Abstract
We have analyzed the plasma membrane association of the SV40 large tumor antigen (large T) in SV40-transformed BALB/c mouse tumor cells (mKSA). Isolated plasma membranes were subfractionated: treatment with the non-ionic detergent Nonidet P40 (NP40) resulted in a NP40-resistant plasma membrane lamina, which could be further extracted with the zwitterionic detergent Empigen BB. Analysis of the different plasma membrane fractions revealed that only about one third of large T associated with isolated plasma membranes could be solubilized with NP40. The residual plasma membrane-associated large T was tightly bound to the NP40-resistant lamina of the plasma membrane from which it was released by treatment with the zwitterionic detergent Empigen BB. Further evidence for a specific interaction of a distinct subclass of large T with the plasma membrane was provided by showing that only T associated with the NP40-resistant lamina of the plasma membrane contained covalently bound fatty acid. Neither nuclear large T nor large T in the NP40-soluble plasma membrane fraction could be labeled with [3H]palmitic acid. Our results indicate that an acylated subclass of large T interacts specifically with a structure of the plasma membrane, suggesting that it might be involved in a membrane-dependent biological function.
Full text
PDF

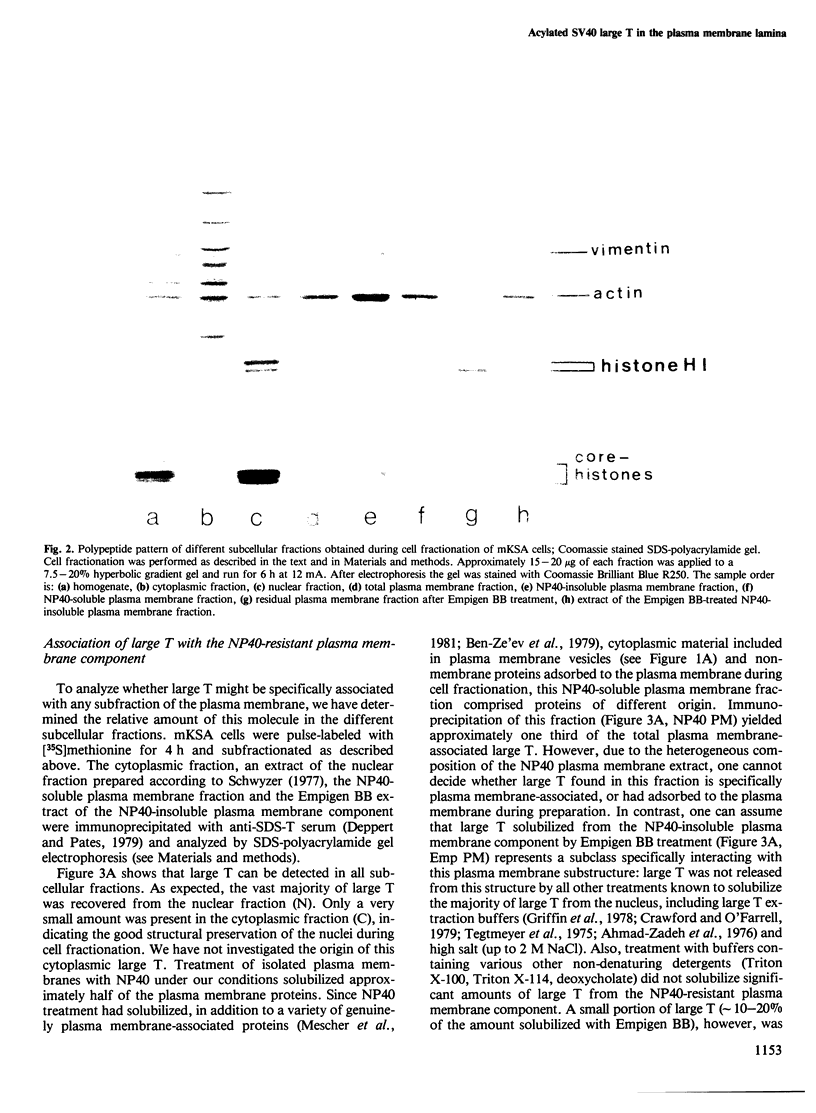
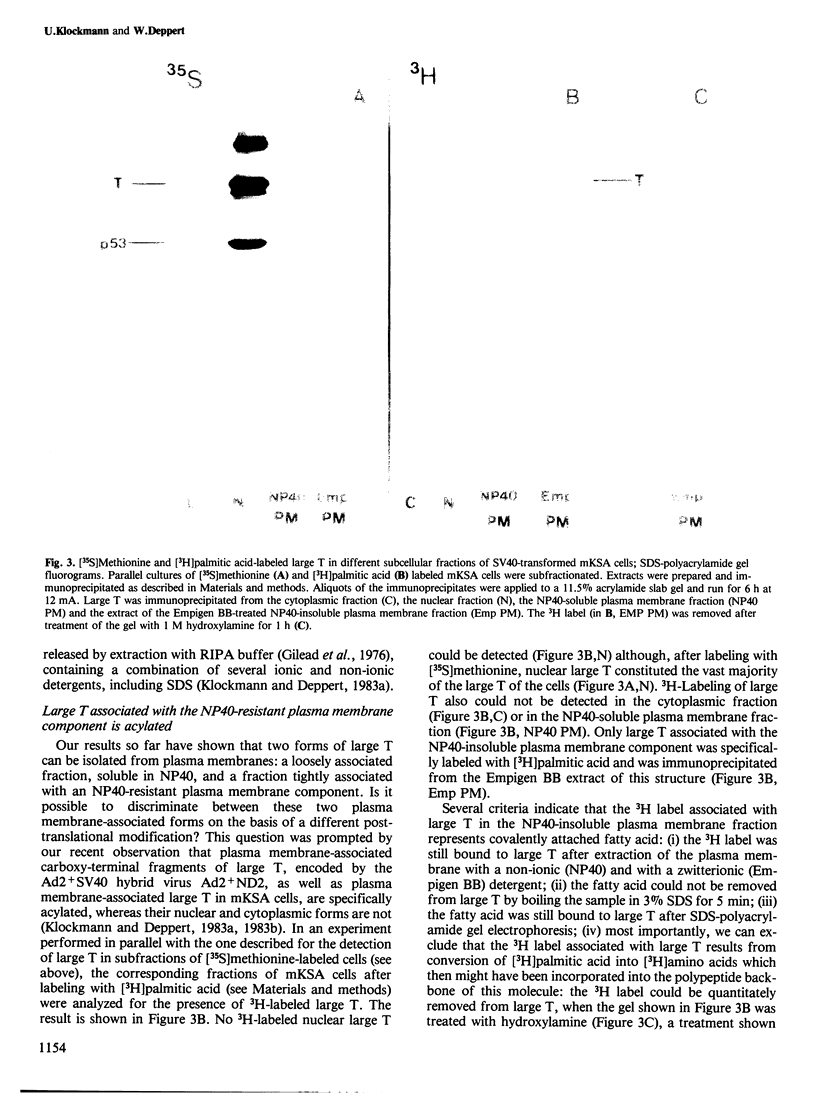



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad-Zadeh C., Allet B., Greenblatt J., Weil R. Two forms of simian-virus-40-specific T-antigen in abortive and lytic infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1097–1101. doi: 10.1073/pnas.73.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Duerr A., Solomon F., Penman S. The outer boundary of the cytoskeleton: a lamina derived from plasma membrane proteins. Cell. 1979 Aug;17(4):859–865. doi: 10.1016/0092-8674(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boschek C. B., Jockusch B. M., Friis R. R., Back R., Grundmann E., Bauer H. Early changes in the distribution and organization of microfilament proteins during cell transformation. Cell. 1981 Apr;24(1):175–184. doi: 10.1016/0092-8674(81)90513-4. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Gurney E. G. Time-dependent maturation of the simian virus 40 large T antigen-p53 complex studied by using monoclonal antibodies. J Virol. 1982 Nov;44(2):565–573. doi: 10.1128/jvi.44.2.565-573.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Hakomori S. A new cell surface, detergent-insoluble glycoprotein matrix of human and hamster fibroblasts. The role of disulfide bonds in stabilization of the matrix. J Biol Chem. 1981 Jul 10;256(13):6953–6960. [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., O'Farrell P. Z. Effect of alkylation on the physical properties of simian virus 40 T-antigen species. J Virol. 1979 Feb;29(2):587–596. doi: 10.1128/jvi.29.2.587-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Hanke K., Henning R. Simian virus 40 T-antigen-related cell surface antigen: serological demonstration on simian virus 40-transformed monolayer cells in situ. J Virol. 1980 Aug;35(2):505–518. doi: 10.1128/jvi.35.2.505-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Pates R. Cell surface location of simian virus 40-specific proteins on HeLa cells infected with adenovirus type 2-simian virus 40 hybrid viruses Ad2+ND1 and Ad2+ND2. J Virol. 1979 Aug;31(2):522–536. doi: 10.1128/jvi.31.2.522-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W. Simian virus 40 T- and U-antigens: immunological characterization and localization in different nuclear subfractions of simian virus 40-transformed cells. J Virol. 1979 Feb;29(2):576–586. doi: 10.1128/jvi.29.2.576-586.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walter G. Domains of simian virus 40 large T-antigen exposed on the cell surface. Virology. 1982 Oct 15;122(1):56–70. doi: 10.1016/0042-6822(82)90377-4. [DOI] [PubMed] [Google Scholar]
- Deppert W., Walter G., Linke H. Simian virus 40 tumor-specific proteins: subcellular distribution and metabolic stability in HeLa cells infected with nondefective adenovirus type 2-simian virus 40 hybrid viruses. J Virol. 1977 Mar;21(3):1170–1186. doi: 10.1128/jvi.21.3.1170-1186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walter G. Simian virus to (SV40) tumor-specific proteins in nucleus and plasma membrane of HeLa cells infected by adenovirus 2-SV40 hybrid virus Ad2+ND2. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2505–2509. doi: 10.1073/pnas.73.7.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Westphal K. H., Brauer D., Cörlin D. Subclasses of simian virus 40 large T antigen: differential binding of two subclasses of T antigen from productively infected cells to viral and cellular DNA. EMBO J. 1982;1(9):1023–1028. doi: 10.1002/j.1460-2075.1982.tb01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Scheller A., Barnet B., Hantzopoulos P., Oren M., Prives C. Different forms of simian virus 40 large tumor antigen varying in their affinities for DNA. J Virol. 1982 May;42(2):456–466. doi: 10.1128/jvi.42.2.456-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Complex of simian virus 40 large tumor antigen and 48,000-dalton host tumor antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):105–109. doi: 10.1073/pnas.78.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Light S., Livingston D. M. Measurements of the molecular size of the simian virus 40 large T antigen. J Virol. 1978 Jul;27(1):218–226. doi: 10.1128/jvi.27.1.218-226.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Pim D. C., Crawford L. V. Complex of simian virus 40 large-T antigen and host 53,000-molecular-weight protein in monkey cells. J Virol. 1981 Feb;37(2):564–573. doi: 10.1128/jvi.37.2.564-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R., Lange-Mutschler J., Deppert W. SV40-transformed cells express SV40 T antigen-related antigens on the cell surface. Virology. 1981 Jan 30;108(2):325–337. doi: 10.1016/0042-6822(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cellular location of viral transforming proteins. Cell. 1980 Oct;21(3):601–602. doi: 10.1016/0092-8674(80)90421-3. [DOI] [PubMed] [Google Scholar]
- Ito Y., Brocklehurst J. R., Dulbecco R. Virus-specific proteins in the plasma membrane of cells lytically infected or transformed by pol-oma virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4666–4670. doi: 10.1073/pnas.74.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y. Polyoma virus-specific 55K protein isolated from plasma membrane of productively infected cells is virus-coded and important for cell transformation. Virology. 1979 Oct 15;98(1):261–266. doi: 10.1016/0042-6822(79)90545-2. [DOI] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylated simian virus 40-specific proteins in the plasma membrane of HeLa cells infected with adenovirus 2-simian virus 40 hybrid virus Ad2+ND2. Virology. 1983 Apr 30;126(2):717–720. doi: 10.1016/s0042-6822(83)80029-4. [DOI] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylation: a new post-translational modification specific for plasma membrane-associated simian virus 40 large T-antigen. FEBS Lett. 1983 Jan 24;151(2):257–259. doi: 10.1016/0014-5793(83)80081-7. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R., Hanafusa H. Changes in amino-terminal sequences of pp60src lead to decreased membrane association and decreased in vivo tumorigenicity. Cell. 1982 Apr;28(4):889–896. doi: 10.1016/0092-8674(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Goldberg A. R. Evidence that the src gene product of Rous sarcoma virus is membrane associated. Virology. 1980 Feb;101(1):25–40. doi: 10.1016/0042-6822(80)90480-8. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Mitchell R. L., Lau A. F., Faras A. J. Association of pp60src and src protein kinase activity with the plasma membrane of nonpermissive and permissive avian sarcoma virus-infected cells. J Virol. 1980 Dec;36(3):805–815. doi: 10.1128/jvi.36.3.805-815.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange-Mutschler J., Henning R. Cell surface binding affinity of simian virus and 40 T-antigen. Virology. 1982 Feb;117(1):173–185. doi: 10.1016/0042-6822(82)90517-7. [DOI] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M. F., Jose M. J., Balk S. P. Actin-containing matrix associated with the plasma membrane of murine tumour and lymphoid cells. Nature. 1981 Jan 15;289(5794):139–144. doi: 10.1038/289139a0. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981 May 25;256(10):4715–4718. [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Gaudray P., Canning M., Trejo-Avila L., Cuzin F. Two polyoma virus gene functions involved in the expression of the transformed phenotype in FR 3T3 rat cells. I. Localization of a transformation maintenance function in the proximal half of the large T coding region. Virology. 1981 Oct 30;114(2):489–500. doi: 10.1016/0042-6822(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Dorai H., Arakere G., Benjamin T. L. Polyoma virus middle T antigen: relationship to cell membranes and apparent lack of ATP-binding activity. Mol Cell Biol. 1982 Oct;2(10):1187–1198. doi: 10.1128/mcb.2.10.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Magee A. I., Schmidt M. F. Fatty acid acylation of proteins in cultured cells. J Biol Chem. 1980 Nov 10;255(21):10021–10024. [PubMed] [Google Scholar]
- Schlesinger M. J. Proteolipids. Annu Rev Biochem. 1981;50:193–206. doi: 10.1146/annurev.bi.50.070181.001205. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F. Acylation of viral spike glycoproteins: a feature of enveloped RNA viruses. Virology. 1982 Jan 15;116(1):327–338. doi: 10.1016/0042-6822(82)90424-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Soule H. R., Butel J. S. Subcellular Localization of simian virus 40 large tumor antigen. J Virol. 1979 May;30(2):523–532. doi: 10.1128/jvi.30.2.523-532.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. R., Lanford R. E., Butel J. S. Antigenic and immunogenic characteristics of nuclear and membrane-associated simian virus 40 tumor antigen. J Virol. 1980 Feb;33(2):887–901. doi: 10.1128/jvi.33.2.887-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Different structural systems of the nucleus are targets for SV40 large T antigen. Cell. 1983 May;33(1):173–181. doi: 10.1016/0092-8674(83)90346-x. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Trejo-Avila L., Gaudray P., Cuzin F. Two polyoma virus gene functions involved in the expression of the transformed phenotype in FR 3T3 rat cells. II. The presence of the 56K middle-T protein in the cell membrane is not sufficient for maintenance. Virology. 1981 Oct 30;114(2):501–506. doi: 10.1016/0042-6822(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Jay G., Pastan I. Localization of the ASV src gene product to the plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1979 Sep;18(1):125–134. doi: 10.1016/0092-8674(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Baltimore D. Preparation of syngeneic tumor regressor serum reactive with the unique determinants of the Abelson murine leukemia virus-encoded P120 protein at the cell surface. J Virol. 1979 Sep;31(3):776–784. doi: 10.1128/jvi.31.3.776-784.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]