtb/helminth co-infected patients from SA or TZ. To address this issue, we plotted the percentage of Mtb-specific CD4 T cells producing IFN-γ or IL-4/5/13 against the percentage memory CD4 T cells expressing T-bethigh or Gata-3 from the same patients (Fig 2D and 2E). The cumulative data showed that the percentage of IFN-γ-producing Mtb-specific CD4 T cells directly correlated with the percentage of T-bethigh memory CD4 T cells (r = 0.3802, P = 0.0085) (Fig 2D) and the percentage of IL-4/5/13-producing Mtb-specific CD4 T cells directly correlated with the percentage of memory CD4 T cells expressing Gata-3 (r = 0.3782, P<0.0088) (Fig 2E).
Taken together, these data indicate that TB patients from TZ have a mixed Th1/Th2 cytokine profile associated with increased Gata-3 and reduced T-bethigh expression.
Reduced proportion of patients with detectable Mtb-specific CD8 T cells in Mtb/helminth co-infected patients
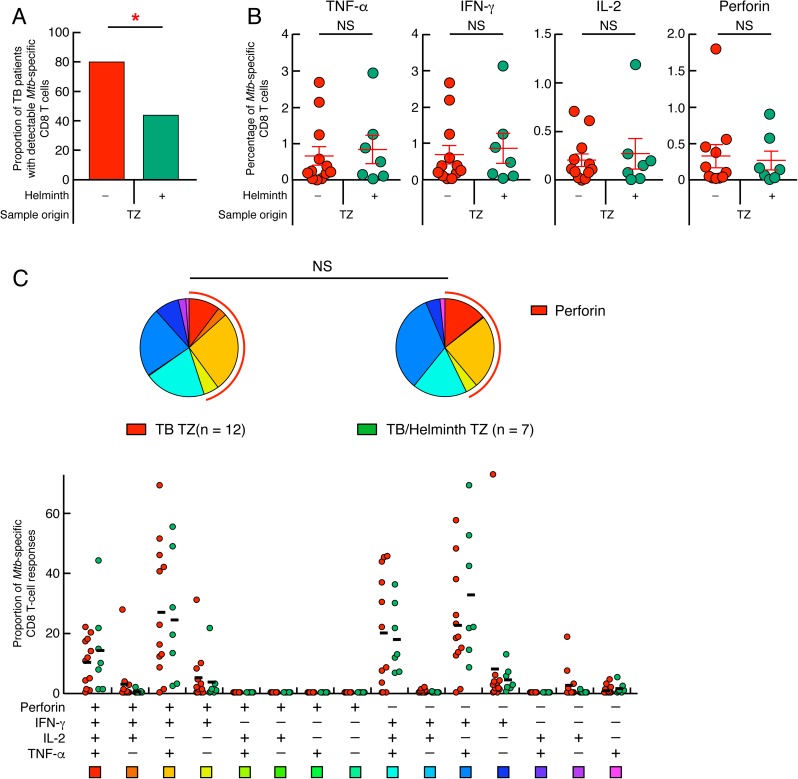
Ongoing helminth infections has been shown to interfere with CD8 T cells responses targeting viruses in mouse models [51, 52]. Our group and others has recently shown that Mtb-specific CD8 T cells were more frequently detected in patients with TB disease as compared to those with latent Mtb infection [53]. Based on this observation, a recent diagnostic test i.e. QuantiFERON TB PLUS proposes optimized CD8-TB-specific-peptides stimulation [54, 55]. This prompted us to investigate whether ongoing helminth infections would influence the proportion of TB patients with detectable Mtb-specific CD8 T cells. To address this issue, the ability of Mtb-specific CD8 T cells to produce IFN-γ, TNF-α, IL-2 and perforin was assessed in 16 TB patients and 23 Mtb/helminth co-infected patients from TZ by flow cytometry. As shown in Fig 3A, the proportion of subjects with detectable Mtb-specific CD8 T cells was significantly reduced in Mtb/helminth co-infected patients from TZ as compared to TB patients from TZ (43.7% versus 80%, respectively; P<0.05; Fig 3A). The frequency of cytokine- and perforin-producing Mtb-specific CD8 T cells and the functional profile of Mtb-specific CD8 T-cell responses did not differ significantly between TB and Mtb/helminth co-infected patients from TZ with detectable Mtb-specific CD8 T cells (Fig 3B) and the predominant CD8 T-cell population was IFN-γ+IL-2-TNF-α+Perforin- (Fig 3C).
Fig 3. Reduced proportion of patients with detectable Mtb-specific CD8 T cells in Mtb/helminth co-infected patients.
(A) Proportion of TB patients with detectable Mtb-specific CD8 T cells of TB and Mtb/helminth patients from TZ (n = 12 and 7, respectively). (B) Percentage of Mtb-specific CD8 T-cell responses producing TNF-α, IFN-γ, IL-2 and/or perforin of TB patients (n = 12) and Mtb/helminth patients (n = 7) from TZ. TB patients were color coded; TB patients from TZ, red and Mtb/helminth co-infected patients, green. (C) Functional profile of Mtb-specific CD8 T cells of TB patients and Mtb/helminth co-infected patients from TZ. Red and green circles represent the proportion of Mtb-specific CD8 T cells producing TNF-α, IFN-γ, IL-2 and/or perforin of TB and Mtb/helminth patients from TZ, respectively (C). Red arcs identify perforin producing cell populations (C). Red stars indicate statistical significance (* = P<0.05). Statistical significance (P<0.05) was calculated using Chi square test (A) and Mann-Whitney test (B). Statistical analyses of the global cytokine profiles (pie charts, C) were performed by partial permutation tests using the SPICE software as described [97].
In summary, these data indicate that ongoing helminth infection reduced the proportion of TB cases with detectable Mtb-specific CD8 T cells.
Differences in systemic inflammation markers in patients from TZ and SA
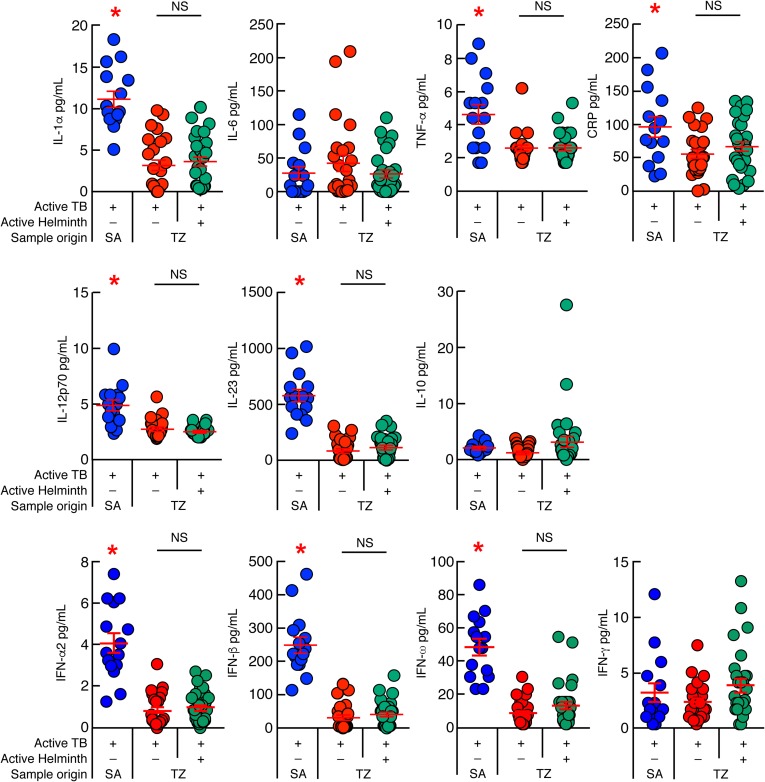
One of the objectives of the present study was to determine whether ongoing helminth infection may influence the levels of systemic inflammation markers. In order to address this issue, we first assessed the serum levels of IL-1α, IL-6, TNF-α, IL-10, IL-12p70, IFN-α2, IFN-β, IFN-ω, IFN-γ, IL-23 and CRP of TB patients from TZ and SA by multiplex bead array analyses (Fig 4). The cumulative data indicated that the serum levels of IL-1α, TNF-α, IL-12p70, IFN-α2, IFN-β, IFN-ω, IL-23 and CRP were significantly increased in TB patients from SA as compared to TB patients from TZ (P<0.05) (Fig 4). However, the levels of IFN-γ, IL-6 and IL-10 were not statistically different between TB patients from SA and TZ (P>0.05; Fig 4). Interestingly, ongoing helminth infection did not further influence the serum levels of cytokines and CRP of TB patients from TZ (P>0.05) (Fig 4).
Fig 4. Differences in systemic inflammation markers in patients from TZ and SA.
Serum levels of IL-1α, IL-6, TNF-α, CRP, IL-12p70, IL-23, IL-10, IFN-α2, IFN-β, IFN-ω and IFN-γ of TB patients from SA (n = 15), TB and Mtb/helminth co-infected patients from TZ (n = 25 and 30, respectively). TB patients were color coded; TB patients from SA, blue; TB patients from TZ, red and Mtb/helminth co-infected patients, green. Red stars indicate statistical significance. Statistical significance (* = P<0.05) was calculated using one way Anova Kruskal-Wallis test followed by a Mann–Whitney test.
Taken together, these data suggest that TB patients from SA and TZ showed differences in serum cytokine profile. In comparison to Tanzanian TB cases, South African patients showed a more pronounced pro-inflammatory serum cytokine profile associated with high levels of type I IFNs.
Th1 cytokine profiles are associated with elevated systemic inflammation markers
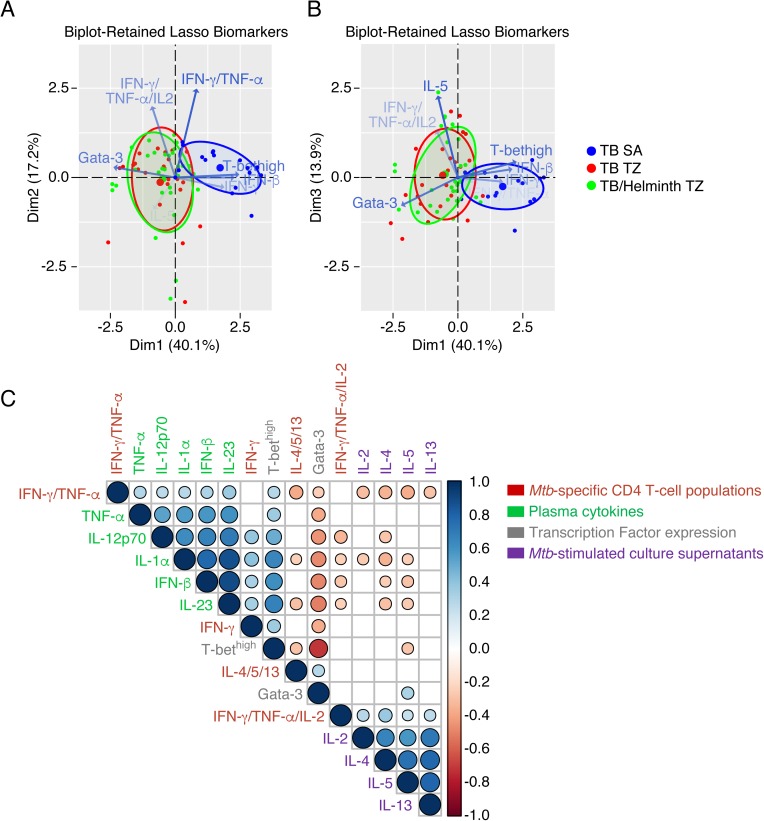
To better identify the immunological parameters associated with Mtb-specific immune signature in TB patients and the influence of ongoing helminth infection, a principal component analysis (PCA) was performed. The results indicated that the Mtb-specific immune profile of TB and Mtb/helminth co-infected patients from TZ clustered away from that of TB patients from SA with a percentage of discrimination reaching about 70% (Fig 5A and 5B). In depth analysis revealed that the differentially expressed immune parameters contributing the most to discriminate Mtb-specific immune response of TB patients from TZ and SA were the percentages of CD4 T cells expressing Gata-3 and T-bethigh, the proportion of polyfunctional TNF-α/IFN-γ/IL-2, dual TNF-α/IFN-γ and single IFN-γ Mtb-specific CD4 T cells among total Mtb-specific CD4 T-cell responses, levels of IL-5 in Mtb-stimulated culture supernatants and IFN-β serum levels (Fig 5A and 5B and Table 2). Again, this analysis did not allow discriminating Mtb-specific immune response of TB and Mtb/helminth patients from TZ (Fig 5A and 5B).
Fig 5. Th1 cytokine profiles are associated with elevated systemic inflammation markers.
Principal component analysis (PCA) of Mtb-specific immunity determined in TB patients from SA (n = 15), TB and Mtb/helminth co-infected patients from TZ (n = 25 and 30, respectively). Blue, red and green symbols represent TB patients from SA, TB patients from TZ, and Mtb/helminth co-infected patients, respectively. Blue, red and green ellipses represent clusters formed by 70% of TB patients from SA, TB patients and Mtb/helminth co-infected patients from TZ, respectively. Blue arrows represent the differentially expressed immune parameters that significantly contributed (P<0.05) to discriminate Mtb-specific immune signatures (A and B). (C) Correlogram of imputed Spearman-Rank-Correlation between the proportion of triple IFN-γ/TNF-α/IL-2, dual IFN-γ/TNF-α, single IFN-γ and single IL-4/5/13 Mtb-specific CD4 T cells among total Mtb-specific CD4 T-cell responses, levels of IL-2, IL-4, IL-5 and IL-13 detected in Mtb-stimulated culture supernatants, percentages of memory CD4 T cells expressing Gata-3 or T-bethigh, serum levels of IL-1α, TNF-α, IL-12p70, IFN-β and IL-23 determined in TB patients from SA and TZ (n = 70). Statistical significance was calculated using non parametric test. Empty squares correspond to non significant correlations (P>0.05), blue circles indicate direct correlations and red circles indicate inverse correlations (P<0.05). Blue and red circle sizes indicate statistical amplitude of correlation.
Table 2. Contribution of the major differentially expressed immune parameters discriminating Mtb-specific immune signatures of TB patients from TZ and SA identified by principal component analysis.
| Axes | Variance (%) | Immune parameters | Correlation | P value |
|---|---|---|---|---|
| Dim1 | 36.6% | T-bethigh | 0.827 | 3.4E-19 |
| Dim1 | 36.6% | IFN-β | 0.767 | 3.9E-15 |
| Dim1 | 36.6% | IFN-γ | 0.635 | 2.1E-09 |
| Dim1 | 36.6% | IFN-γ/TNF-α | 0.277 | 0.018 |
| Dim1 | 36.6% | IL-5 | -0.284 | 0.018 |
| Dim1 | 36.6% | IFN-γ/TNF-α/IL2 | -0.303 | 0.010 |
| Dim1 | 36.6% | Gata-3 | -0.800 | 3.5E-17 |
| Dim2 | 18% | IFN-γ/TNF-α | 0.835 | 7.5E-20 |
| Dim2 | 18% | IFN-γ/TNF-α/IL2 | 0.670 | 1.2E-10 |
| Dim2 | 18% | IL-5 | -0.302 | 0.010 |
| Dim3 | 15% | IL-5 | 0.838 | 4.2E-20 |
| Dim3 | 15% | IFN-γ/TNF-α/IL2 | 0.483 | 1.7E-05 |
| Dim3 | 15% | Gata-3 | -0.288 | 0.014 |
Abbreviations: T-bethigh, percentage of memory CD4 T cells expressing high level of T-bet; IFN-β, serum level of IFN-β; IFN-γ, proportion of single IFN-γ producing Mtb-specific CD4 T cells; IFN-γ/TNF-α, proportion of dual IFN-γ/TNF-α producing Mtb-specific CD4 T cells; IL-5, level of IL-5 detected in supernatants of Mtb-stimulated cell cultures; IFN-γ/TNF-α/IL-2, proportion of polyfunctional IFN-γ/TNF-α/IL-2 producing Mtb-specific CD4 T cells; Gata-3, percentage of memory CD4 T cells expressing Gata-3.
We next performed multiparametric statistical analysis to investigate the potential associations between the four major represented Mtb-specific CD4 T-cell populations i.e. triple IFN-γ/IL-2/TNF-α, dual IFN-γ/TNF-α, single IFN-γ and single IL-4/5/13 Mtb-specific CD4 T cells, the levels of cytokine detected in Mtb-stimulated culture supernatants, i.e. IL-2, IL-4, IL-5 and IL-13, the percentages of memory CD4 T cells expressing Gata-3 or T-bethigh, and the serum levels of IL-1α, TNF-α, IL-12p70, IFN-β and IL-23 in TB patients from SA and TZ (Fig 5C). The combined data indicated that IFN-β serum concentrations positively correlated with i) T-bethigh memory CD4 T cells, ii) higher proportion of Mtb-specific Th1 cells (single IFN-γ and dual IFN-γ/TNF-α), iii) higher serum levels of pro-inflammatory cytokines (IL-1α and TNF-α), IL-12p70 and IL-23 and negatively correlated with a) Gata-3 expression on memory CD4 T cells, b) higher proportion of polyfunctional Mtb-specific CD4 T cells and c) type 2 cytokine secretion (IL-5 and IL-13) (P<0.05) (Fig 5C).
Taken together, the data indicate that the serum cytokine profile and the Mtb-specific immune signatures of TB patients from SA and TZ are significantly different and that Th1 cytokine profiles are positively associated with TB-induced systemic inflammation and higher serum levels of type I IFNs.
Ongoing helminth infection is not associated with reduced anti-mycobacterial treatment outcome
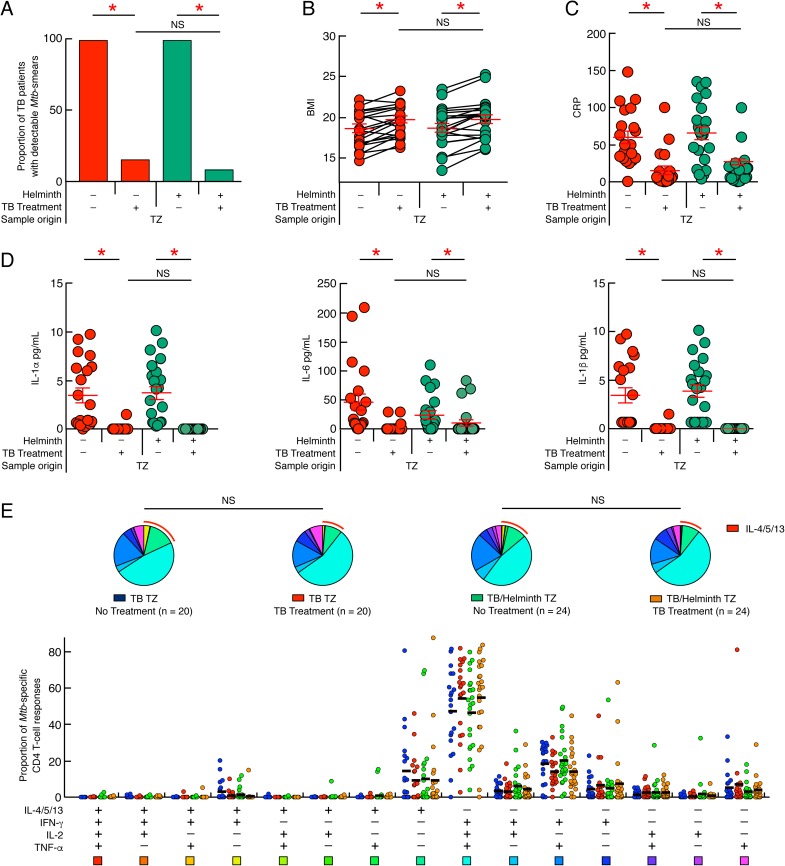
We then assessed the influence of ongoing helminth infection on TB drug treatment efficiency in the Tanzanian cohort. To address this issue, we first assessed the presence of Mtb in the sputum of treated individuals based on sputum smear microscopy. The cumulative data showed a significant reduction in the proportion of patients with detectable Mtb in the sputum following 60 days of drug treatment (P<0.05; Fig 6A). The presence of an ongoing helminth infection did not influence the effect of anti-mycobacterial drug treatment in relation to sputum detectable Mtb (P>0.05) (Fig 6A), suggesting that ongoing helminth infection was not associated with reduced anti-mycobacterial treatment efficiency.
Fig 6. Influence of ongoing helminth infection on TB treatment efficiency.
(A) Proportion of TB patients (n = 20) and Mtb/helminth (n = 24) co-infected patients with detectable Mtb prior to and following 60 days of TB treatment. Impact of TB treatment initiation on BMI (B), serum levels of CRP (C) and serum levels of IL-1α, IL-6 and IL-1β (D) of TB (n = 20) and Mtb/helminth (n = 22) co-infected patients from TZ prior to and following 60 days of TB treatment. Red and green circles represent TB and Mtb/helminth co-infected patients from TZ, respectively. (E) Functional profile of Mtb-specific CD4 T cells of TB patients (n = 20) and Mtb/helminth (n = 24) co-infected patients from TZ prior to and following 60 days of TB treatment. Blue, red, green and orange circles represent the proportion of Mtb-specific CD4 T cells producing TNF-α, IFN-γ, IL-2 and/or IL-4/5/13 in untreated TB patients, 60 days treated TB patients, untreated Mtb/helminth co-infected patients and 60 days treated Mtb/helminth co-infected patients recruited from TZ, respectively (E). TB patients were color coded; untreated TB patients, blue; treated TB patients, red; untreated Mtb/helminth co-infected patients, green; treated Mtb/helminth co-infected patients, orange. Red arcs identify IL-4/5/13 producing cell populations (E). Red stars indicate statistical significance (* = P<0.05). Statistical significance (P<0.05) was calculated using Chi square test (A), Wilcoxon signed-rank test (B and C) and one way Anova Kruskal-Wallis test followed by a Mann-Whitney test (D). Statistical analyses of the global cytokine profiles (pie charts, E) were performed by partial permutation tests using the SPICE software as described [97].
In addition, 60 days of anti-mycobacterial treatment was associated with a significant increase of the body mass index (BMI) and significant decrease of serum levels of CRP and pro-inflammatory cytokines i.e. IL-1α, IL-6, and IL-1β (P<0.05; Fig 6B–6D), irrespective of the presence or absence of ongoing helminth infection (P>0.05) (Fig 6B–6D).
We next assessed the impact of TB treatment initiation on Mtb-specific T-cell immunity in TB and Mtb/helminth patients. The functional profiles of Mtb-specific CD4 T-cell responses and the transcription factor expression profiles were assessed using aforementioned experimental strategies. The cumulative data indicated that the initiation of efficient TB treatment did not significantly influence 1) the Mtb-specific CD4 T-cell functional profiles (P>0.05) (Fig 6E), 2) the nature and the amount of Th2 cytokines produced i.e. IL-4 and/or IL-5 and/or IL-13 (P>0.05) (S6A Fig) and 3) the transcription factor profile of TB and Mtb/helminth infected patients from TZ (P>0.05) (S6B Fig). Indeed, two months after TB treatment, the cytokine profile of Mtb-specific CD4 T cells was still dominated by polyfunctional IFN-γ/IL-2/TNF-α and Th2 Mtb-specific CD4 T cells (Fig 6E).
In summary, two months of TB treatment did not change the functional profile of Mtb-specific CD4 T cells. These data provide evidence that TB treatment outcome during this follow up period was not influenced by presence or absence of ongoing helminth infection.
Discussion
Helminth infections have been shown to impact the control of virus replication in mouse models [52, 56] and interfere with vaccine induced immune responses [57, 58]. Since helminths and Mtb are co-endemic in several regions of the world, including Tanzania [1], we hypothesized that ongoing helminth infection may influence and potentially modulate the functional profile of Mtb-specific T-cell responses. Our hypothesis is founded on the observations that helminths stimulate Th2 type immune responses and previous studies that demonstrated that the cytokine micro-environment may influence, at least in vitro, the functional profile of antigen-specific CD4 T cell responses [59, 60].
In the present study, we provide evidence that a significant proportion of Mtb-specific CD4 T cells in patients with active TB disease from TZ have a Th2 cytokine profile as indicated by the production of IL-4/IL-5/IL-13 and expression of the Th2 cell lineage transcription factor Gata-3. Mtb-specific CD4 T-cells comprised Th1 and Th2 cells with a polyfunctional cytokine profile, and the increased frequency of Gata-3+ memory CD4 T cells was associated with reduced frequency of T-bethigh+ memory CD4 T cells. Interestingly, Mtb-specific CD4 T cells in patients with active TB disease from SA had a typical Th1 profile encompassing single IFN-γ and dual IFN-γ/TNF-α cells. Taken together, these results demonstrate that active TB disease induced the generation of mixed Th1/Th2 Mtb-specific CD4 T cells in patients from TZ whereas a typical Th1 Mtb-specific CD4 T cell response was generated in patients from SA. Further investigations would be needed to determine whether TB-uninfected individuals from TZ and SA harbor different basic response to TB antigens.
These observations are of high interest since the assessment of Mtb-specific CD4 T-cell cytokine profile is consistently proposed to allow the discrimination between active and latent Mtb infections [61–64]. Among, these parameters, high proportions of IL-2-producing Mtb-specific CD4 T cells (in association with Th1 cytokines i.e IFN-γ and/or TNF-α) are associated with individuals with LTBI and therefore Mtb containment, while high proportion of Mtb-specific CD4 T cells producing Th1 cytokines in absence of IL-2 is associated with patients suffering from active TB disease [62–65]. Indeed, reduced capacity to produce IL-2 is usually associated with high antigen load, persistent T-cell stimulation, expression of co-inhibitory molecules and differentiation towards effector memory and/or terminally differentiated effector memory [66, 67]. Interestingly, CD4 T cells coexpressing IFN-γ and TNF-α and harboring a phenotype of effector-memory response were associated with active tuberculosis in HIV-uninfected [68, 69] and HIV-infected TB patients [67]. Of note, polyfunctional helminth-specific CD4 T-cell responses were also recently associated to helminth biological activity [47, 70].
As mentioned above, the study hypothesis was that helminth infection might influence the functional profile of Mtb-specific CD4 T cells. In this context, about 67% of patients from TZ either had an ongoing active helminth infection or had evidence of previous helminth exposure/infection. However, about 11% of the patients from SA also showed a positive serology for previous helminth exposure/infection with helminth but no sign of generation of Mtb-specific Th2 CD4 T cells. Despite this discordance between positive serology for helminth exposure and lack of the generation of Mtb-specific Th2 CD4 T cells in patients from SA, we cannot exclude that the frequency and/or severity of helminth re-infection/exposure in patients from TZ is higher as when compared to South African patients and therefore responsible for the mixed Th1/Th2 functional profile of Mtb-specific CD4 T cells [71, 72]. In addition, we cannot exclude that human or Mtb genetic diversity and other yet unknown environmental factors may contribute to the generation of mixed Th1/Th2 functional cytokine profile and/or influence the durability of the Th2 response after parasite infection is cleared [73] in patients from TZ.
Consistent with previous studies [53, 74], about 80% of TB patients from TZ had Mtb-specific CD8 T cell responses detected by polychromatic flow cytometry. Interestingly, this proportion was significantly reduced (about two fold) in the presence of ongoing helminth infections. These results provide additional evidence that helminth infections may significantly influence the generation of distinct T cell subsets in active TB disease. This important finding echoes with recent data demonstrating that ongoing helminth infection impairs virus-specific T-cell immunity via a STAT-6-dependent alternative activation of macrophages differentiation [52].
In contrast to Tanzanian TB cases, the South African TB patients were characterized by significantly higher serum levels of pro-inflammatory cytokines (IL-1α and TNF-α) in combination with IL-12p70 and type I IFNs. Interestingly, statistical analyses revealed that elevated serum levels of IFN-β were associated with elevated serum levels of pro-inflammatory cytokines and Th1 Mtb-specific CD4 T cells lacking IL-2 co-production. These IFN-β serum levels were inversely correlated with polyfunctional Mtb-specific CD4 T cells and Th2 cytokines detected by polychromatic flow cytometry and in cell culture supernatants. Based on these findings, the so far unappreciated role of IFN-β (and type I IFNs in general) in coordinating TB specific immunity needs to be further explored.
The role of Th1, Th2 and CD8 T cells in the control of Mtb infection and the progression of TB disease is under intense debate. It has been clearly demonstrated that functional impairment of the IL-12p70/IFN-γ axis predisposes to the development of mycobacterial disease [7–10], probably by compromising the phagocytic and cytolytic capacity of macrophages primed with IFN-γ [75]. The role of Mtb-specific CD8 T cells is however still controversial. Some studies indicate that Mtb-specific CD8 T cells may play an important role in protective immunity against TB via the production of perforin and/or cytolysin [76–80], while others indicate that the presence of Mtb-specific CD8 T cells may be detrimental [81], since Mtb-specific CD8 T cells were enriched in TB patients as compared to individuals with LTBI [53]. Excessive IFN-γ production by CD4 or CD8 T cells may in fact favour Mtb transmission via inflammation mediated mucosal damage enabling access of Mtb bacilli to airways [81].
The dual role of IFN-γ in the protection or progression of TB disease may be linked to macrophage hypo-responsiveness to IFN-γ [82], also called progressive exhaustion, which can be mediated by Mtb-induced type I IFNs [83, 84]. Indeed, recent studies demonstrated that Mtb-induced type I IFN might be detrimental, since TB patients with reduced/absent type I IFN signature had reduced bacterial load and/or improved host survival [85–87]. Interestingly, other studies indicate that type I IFN responses enhance CD4 T-cell differentiation towards Th1 [88], enhance CD8 T-cell responses [89] and interfere with IL-23-mediated Th17 cell differentiation and IL-4-mediated Th2-cell differentiation by inhibiting Gata-3 expression [88, 90, 91], supporting our observations.
The reason why TB patients from TZ had lower levels of type I IFNs remains unclear and needs to be further investigated. However, one could postulate that the genetic background of the Mtb strains isolated from TZ and SA, the antigen load and the genetic background of the individuals living in TZ versus SA might be associated with this profile, since the level of Mtb-induced type I IFN production might be strain dependent [92]. Of note, these parameters were not evaluated in the present study and would require further evaluation.
Finally, we did not observe any influence of ongoing helminth infection on the efficacy of TB therapy. Interestingly, after sixty days of treatment, we did not observe changes in Mtb-specific CD4 T-cell cytokine and transcription factor expression profile but strongly reduced CRP serum levels and pro-inflammatory cytokine circulation in combination with improved BMI.
In conclusion, we provide evidence that the generation of Mtb-specific CD4 and CD8 T cell responses, may be substantially influenced by co-infectious agents and possibly genetic and environmental factors resulting in pronounced variations in the qualitative and quantitative profile of pathogen-specific responding T cells in human populations.
Materials and methods
Study group, helminth diagnosis and cell isolation
In total, 72 subjects were recruited to participate in this study. No statistical method was used to predetermine sample size. Fifty-five subjects were recruited at the Mwananyamala Hospital, Dar es Salaam, and the TB clinic of Bagamoyo (TZ). TB patients (n = 55) were selected based on sputum smear microscopy confirmed by GeneXpert assay and HIV infection was ruled out by rapid serological test (Alere Determine HIV-1/2 test). The diagnosis of ongoing and/or past helminth infection was based on assays performed on feces (Kato-Katz thick smear, FLOTAC and Baermann assays), urine (urine filtration), whole blood (Immuno-chromatography) and serum (ELISA) samples at date of blood sample collection and the assay used depended on the helminth species and on the site of sample collection. All TB patients from TZ were screened for active soil-transmitted helminths (hookworms, Ascaris lumbricoides, Trichuris trichiura) and S. mansoni infections using the Kato-Katz thick smear and FLOTAC methods performed on one stool sample at date of blood sample collection, for active S. stercoralis infection using the Baermann technique and for active S. haematobium infection using the urine filtration method. Binax NOW ICT test card were used to detect W. Bancrofti antigen on blood sample [93, 94]. In addition, serology for 7 different helminths (Echinococcus spp, Fasciola hepatica, Filaria, Schistosoma spp, S. stercoralis, Toxocara spp and Trichinella spp) was performed by ELISA. The serodiagnostic helminth screening ELISA is routinely performed at the diagnostic centre of the Swiss Tropical and Public Health Institute and detects helminth specific IgG. A total of 30 Mtb/helminth co-infected patients were recruited. TB patient co-infected with only one helminth species were infected with S. mansoni (n = 7), W. bancrofti (n = 6), hookworms (n = 2), S. haematobium (n = 2) or S. stercoralis (n = 2). Eleven TB patients (36%) were co-infected with multiple helminth species. Blood samples were collected prior to and following 60 days of anti-mycobacterial treatment (“fixed dose combination” consisting of Rifampicin, Isoniazid, Pyrazinamide and Ethambutol, (RHZE)) from 20 TB and 24 Mtb/helminth co-infected patients recruited in TZ. In addition, seventeen subjects were enrolled at the field site of the South African Tuberculosis Vaccine Initiative in the Boland Overberg region of the Western Cape Province of SA (SA). TB disease was diagnosed by positive sputum Xpert MTB/RIF and HIV infection was ruled out by rapid serological test. PBMCs were collected as part of a cross-sectional study, in HIV-negative participants before commencing treatment for TB. Diagnosis of helminth exposure was performed using ELISA detecting helminth-specific IgG (Echinococcus spp, Fasciola hepatica, Filaria, Schistosoma spp, S. stercoralis, Toxocara spp and Trichinella spp).
Ethics statement
All participants were adults and provided written informed consent and the study protocol was approved for TZ by the Ethikkomission beider Basel (EKBB; Basel, Switzerland; reference number 257/08), the Ifakara Health Institute Institutional Review Board and the National Institute for Medical Research (NIMR; Dar es Salaam, United Republic of Tanzania; reference number NIMR/HQ/R.8a/Vol.IX/1098). For SA, the Human Research Ethics Committee of the University of Cape Town granted the study protocol approval.
Antibodies
The following monoclonal antibodies (mAbs) were used in different combinations. CD3-APC-H7 (CloneSK7), CD4-PECF594 or CD4-APC (Clone RPA-T4), CD8-PB (Clone RPA-T8), IFN-γ-AF700 or IFN-γ-APC (Clone B27), TNF-α-PeCy-7 (Clone MAb11), IL-4-PE (Clone 3010.211), IL-2-PE (Clone MQ1-17H12), Gata-3-PeCy-7 (Clone L50-823), all from Becton Dickinson (BD); CD45RA-BV711 (Clone HI100), IL-2-PerCpCy5.5 (Clone MQ1-17H12), IL-5-PE (Clone TRFK5), IL-13-PE (Clone JES10-5A2), T-bet-PerCpCy5.5 (Clone 4B10) were purchased from BioLegend; CD8-Efluor625NC (Clone RPA-T8) from eBioscience; perforin-FITC (Clone B-D48) from Diaclone.
Antigens
Mtb-derived CFP-10 and ESAT-6 peptide pools are composed of 15-mers overlapping by 11 amino-acids encompassing the entire sequences of the proteins and all peptides were HPLC purified (>90% purity).
Ex vivo assessment of CD4 T-cell cytokine profile by ICS
PBMCs were stimulated overnight in complete media (RPMI (Invitrogen), 10% fetal calf serum (FCS; Invitrogen), 100 µg/ml penicillin, 100 unit/ml streptomycin (BioConcept)) with ESAT-6 and CFP-10 peptide pools (1 µg/ml) or with Staphyloccocus enterotoxin B (SEB; 250 ng/mL) or unstimulated in the presence of Golgiplug (1 μl/ml; BD) as previously described [95]. At the end of the stimulation period, cells were washed and stained (20 min; 4°C) for dead cells using the Aqua LIVE/DEAD stain kit (Invitrogen), washed and stained (20 min; 4°C) with mAbs to CD3, CD4, CD8 and CD45RA. Cells were then permeabilized (30 min; 20°C) (Cytofix/Cytoperm, BD) and stained (20 min; 20°C) with mAbs to TNF-α, IFN-γ, IL-2, IL-4, IL-5 and IL-13.
Ex vivo assessment of CD8 T-cell cytokine profile by ICS
PBMCs were stimulated overnight in complete media (RPMI (Invitrogen), 10% fetal calf serum (FCS; Invitrogen), 100 µg/ml penicillin, 100 unit/ml streptomycin (BioConcept)) with ESAT-6 and CFP-10 peptide pools (1 µg/ml) or Staphyloccocus enterotoxin B (SEB; 250 ng/mL) or unstimulated in the presence of Golgiplug (1 μl/ml; BD). At the end of the stimulation period, cells were washed and stained (20 min; 4°C) for dead cells using the Aqua LIVE/DEAD stain kit, then permeabilized (30 min; 20°C) (Cytofix/Cytoperm, BD) and stained (20 min; 20°C) with mAbs to CD3, CD4, CD8, TNF-α, IFN-γ, IL-2 and perforin.
Assessment of T-bet, Gata-3 expression
PBMCs were washed, stained (20 min; 4°C) for dead cells using the Aqua LIVE/DEAD stain kit, then washed and stained (20 min; 4°C) for CD3, CD4, CD8, CD45RA. Cells were then washed, permeabilized (45 min; 4°C) (Foxp3 Fixation/Permeabilization Kit; eBioscience) and stained (20 min; 4°C) with mAbs to T-bet and Gata-3.
Assessment of Mtb-stimulated culture supernatant cytokine profile by luminex assay
PBMCs (2x105 cells) were stimulated for 24 hours in complete media (RPMI (Invitrogen), 10% fetal calf serum (FCS; Invitrogen), 100 µg/ml penicillin, 100 unit/ml streptomycin (BioConcept)) with ESAT-6 and CFP-10 peptide pools (1 µg/ml) or with Staphyloccocus enterotoxin B (SEB; 250 ng/mL) or left unstimulated (negative control). At the end of the stimulation period, culture supernatants were collected and levels of TNF-α, IFN-γ, IL-2, IL-4, IL-5, IL-13, IL-10, IL-17A and IL-17F were assessed cells by luminex assay (ProcartaPlex Mix&Match Human plex, eBioscience).
Assessment of serum cytokine profile
Serum levels of IL-1α, IL-6, TNF-α, IL-12p70, IL-23, IL-10, IFN-α2, IFN-β, IFN-ω and IFN-γ was assessed by luminex assay (ProcartaPlex Mix&Match Human plex, eBioscience) and CRP was assessed by nephelemetry (CardioPhasehsCRP, Siemens Healthcare Diagnostics Products GmbH) as previously described [96].
Flow cytometry analyses
Cells were fixed with CellFix (BD), acquired on an LSRII SORP (4 lasers: 405, 488, 532 and 633 nm) and analyzed using FlowJo (version 9.7.7) (Tree star Inc, Ashland, OR, USA). Frequencies of cytokine-producing Mtb-specific T cells and cytokine profile of Mtb-specific T-cell responses were analyzed using SPICE software (version 5.34) following background subtraction. When required, analysis and presentation of distributions was performed using SPICE, downloaded from <http://exon.niaid.nih.gov/spice> [97].
Statistical analyses
Statistical significance (P values) was obtained either using two-tailed Chi-square analysis for comparison of positive proportions or using one-way ANOVA (Kruskal-Wallis test) followed by Mann–Whitney test or Wilcoxon Matched-pairs two-tailed Signed Rank test for multiple comparisons or Spearman rank test for correlations using GraphPad Prism version 7 (San Diego, CA). Statistical analyses of global cytokine profiles (pie charts) were performed by partial permutation tests using the SPICE software as described [97]. Principal component analysis (PCA) was performed using the R ‘‘stats” package. To normalize data distribution, the values of each parameter were first log transformed. Data were then filtered using Lasso method [98].
Supporting information
(PDF)
(PDF)
Proportion of SEA-specific CD4 T-cell responses producing IFN-γ, IL-4/5/13, TNF-α and/or IL-2 of TB patients from TZ (n = 7). All the possible combinations of the responses are shown on the x axis and the percentage of the functionally distinct cell populations within the SEA-specific CD4 T-cell populations are shown on the y axis. Responses are grouped and color-coded on the basis of the number of functions. The pie chart summarizes the data, and each slice corresponds to the fraction of SEA-specific CD4 T cell response with a given number of functions within the responding CD4 T-cell population. Bars correspond to the fractions of different functionally distinct CD4 T-cell populations within the total CD4 T cells. Red arcs correspond to IL-4/5/13-producing CD4 T-cell populations.
(PDF)
(A) Proportion of Mtb-specific CD4 T cells producing IL-4/5/13 among total Mtb-specific CD4 T-cell responses assessed in TB patients from TZ infected with one helminth (n = 19) as compared to TB patients from TZ infected with more than one helminth (n = 11) or (B) between TB patients from TZ infected with helminth species harbouring (hookworm and S. stercoralis) or not (S. mansoni, S. haematobium and W. bancrofti) lung migration capacity. (C) Proportion of Mtb-specific CD4 T cells producing IL-4/5/13 among total Mtb-specific CD4 T-cell responses assessed in TB patients from SA with (n = 2) or without past helminth exposure (n = 15). (D) Proportion of Mtb-specific CD4 T cells producing IL-4/5/13 among total Mtb-specific CD4 T-cell responses assessed in TB patients from TZ without ongoing helminth infection but with (n = 7) or without past helminth exposure (n = 18). (E) Levels of IL-4, IL-5 and IL-13 secreted in Mtb-stimulated culture supernatants in TB patients from TZ infected with one helminth (n = 19) and TB patients from TZ infected with more than one helminth (n = 10) or (F) between TB patients from TZ infected with helminth species harbouring or not lung migration capacity. (G) Levels of IL-4, IL-5 and IL-13 secreted in Mtb-stimulated culture supernatants in TB patients from SA with (n = 2) or without past helminth exposure (n = 10). (H) Levels of IL-4, IL-5 and IL-13 secreted in Mtb-stimulated culture supernatants in TB patients from TZ without ongoing helminth infection but with (n = 6) or without past helminth exposure (n = 15). (I) Percentage of memory CD4 T cells (CD45RA-) expressing Gata-3 of TB patients from TZ infected with one helminth (n = 8) and TB patients from TZ infected with more than one helminth (n = 5) or (J) between TB patients from TZ infected with helminth species harbouring or not lung migration capacity. (K) Percentage of memory CD4 T cells (CD45RA-) expressing Gata-3 of TB patients from SA with (n = 2) or without past helminth exposure (n = 10). (L) Percentage of memory CD4 T cells (CD45RA-) expressing Gata-3 of TB patients from TZ without ongoing helminth infection but with (n = 6) or without past helminth exposure (n = 8). Helminth species were color coded (A, E and I); W. bancrofti, orange; hookworms, green; S. mansoni, red, S. haematobium, yellow, S. stercolaris, violet, and patients infected with more than one helminth species in light blue. Statistical significance (P<0.05) was calculated by Mann-Whitney test.
(PDF)
(A) Correlation between the percentage of memory CD4 T cell expressing Tbethigh and the percentage of memory CD4 T cell expressing Gata-3 in TB patients from SA (n = 12), TB patient (n = 14) and Mtb/helminth co-infected patients (n = 13) from TZ. TB patients were color coded; TB patients from SA, blue; TB patients from TZ, red and Mtb/helminth co-infected patients, green. Statistical significance (P<0.05) was calculated using Spearman rank test.
(PDF)
(A) Levels of IL-4, IL-5 and IL-13 produced in Mtb-stimulated culture supernatants of TB (n = 13) and Mtb/helminth co-infected patients from TZ (n = 18) assessed by luminex assay. (B) Percentage of memory (CD45RA-) CD4 T cells expressing Gata-3 or T-bethigh of TB (n = 11) and TB/helminth co-infected patients (n = 8) from TZ. TB patients were color coded; TB patients, red and Mtb/helminth co-infected patients, green.
(PDF)
Acknowledgments
We would like to thank the study participants in TZ and SA for their willingness to donate blood samples for these studies. The invaluable work of Julian Rothen and Tobias Schindler in establishing the helminth infection status of the Tanzanian cohort in Bagamoyo is acknowledged. Isabelle Zenklusen, Philipp Mächler, Federica Klaus and Jerry Hela facilitated clinical data cleaning and entry of the Tanzanian patients.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research leading to these results has received funding from the [European Community's] Seventh Framework Programme ([FP7/2007-2013]) under EC-GA n° [241642]. This work was also supported by Swiss National Science Foundation Grants 320030_173071 and by the Strategic Health Innovation Partnerships (SHIP) Unit of the South African Medical Research Council with funds received from the South African Department of Science and Technology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14(11):1118–26. doi: 10.1038/ni.2736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11(6):375–88. doi: 10.1038/nri2992 . [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118(4):1311–21. doi: 10.1172/JCI34261. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. Lancet. 2015. doi: 10.1016/S0140-6736(15)00151-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling . PLoS Med. 2016;13(10):e1002152 doi: 10.1371/journal.pmed.1002152 ; PubMed Central PMCID: PMCPMC5079585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–80. doi: 10.1093/cid/cit790 . [DOI] [PubMed] [Google Scholar]
- 7.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche MC, Dupuis S, et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nature genetics. 1999;21(4):370–8. doi: 10.1038/7701 . [DOI] [PubMed] [Google Scholar]
- 8.de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–8. . [DOI] [PubMed] [Google Scholar]
- 9.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280(5368):1432–5. . [DOI] [PubMed] [Google Scholar]
- 10.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335(26):1956–61. doi: 10.1056/NEJM199612263352604 . [DOI] [PubMed] [Google Scholar]
- 11.Granich R, Akolo C, Gunneberg C, Getahun H, Williams P, Williams B. Prevention of tuberculosis in people living with HIV. Clin Infect Dis. 2010;50 Suppl 3:S215–22. doi: 10.1086/651494 . [DOI] [PubMed] [Google Scholar]
- 12.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110 . [DOI] [PubMed] [Google Scholar]
- 13.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–93. Epub 1996/10/31. doi: 10.1038/383787a0 . [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–46. Epub 2007/05/09. doi: 10.1038/ni1467 . [DOI] [PubMed] [Google Scholar]
- 15.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–61. Epub 2007/07/20. doi: 10.1084/jem.20070663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. Epub 2001/03/13. doi: 10.1146/annurev.immunol.19.1.93 . [DOI] [PubMed] [Google Scholar]
- 17.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11(5):343–54. doi: 10.1038/nri2960 . [DOI] [PubMed] [Google Scholar]
- 18.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;147(8):2713–6. . [PubMed] [Google Scholar]
- 19.Pearce EJ, C MK, Sun J, J JT, McKee AS, Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev. 2004;201:117–26. doi: 10.1111/j.0105-2896.2004.00187.x . [DOI] [PubMed] [Google Scholar]
- 20.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173(2):1224–31. . [DOI] [PubMed] [Google Scholar]
- 21.Anthony RM, Rutitzky LI, Urban JF Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7(12):975–87. doi: 10.1038/nri2199 ; PubMed Central PMCID: PMC2258092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romagnani S. Type 1 T helper and type 2 T helper cells: functions, regulation and role in protection and disease. Int J Clin Lab Res. 1991;21(2):152–8. . [DOI] [PubMed] [Google Scholar]
- 23.Romagnani S. Regulation of the T cell response. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2006;36(11):1357–66. doi: 10.1111/j.1365-2222.2006.02606.x . [DOI] [PubMed] [Google Scholar]
- 24.Callard RE. Decision-making by the immune response. Immunol Cell Biol. 2007;85(4):300–5. doi: 10.1038/sj.icb.7100060 . [DOI] [PubMed] [Google Scholar]
- 25.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123(3):326–38. doi: 10.1111/j.1365-2567.2007.02719.x. 3439; PubMed Central PMCID: PMCPMC2433332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999;5(4):285–94. . [DOI] [PubMed] [Google Scholar]
- 27.Metenou S, Babu S, Nutman TB. Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS. 2012;7(3):231–8. doi: 10.1097/COH.0b013e3283522c3d ; PubMed Central PMCID: PMC3431797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rougemont A, Boisson-Pontal ME, Pontal PG, Gridel F, Sangare S. Tuberculin skin tests and B.C.G. vaccination in hyperendemic area of onchocerciasis. Lancet. 1977;1(8006):309 . [DOI] [PubMed] [Google Scholar]
- 29.Gebreegziabiher D, Desta K, Howe R, Abebe M. Helminth infection increases the probability of indeterminate QuantiFERON gold in tube results in pregnant women. BioMed research international. 2014;2014:364137 doi: 10.1155/2014/364137 ; PubMed Central PMCID: PMC3950403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas TA, Mondal D, Noor Z, Liu L, Alam M, Haque R, et al. Malnutrition and helminth infection affect performance of an interferon gamma-release assay. Pediatrics. 2010;126(6):e1522–9. doi: 10.1542/peds.2010-0885 ; PubMed Central PMCID: PMC3403682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Soelen N, Mandalakas AM, Kirchner HL, Walzl G, Grewal HM, Jacobsen M, et al. Effect of Ascaris Lumbricoides specific IgE on tuberculin skin test responses in children in a high-burden setting: a cross-sectional community-based study. BMC infectious diseases. 2012;12:211 doi: 10.1186/1471-2334-12-211 ; PubMed Central PMCID: PMC3482567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipner EM, Gopi PG, Subramani R, Kolappan C, Sadacharam K, Kumaran P, et al. Coincident filarial, intestinal helminth, and mycobacterial infection: helminths fail to influence tuberculin reactivity, but BCG influences hookworm prevalence. The American journal of tropical medicine and hygiene. 2006;74(5):841–7. . [PubMed] [Google Scholar]
- 33.Neto LM, Oliveira Rde V, Totino PR, Sant'Anna FM, Coelho Vde O, Rolla VC, et al. Enteroparasitosis prevalence and parasitism influence in clinical outcomes of tuberculosis patients with or without HIV co-infection in a reference hospital in Rio de Janeiro (2000–2006). The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2009;13(6):427–32. . [DOI] [PubMed] [Google Scholar]
- 34.Zevallos K, Vergara KC, Vergara A, Vidal C, Garcia HH, Evans CA. Tuberculin skin-test reactions are unaffected by the severity of hyperendemic intestinal helminth infections and co-infections. The American journal of tropical medicine and hygiene. 2010;83(2):319–25. doi: 10.4269/ajtmh.2010.10-0073 ; PubMed Central PMCID: PMC2911178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Zalm MM, van Soelen N, Mandalakas AM, Jacobsen M, Detjen AK, Marx FM, et al. The Effect of Deworming on Tests of Tuberculosis Infection in Children With Recent Tuberculosis Exposure: A Randomized Controlled Trial. The Pediatric infectious disease journal. 2016;35(6):622–7. doi: 10.1097/INF.0000000000001115 . [DOI] [PubMed] [Google Scholar]
- 36.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Tropical medicine & international health: TM & IH. 2006;11(4):551–8. doi: 10.1111/j.1365-3156.2006.01578.x . [DOI] [PubMed] [Google Scholar]
- 37.Brown M, Miiro G, Nkurunziza P, Watera C, Quigley MA, Dunne DW, et al. Schistosoma mansoni, nematode infections, and progression to active tuberculosis among HIV-1-infected Ugandans. The American journal of tropical medicine and hygiene. 2006;74(5):819–25. . [PubMed] [Google Scholar]
- 38.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, et al. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162(11):6843–8. . [PubMed] [Google Scholar]
- 39.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol. 2001;123(2):219–25. PubMed Central PMCID: PMC1905995. doi: 10.1046/j.1365-2249.2001.01446.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine. 2008;26(31):3897–902. doi: 10.1016/j.vaccine.2008.04.083 . [DOI] [PubMed] [Google Scholar]
- 41.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208(9):1863–74. doi: 10.1084/jem.20091473 ; PubMed Central PMCID: PMC3171086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George PJ, Anuradha R, Kumaran PP, Chandrasekaran V, Nutman TB, Babu S. Modulation of mycobacterial-specific Th1 and Th17 cells in latent tuberculosis by coincident hookworm infection. J Immunol. 2013;190(10):5161–8. doi: 10.4049/jimmunol.1203311 ; PubMed Central PMCID: PMCPMC3646958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George PJ, Anuradha R, Kumar NP, Sridhar R, Banurekha VV, Nutman TB, et al. Helminth infections coincident with active pulmonary tuberculosis inhibit mono- and multifunctional CD4+ and CD8+ T cell responses in a process dependent on IL-10. PLoS Pathog. 2014;10(9):e1004375 doi: 10.1371/journal.ppat.1004375 ; PubMed Central PMCID: PMC4161445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anuradha R, Munisankar S, Bhootra Y, Dolla C, Kumaran P, Nutman TB, et al. Anthelmintic Therapy Modifies the Systemic and Mycobacterial Antigen-Stimulated Cytokine Profile in Helminth-Latent Mycobacterium tuberculosis Coinfection. Infect Immun. 2017;85(4). doi: 10.1128/IAI.00973-16 ; PubMed Central PMCID: PMCPMC5364310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anuradha R, Munisankar S, Bhootra Y, Dolla C, Kumaran P, Nutman TB, et al. Modulation of Mycobacterium tuberculosis-specific humoral immune responses is associated with Strongyloides stercoralis co-infection. PLoS Negl Trop Dis. 2017;11(5):e0005569 doi: 10.1371/journal.pntd.0005569 ; PubMed Central PMCID: PMCPMC5426788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldas IR, Campi-Azevedo AC, Oliveira LF, Silveira AM, Oliveira RC, Gazzinelli G. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta tropica. 2008;108(2–3):109–17. doi: 10.1016/j.actatropica.2008.05.027 . [DOI] [PubMed] [Google Scholar]
- 47.Petrone L, Vanini V, Petruccioli E, Ettorre GM, Schinina V, Busi Rizzi E, et al. Polyfunctional Specific Response to Echinococcus Granulosus Associates to the Biological Activity of the Cysts. PLoS Negl Trop Dis. 2015;9(11):e0004209 doi: 10.1371/journal.pntd.0004209 ; PubMed Central PMCID: PMCPMC4648505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292(5523):1907–10. doi: 10.1126/science.1059835 . [DOI] [PubMed] [Google Scholar]
- 49.Ting CN, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384(6608):474–8. doi: 10.1038/384474a0 . [DOI] [PubMed] [Google Scholar]
- 50.Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, et al. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci U S A. 2009;106(42):17876–81. doi: 10.1073/pnas.0909357106 ; PubMed Central PMCID: PMC2764903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Actor JK, Shirai M, Kullberg MC, Buller RM, Sher A, Berzofsky JA. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci U S A. 1993;90(3):948–52. Epub 1993/02/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, et al. Coinfection. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science. 2014;345(6196):578–82. doi: 10.1126/science.1256942 ; PubMed Central PMCID: PMC4548887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, et al. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol. 2013;43(6):1568–77. doi: 10.1002/eji.201243262 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, et al. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J. 2016;47(5):1587–90. doi: 10.1183/13993003.02033-2015 . [DOI] [PubMed] [Google Scholar]
- 55.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, et al. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. The Journal of infection. 2016;73(6):588–97. doi: 10.1016/j.jinf.2016.09.008 . [DOI] [PubMed] [Google Scholar]
- 56.Reese TA, Wakeman BS, Choi HS, Hufford MM, Huang SC, Zhang X, et al. Helminth infection reactivates latent gamma-herpesvirus via cytokine competition at a viral promoter. Science. 2014;345(6196):573–7. doi: 10.1126/science.1254517 ; PubMed Central PMCID: PMC4531374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cooper PJ, Chico ME, Losonsky G, Sandoval C, Espinel I, Sridhara R, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis. 2000;182(4):1199–206. doi: 10.1086/315837 . [DOI] [PubMed] [Google Scholar]
- 58.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun. 2004;72(5):2598–604. PubMed Central PMCID: PMC387878. doi: 10.1128/IAI.72.5.2598-2604.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212 ; PubMed Central PMCID: PMCPMC3502616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130(1):1–9. doi: 10.1111/j.1365-2567.2010.03259.x ; PubMed Central PMCID: PMCPMC2855787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marin ND, Paris SC, Rojas M, Garcia LF. Functional profile of CD4+ and CD8+ T cells in latently infected individuals and patients with active TB. Tuberculosis (Edinb). 2013;93(2):155–66. doi: 10.1016/j.tube.2012.12.002 . [DOI] [PubMed] [Google Scholar]
- 62.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40(8):2211–20. doi: 10.1002/eji.201040455 . [DOI] [PubMed] [Google Scholar]
- 63.Sester U, Fousse M, Dirks J, Mack U, Prasse A, Singh M, et al. Whole-blood flow-cytometric analysis of antigen-specific CD4 T-cell cytokine profiles distinguishes active tuberculosis from non-active states. PLoS One. 2011;6(3):e17813 doi: 10.1371/journal.pone.0017813 ; PubMed Central PMCID: PMC3058054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, et al. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nature medicine. 2011;17(3):372–6. Epub 2011/02/22. doi: 10.1038/nm.2299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Portevin D, Moukambi F, Clowes P, Bauer A, Chachage M, Ntinginya NE, et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. The Lancet Infectious diseases. 2014;14(10):931–8. doi: 10.1016/S1473-3099(14)70884-9 . [DOI] [PubMed] [Google Scholar]
- 66.Jayaraman P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, Madi A, et al. TIM3 Mediates T Cell Exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog. 2016;12(3):e1005490 doi: 10.1371/journal.ppat.1005490 ; PubMed Central PMCID: PMC4788425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiacchio T, Petruccioli E, Vanini V, Cuzzi G, Pinnetti C, Sampaolesi A, et al. Polyfunctional T-cells and effector memory phenotype are associated with active TB in HIV-infected patients. The Journal of infection. 2014;69(6):533–45. doi: 10.1016/j.jinf.2014.06.009 . [DOI] [PubMed] [Google Scholar]
- 68.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009;39(3):723–9. doi: 10.1002/eji.200838693 . [DOI] [PubMed] [Google Scholar]
- 69.Petruccioli E, Petrone L, Vanini V, Sampaolesi A, Gualano G, Girardi E, et al. IFNgamma/TNFalpha specific-cells and effector memory phenotype associate with active tuberculosis. The Journal of infection. 2013;66(6):475–86. doi: 10.1016/j.jinf.2013.02.004 . [DOI] [PubMed] [Google Scholar]
- 70.Gazzinelli-Guimaraes PH, Bonne-Annee S, Fujiwara RT, Santiago HC, Nutman TB. Allergic Sensitization Underlies Hyperreactive Antigen-Specific CD4+ T Cell Responses in Coincident Filarial Infection. J Immunol. 2016;197(7):2772–9. doi: 10.4049/jimmunol.1600829 ; PubMed Central PMCID: PMCPMC5026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Speich B, Moser W, Ali SM, Ame SM, Albonico M, Hattendorf J, et al. Efficacy and reinfection with soil-transmitted helminths 18-weeks post-treatment with albendazole-ivermectin, albendazole-mebendazole, albendazole-oxantel pamoate and mebendazole. Parasit Vectors. 2016;9:123 doi: 10.1186/s13071-016-1406-8 ; PubMed Central PMCID: PMCPMC4776366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karagiannis-Voules DA, Biedermann P, Ekpo UF, Garba A, Langer E, Mathieu E, et al. Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: a systematic review and geostatistical meta-analysis. The Lancet Infectious diseases. 2015;15(1):74–84. doi: 10.1016/S1473-3099(14)71004-7 . [DOI] [PubMed] [Google Scholar]
- 73.Anuradha R, Munisankar S, Dolla C, Kumaran P, Nutman TB, Babu S. Parasite Antigen-Specific Regulation of Th1, Th2, and Th17 Responses in Strongyloides stercoralis Infection. J Immunol. 2015;195(5):2241–50. doi: 10.4049/jimmunol.1500745 ; PubMed Central PMCID: PMCPMC4546867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rozot V, Patrizia A, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, et al. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis. 2015;60(3):432–7. doi: 10.1093/cid/ciu795 ; PubMed Central PMCID: PMC4293395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herbst S, Schaible UE, Schneider BE. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS One. 2011;6(5):e19105 doi: 10.1371/journal.pone.0019105 ; PubMed Central PMCID: PMC3085516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brighenti S, Andersson J. Induction and regulation of CD8+ cytolytic T cells in human tuberculosis and HIV infection. Biochemical and biophysical research communications. 2010;396(1):50–7. doi: 10.1016/j.bbrc.2010.02.141 . [DOI] [PubMed] [Google Scholar]
- 77.Lalvani A, Brookes R, Wilkinson RJ, Malin AS, Pathan AA, Andersen P, et al. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1998;95(1):270–5. ; PubMed Central PMCID: PMC18198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5(4):e1000392 doi: 10.1371/journal.ppat.1000392 ; PubMed Central PMCID: PMC2663842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazzaccaro RJ, Stenger S, Rock KL, Porcelli SA, Brenner MB, Modlin RL, et al. Cytotoxic T lymphocytes in resistance to tuberculosis. Adv Exp Med Biol. 1998;452:85–101. . [DOI] [PubMed] [Google Scholar]
- 80.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119(5):1167–77. doi: 10.1172/JCI38482 ; PubMed Central PMCID: PMC2673881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 2012;8(5):e1002607 doi: 10.1371/journal.ppat.1002607 ; PubMed Central PMCID: PMC3349743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. doi: 10.1038/nri3787 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339(6126):1448–53. doi: 10.1126/science.1233665 ; PubMed Central PMCID: PMC3653587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–7. doi: 10.1038/nature09247 ; PubMed Central PMCID: PMC3492754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25(11):694–701. doi: 10.1089/jir.2005.25.694 . [DOI] [PubMed] [Google Scholar]
- 86.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol. 2007;179(1):522–31. . [DOI] [PubMed] [Google Scholar]
- 87.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178(5):3143–52. . [DOI] [PubMed] [Google Scholar]
- 88.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581 ; PubMed Central PMCID: PMC4084561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100(26):15818–23. doi: 10.1073/pnas.2636938100 ; PubMed Central PMCID: PMC307651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huber JP, Ramos HJ, Gill MA, Farrar JD. Cutting edge: Type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J Immunol. 2010;185(2):813–7. doi: 10.4049/jimmunol.1000469 ; PubMed Central PMCID: PMC2927323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber JP, Farrar JD. Regulation of effector and memory T-cell functions by type I interferon. Immunology. 2011;132(4):466–74. doi: 10.1111/j.1365-2567.2011.03412.x ; PubMed Central PMCID: PMC3075500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiens KE, Ernst JD. The Mechanism for Type I Interferon Induction by Mycobacterium tuberculosis is Bacterial Strain-Dependent. PLoS Pathog. 2016;12(8):e1005809 doi: 10.1371/journal.ppat.1005809 ; PubMed Central PMCID: PMC4976988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, Lweno O, et al. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. The American journal of tropical medicine and hygiene. 2014;90(3):535–45. doi: 10.4269/ajtmh.13-0268 ; PubMed Central PMCID: PMCPMC3945701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chesnais CB, Missamou F, Pion SD, Bopda J, Louya F, Majewski AC, et al. Semi-quantitative scoring of an immunochromatographic test for circulating filarial antigen. The American journal of tropical medicine and hygiene. 2013;89(5):916–8. doi: 10.4269/ajtmh.13-0245 ; PubMed Central PMCID: PMCPMC3820335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perreau M, Rozot V, Welles HC, Belluti-Enders F, Vigano S, Maillard M, et al. Lack of Mycobacterium tuberculosis-specific interleukin-17A-producing CD4+ T cells in active disease. Eur J Immunol. 2013;43(4):939–48. doi: 10.1002/eji.201243090 . [DOI] [PubMed] [Google Scholar]
- 96.Perreau M, Vigano S, Bellanger F, Pellaton C, Buss G, Comte D, et al. Exhaustion of bacteria-specific CD4 T cells and microbial translocation in common variable immunodeficiency disorders. J Exp Med. 2014;211(10):2033–45. doi: 10.1084/jem.20140039 ; PubMed Central PMCID: PMC4172212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79(2):167–74. Epub 2011/01/26. doi: 10.1002/cyto.a.21015 ; PubMed Central PMCID: PMC3072288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tibshirani R, Bien J, Friedman J, Hastie T, Simon N, Taylor J, et al. Strong rules for discarding predictors in lasso-type problems. Journal of the Royal Statistical Society Series B, Statistical methodology. 2012;74(2):245–66. doi: 10.1111/j.1467-9868.2011.01004.x ; PubMed Central PMCID: PMC4262615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Proportion of SEA-specific CD4 T-cell responses producing IFN-γ, IL-4/5/13, TNF-α and/or IL-2 of TB patients from TZ (n = 7). All the possible combinations of the responses are shown on the x axis and the percentage of the functionally distinct cell populations within the SEA-specific CD4 T-cell populations are shown on the y axis. Responses are grouped and color-coded on the basis of the number of functions. The pie chart summarizes the data, and each slice corresponds to the fraction of SEA-specific CD4 T cell response with a given number of functions within the responding CD4 T-cell population. Bars correspond to the fractions of different functionally distinct CD4 T-cell populations within the total CD4 T cells. Red arcs correspond to IL-4/5/13-producing CD4 T-cell populations.
(PDF)
(A) Proportion of Mtb-specific CD4 T cells producing IL-4/5/13 among total Mtb-specific CD4 T-cell responses assessed in TB patients from TZ infected with one helminth (n = 19) as compared to TB patients from TZ infected with more than one helminth (n = 11) or (B) between TB patients from TZ infected with helminth species harbouring (hookworm and S. stercoralis) or not (S. mansoni, S. haematobium and W. bancrofti) lung migration capacity. (C) Proportion of Mtb-specific CD4 T cells producing IL-4/5/13 among total Mtb-specific CD4 T-cell responses assessed in TB patients from SA with (n = 2) or without past helminth exposure (n = 15). (D) Proportion of Mtb-specific CD4 T cells producing IL-4/5/13 among total Mtb-specific CD4 T-cell responses assessed in TB patients from TZ without ongoing helminth infection but with (n = 7) or without past helminth exposure (n = 18). (E) Levels of IL-4, IL-5 and IL-13 secreted in Mtb-stimulated culture supernatants in TB patients from TZ infected with one helminth (n = 19) and TB patients from TZ infected with more than one helminth (n = 10) or (F) between TB patients from TZ infected with helminth species harbouring or not lung migration capacity. (G) Levels of IL-4, IL-5 and IL-13 secreted in Mtb-stimulated culture supernatants in TB patients from SA with (n = 2) or without past helminth exposure (n = 10). (H) Levels of IL-4, IL-5 and IL-13 secreted in Mtb-stimulated culture supernatants in TB patients from TZ without ongoing helminth infection but with (n = 6) or without past helminth exposure (n = 15). (I) Percentage of memory CD4 T cells (CD45RA-) expressing Gata-3 of TB patients from TZ infected with one helminth (n = 8) and TB patients from TZ infected with more than one helminth (n = 5) or (J) between TB patients from TZ infected with helminth species harbouring or not lung migration capacity. (K) Percentage of memory CD4 T cells (CD45RA-) expressing Gata-3 of TB patients from SA with (n = 2) or without past helminth exposure (n = 10). (L) Percentage of memory CD4 T cells (CD45RA-) expressing Gata-3 of TB patients from TZ without ongoing helminth infection but with (n = 6) or without past helminth exposure (n = 8). Helminth species were color coded (A, E and I); W. bancrofti, orange; hookworms, green; S. mansoni, red, S. haematobium, yellow, S. stercolaris, violet, and patients infected with more than one helminth species in light blue. Statistical significance (P<0.05) was calculated by Mann-Whitney test.
(PDF)
(A) Correlation between the percentage of memory CD4 T cell expressing Tbethigh and the percentage of memory CD4 T cell expressing Gata-3 in TB patients from SA (n = 12), TB patient (n = 14) and Mtb/helminth co-infected patients (n = 13) from TZ. TB patients were color coded; TB patients from SA, blue; TB patients from TZ, red and Mtb/helminth co-infected patients, green. Statistical significance (P<0.05) was calculated using Spearman rank test.
(PDF)
(A) Levels of IL-4, IL-5 and IL-13 produced in Mtb-stimulated culture supernatants of TB (n = 13) and Mtb/helminth co-infected patients from TZ (n = 18) assessed by luminex assay. (B) Percentage of memory (CD45RA-) CD4 T cells expressing Gata-3 or T-bethigh of TB (n = 11) and TB/helminth co-infected patients (n = 8) from TZ. TB patients were color coded; TB patients, red and Mtb/helminth co-infected patients, green.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.