Abstract
Preventive intervention effects on adolescent alcohol misuse may differ based on genotypes in gene-by-intervention (G x I) interactions, and these G x I interactions may vary as a function of age. The current study uses a novel statistical method, time-varying effect modeling (TVEM), to test an age-varying interaction between a single nucleotide polymorphism in the GABRA2 gene (rs279845) and a preventive intervention in predicting alcohol misuse in a longitudinal study of adolescents (ages 11–20). The preventive intervention was PROSPER, a community-based system for delivery of family and school programs selected from a menu of evidence-based interventions. TVEM results revealed a significant age-varying GABRA2 x Intervention interaction from ages 12–18, with the peak effect size seen around age 13 (IRR=0.50). The intervention significantly reduced alcohol misuse for adolescents with the GABRA2 TT genotype from ages 12.5–17, but did not reduce alcohol use for adolescents with GABRA2 A allele at any age. Differences in intervention effects by GABRA2 genotype were most pronounced from ages 13–16 – a period when drinking is associated with increased risk for alcohol use disorder. Our findings provide additional evidence that intervention effects on adolescent alcohol misuse may differ by genotype, and provide novel evidence that the interaction between GABRA2 and intervention effects on alcohol use may vary with age. Implications for interventions targeting adolescent alcohol misuse are discussed.
Keywords: GABRA2, Preventive Intervention, Adolescent Alcohol Misuse, Age-Varying Effects, Gene x Intervention (G x I) Interaction
Adolescent alcohol misuse (e.g., drinking and drunkenness) is a major public health problem, owing to its high prevalence (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2014) and wide-ranging negative consequences such as motor vehicle accidents, drunk driving, and sexual risk behavior (NIAAA, 2003). At least some alcohol use (e.g., alcohol initiation and lower-frequency use) appears normative during adolescence (Johnston et al., 2014), but such use is sometimes linked to alcohol problems later in life (Chassin, Sher, Hussong, & Curran, 2013). Adolescent alcohol misuse most frequently predicts future alcohol problems when it begins early, i.e., prior to age 15 (Grant & Dawson, 1997). For these reasons, early alcohol misuse has been a target for preventive interventions, which have shown success as indexed by reduced rates of heavy drinking (e.g., 5 or more drinks on a single occasion), drunkenness, and related problem behaviors associated with alcohol use risk, such as aggression (Spoth, Greenberg, & Turrisi, 2008). For example, the PROSPER prevention trial examined the long-term effects of the PROSPER Partnership Model as a delivery system for evidence-based universal family and school interventions selected from a menu. It showed a relative reduction in the rate (RRR) of 10th grade alcohol use initiation by 5% (p=.034; Spoth et al., 2011) and showed a trend-level reduction in past-year drunkenness (RRR=9%, p=.067).
In their review of preventive interventions addressing underage drinking, Spoth and colleagues (2008) acknowledged there is limited evidence that prevention is uniformly efficacious for all adolescents, and called for more research examining risk-related moderation of preventive intervention effects. Emerging research integrating genetics into intervention trials has answered this call, with studies now showing that genotypes associated with risk for alcohol use behaviors may moderate prevention effects. For example, Brody and colleagues (Brody, Beach, Philibert, Chen, & Murry, 2009; Brody et al., 2014; Brody, Chen, & Beach, 2013) found that adolescents with different genetic variants in the dopaminergic (e.g., DRD4), serotonergic (e.g., 5-HTTLPR), and GABAergic (e.g., GABRA2) systems responded differently to intervention effects on conduct problems, alcohol misuse (self-reported past-month drinking and past-month heavy drinking), and other substance misuse (self-reported marijuana and nicotine use). Another study by Cleveland and colleagues (2015) reported that the PROSPER intervention delivery system showed stronger protective effects on 9th grade lifetime alcohol misuse – a composite including having ever (a) had a drink of alcohol, (b) had more than a few sips of a drink, and (c) been drunk from alcohol – for adolescents carrying the DRD4-7R variant who also experienced average to high levels of maternal involvement. These gene-by-intervention (G x I) interaction findings not only shed light on genetically-related differences in prevention responsiveness, they also add powerful evidence supporting the concept of gene-by-environment (G x E) interaction, which suggests that genetic differences may increase sensitivity to environmental effects on youth problem behaviors (Moffitt, Caspi, & Rutter, 2006). G x I interaction studies provide strong evidence for G x E because the intervention “environment” is randomly assigned, which greatly reduces the concern that gene-environment correlations (rGEs) may be masquerading as G x E interactions (Kendler, 2011). In addition, by testing differential effectiveness of prevention and intervention efforts by genotype, G x I interaction studies not only assist in the identification of those youth who may be most responsive to existing intervention efforts, but may conversely help to identify youth who are not as responsive yet remain at risk (Cleveland, Schlomer, Vandenbergh, & Wiebe, in press).
There is now an emerging body of evidence for G x I interactions (see, e.g., meta-analysis by van IJzendoorn & Bakermans-Kranenburg, 2015). As G x I research grows, the issue of developmental variation in genetic susceptibility to environments has received increasing attention (Albert et al., 2015; Cleveland et al., under review; Costello et al., 2013), partly in response to the possibility that developmental changes in gene expression (Lenroot & Giedd, 2011) may alter genetic sensitivity to environmental risks and buffers for alcohol misuse across age. This is especially important to consider given (a) differences in future dependence risk associated with early versus later adolescent alcohol use initiation; (b) differences in ethanol sensitivity across adolescent development (Windle et al., 2008); and (c) changes in the social norms surrounding alcohol use, including less restrictive norms as adolescents age and begin the transition into young adulthood (Schulenberg & Maggs, 2002).
In the current study, we focused on how PROSPER intervention effects on adolescent alcohol misuse (past-month drinking and drunkenness) are moderated by GABRA2 genotype, as well as how this genetically moderated intervention effect changes with age. The GABRA2 gene codes for the alpha-2 subunit of GABAA receptors and is part of a cluster of GABAA receptor subunit-encoding genes on chromosome 4 (McLean, Farb, & Russek, 1995). GABAA receptors are important mediators of alcohol’s behavioral effects (Kumar et al., 2009). Associations have been reported between GABRA2 variants and a number of alcohol-related phenotypes across developmental periods, including adult alcohol dependence (Covault, Gelernter, Hesselbrock, Nellissery, & Kranzler, 2004; Edenberg et al., 2004; Enoch, 2008; Fehr et al., 2006; Soyka et al., 2008), subclinical alcohol-related risk behaviors in late adolescence and young adulthood (i.e., drinking, heavy drinking, and drunkenness; Dick et al., 2014; Trucco, Villafuerte, Heitzeg, Burmeister, & Zucker, 2014) and problem behaviors predictive of alcohol misuse – such as conduct problems and rule-breaking behavior – during early-to-mid adolescence (Trucco et al., 2014). However, associations between GABRA2 SNPs and adolescent and adult alcohol use disorder are not always found (Dick et al., 2006; Onori et al., 2010; Sakai et al., 2010). The GABRA2 gene may also influence sensitivity to environments, including interventions, and this sensitivity may vary with age. GABAA alpha-2 subunit-containing receptors are heavily expressed in reward sensitive areas of the brain, such as the nucleus accumbens and the striatum (Schwarzer et al., 2001). Heightened reward sensitivity is not only associated with increased risk for adolescent substance use (Dawe & Loxton, 2004), but it may also result in greater responsiveness to positively and negatively valenced environmental conditions, as suggested by differential susceptibility theory (e.g., Belsky & Pluess, 2009; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011). Moreover, the differential expression of gamma-aminobutyric acid (GABA) receptor genes across development (Fillman, Duncan, Webster, Elashoff, & Weickert, 2010), the continued maturation of the GABA system during adolescence (Kilb, 2012), and changing associations between GABRA2 variants and externalizing and alcohol-related outcomes across development (Dick et al., 2013; Dick et al., 2014) suggest that associations between the GABRA2 gene variants, alcohol misuse, and responsivity to intervention may be developmentally varying.
We focus on the GABRA2 SNP rs279845 in the current study because this SNP has shown associations with alcohol use phenotypes and externalizing outcomes across a variety of populations (e.g., Dick et al., 2009, 2011; Edenberg et al., 2004; Fehr et al., 2006; Lind et al., 2008; Uhart et al., 2013). The biological function of rs279845 has not yet been assessed; however, the location of rs279845 within the 3rd intron of GABRA2 points to the need to explore its possible role in RNA splicing, which is known to contribute to a significant fraction of genetic variation in disease (Li et al., 2016). To capture the potentially complex age-related variation in the GABRA2 x Intervention interaction, we use time-varying effect modeling (TVEM; Tan, Shiyko, Li, Li, & Dierker, 2012), a novel statistical model that allows associations between variables – including intervention and G x I interaction effects – to vary as complex functions of age. To our knowledge, this paper is the first to use TVEM to estimate G x I interaction by adolescent age. Although we expected, based on prior research and theory, that the GABRA2 x intervention interaction would vary over adolescence, we did not have specific hypotheses regarding the direction or magnitude of this change.
Method
Data were from the PROSPER cohort sequential prevention trial, which included students across 28 school districts in Iowa and Pennsylvania. Half of the districts were randomly assigned to receive preventive interventions targeting adolescent substance use, yielding 14 intervention communities and 14 control communities. Students in all 28 school districts were invited to complete in-school surveys, and 90% participated. Two cohorts of students participated. Pre-intervention assessments were conducted in school during the fall semesters of 2002 and 2003 for cohorts 1 and 2, respectively (first semester of 6th grade). Follow-up in-school surveys were given annually from 0.5 years to 6.5 years past baseline (from spring semester of 6th grade to spring semester of 12th grade). Additionally, a random sample of 2,267 families of youth in Cohort 2 of the PROSPER project were invited to participate in the in-home data collections, which consisted of interviews and parent and adolescent questionnaires in Waves 1 through 5; 979 (43%) participated.
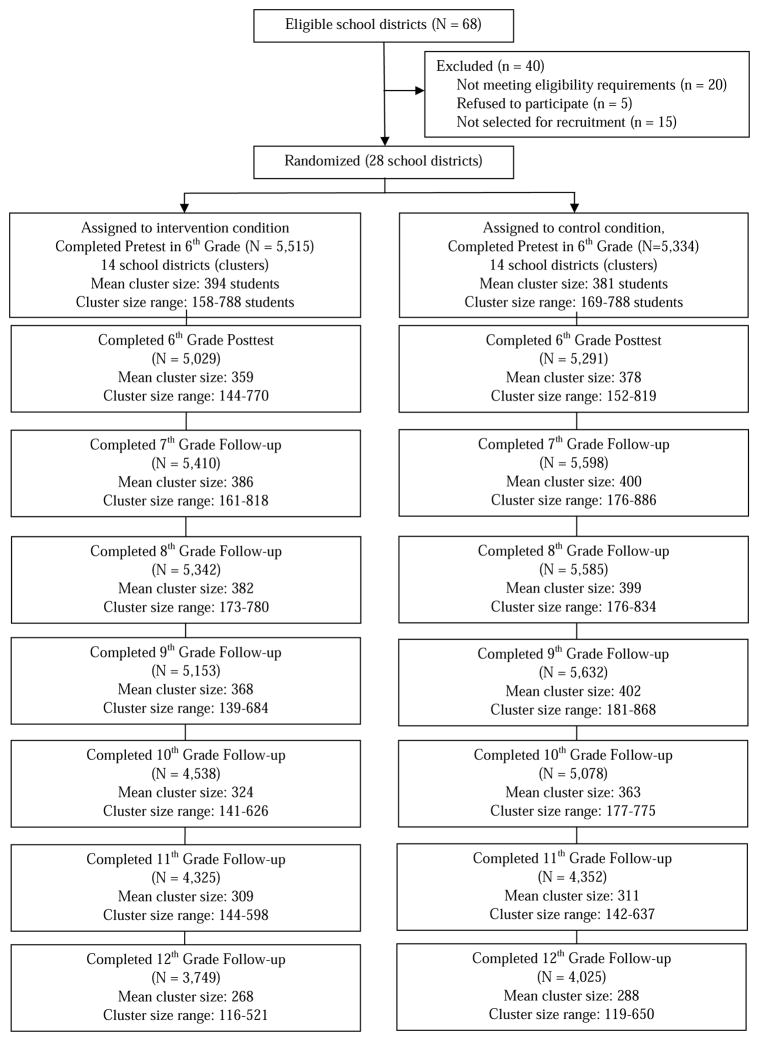
Intervention was provided via the PROSPER delivery system, which supported the delivery of evidence-based preventive intervention through a school-community-university partnership (see Spoth, Greenberg, Bierman, & Redmond, 2004). The intervention consisted of two types of evidence-based programs (chosen from a short menu provided to community teams): (1) a voluntary family-focused intervention (delivered in the spring semester of 6th grade, 2003 and 2004 for Cohorts 1 and 2, respectively) and (2) a school-based intervention (delivered in 7th grade, 2004 and 2005 for Cohorts 1 and 2, respectively). The interventions targeted social norms, personal goal setting, decision-making, peer group affiliation, the parent-child relationship, and family-functioning. High levels of implementation quality were observed (Spoth, 2007; Spoth et al., 2007). See Figure 1 for the PROSPER CONSORT diagram (for design details, see Spoth et al., 2013).
Figure 1.
In-School Survey Total Participation by Wave*
*Reprinted from Preventive Medicine, Vol. 56, Spoth, R., Redmond, C., Shin, C., Greenberg, M., Feinberg, M., & Schainker, L., PROSPER community–university partnership delivery system effects on substance misuse through 6-1/2years past baseline from a cluster randomized controlled intervention trial, p. 190–196, Copyright (2013), with permission from Elsevier.
The gPROSPER sample includes 2,032 youth participating in the larger PROSPER project who provided buccal cell samples for genotyping. Of the 2,032 youth who provided buccal cell samples, 537 did so during the Wave 5 in-home assessment, and the remaining 1,495 provided samples through the mail as part of a young-adult follow-up assessment. The gPROSPER study is adequately powered to detect associations – including main effects and interactions in multiple regression analysis – even with small effect sizes, with over 99% power to detect partial correlations of 0.1 or greater. DNA was collected via buccal cell samples from all gPROSPER youth. Youth were compensated $25 for their participation in the DNA collection. Of the 2,032 participants who provided DNA samples, 94% (1,920) were successfully genotyped for the GABRA2 rs279845 SNP [TaqMan assay # C___8263012_10 conducted on an OpenArray system (Life Technologies, Thermo Fisher)]. For the remaining 6% of participants, the GABRA2 rs279845 genotype could not be determined based on the DNA samples provided. The 1,920 youth successfully genotyped for GABRA2 rs279845 make up the analytic sample of the current study (40% male, 53% intervention condition, 66% Cohort 2; 90% non-Hispanic White, 4% Hispanic, 2% African-American, <1% Native American, 1% Asian, 2% Other). GABRA2 genotype frequencies (21% AA, 49% AT, 30% TT) were in Hardy-Weinberg Equilibrium (χ2(df=2)=0.06, p=.97), consistent with population estimates (see dbSNP, http://www.ncbi.nlm.nih.gov/snp and the 1000 genomes project, http://www.1000genomes.org/). The genotype distribution did not differ by intervention condition, χ2(df=2)=0.28, p=.87, self-reported White versus non-White race/ethnicity, χ2(df=2)=2.23, p=.33, or White versus non-White race/ethnicity identified via ancestry informative genetic markers (see description in Statistical Analysis section), χ2(df=2)=1.60, p=.45; however, the distribution did differ by gender, χ2(df=2)=9.48, p=.009. Follow-up analyses showed male genotype frequencies (24% AA, 50% AT, 27% TT) differed from females (19% AA, 49% AT, 32% TT), but only for the two homozygotes (ps <.014).
Adolescent alcohol misuse was assessed annually (6th–12th grade) via self-report surveys. Adolescents reported annually on past-month drinking (frequency of any drinking in the past month), and past-month drunkenness (frequency of being intoxicated or drunk from alcohol in the past month) on a 1 to 5 scale (1=Not at all, 2=One time, 3=A few times, 4=About once a week, and 5=More than once a week). Over 11,000 person-waves of alcohol misuse data were collected across adolescents in the analytic sample from wave 2 (second semester of 6th grade, M age = 12.2 years) to wave 8 (12th grade, M age = 18.1 years); responses from baseline (Wave 1, first semester of 6th grade; M age = 11.8 years) were excluded from analyses as these responses were provided prior to intervention participation for all adolescents. On average, each adolescent provided 5.8 out of 7 possible alcohol misuse reports across waves 2 to 8 (SD=1.5). Examination of drinking and drunkenness distributions including observations across all person-waves revealed that approximately 73% and 86% of person-waves indicated no drinking or drunkenness respectively. As such, both scales were dichotomized; the overall base rates of drinking and drunkenness were 26.9% and 14.3%, respectively. Finally, drinking and drunkenness indicators were summed to create an adolescent alcohol misuse composite for use in analyses (M=0.41, SD=0.72, Min=0, Max=2). Summation of these and similar indicators has been used previously to evaluate prevention effects on adolescent alcohol use (Brody et al., 2013; Spoth, Redmond, Shin, & Azevedo, 2004).
Statistical Analyses
Overview
The TVEM SAS macro was used for all analyses (available for download at methodology.psu.edu). TVEM is a direct extension of multiple regression that allows regression coefficients (i.e., intercepts and slopes) to be estimated as non-parametric functions of continuous time or age. These functions are non-parametric because TVEM requires no constraints on the shapes of the intercept and slope functions across age. All models used “sandwich” error estimation to adjust for the clustering of observations within adolescents (Williams, 2000). All analyses involving the intervention were intent-to-treat analyses. As such, intervention condition assignment, as opposed to intervention participation, was used as the intervention variable in the current analyses (53% of adolescents in the analytic sample were randomly assigned to intervention).
Preliminary analyses
The biological function of rs279845 is not currently known, and there have previously been inconsistencies as to which rs279845 allele is associated with externalizing and alcohol-related outcomes, with some studies identifying the minor allele (A) as the risk allele (Dick et al., 2014; Lind et al., 2008; Uhart et al., 2013), whereas others have identified the major allele (T) as the risk allele (Dick et al., 2009, 2011; Edenberg et al., 2004). Thus, in the absence of clear prior information that could inform the choice of a genetic analytic model, preliminary analyses were run coding GABRA2 genotype as a dummy-coded categorical variable. The use of dummy codes allows a model-free approach that does not assume additive, recessive, or dominant genotype effects. Results of these preliminary analyses revealed that intervention effects for adolescents with AA and AT genotypes were highly overlapping; thus, these groups were collapsed into a single group (referred to hereafter as A carriers) and comparisons were made between adolescents with TT genotype and A carriers. This type of data-driven approach to genetic model specification has been taken in previous studies (e.g., Trucco, Villafuerte, Heitzeg, Burmeister, & Zucker, 2016).
Testing age-varying GxI using TVEM
TVEM results do not rely on p-values for their interpretation. Thus, our GxI results were evaluated for significance via the following steps. First, each regression coefficient (including both main effects and interactions) and its 95% confidence interval (CI) are estimated as a function of age. Second, these estimates are compared against a hypothesis of no effect (β=0) and are declared significant at ages when their 95% CIs do not include 0. Equation 1 shows the TVEM used in our analyses.
| (1) |
Equation 1 models the alcohol misuse composite using a Poisson distribution (with a logarithmic link) to account for positive skew. The model includes an intercept (β0), a main effect of the GABRA2 TT genotype (β1), a main effect of intervention condition (β2), and an interaction between GABRA2 genotype and intervention condition (β3, the G x I interaction). These coefficients were modeled as continuous functions of age, denoted by the (age) modifier, which allowed us to examine variation in gene, intervention, and G x I effects from the youngest (11.2 years) to the oldest age (20.0 years) included across grades 7–12. Incident rate ratio (IRR) effect sizes and predicted alcohol misuse means were estimated by exponentiating slope and intercept coefficients (IRR=eβ) respectively. Modeling was executed in three steps. First, we estimated the full model as specified in Equation 1. We evaluated the significance of the age-varying G x I interaction effect (β3) by exponentiating the Poisson coefficient to yield an IRR effect size and comparing this estimate to a value of 1 (corresponding to a Poisson β=0). The G x I interaction effect was declared significant at ages where the 95% confidence interval of its IRR effect size did not overlap with 1. Second, we unpacked the interaction by generating genotype-specific intervention slopes describing the age-varying intervention effect for each GABRA2 genotype (separately for A carriers and TT youth) from the model estimates. Third, we generated group-specific intercept functions that described estimated alcohol misuse levels as a function of age for each GABRA2 genotype by intervention condition subgroup.
Additionally, we ran sensitivity analyses to control for population stratification, which refers to differences in allele frequencies across ancestral or ethnic populations that may create spurious associations between genes and behavior (Cardon & Palmer, 2003). Population stratification was assessed and controlled for in the current study via an admixture mapping method using ancestry informative markers (AIMs) described in Cleveland et al. (2015). Using information from 318,000 SNPs, a single principal coordinate (PC1) that explained 73.4% of the common variance in genetic ancestry was derived; further PCs provided little additional explanatory power. PC1 provides a continuous scale of European ancestry based on genetics. We used PC1 to control for population stratification in two ways: (1) by including the PC1 scale as a continuous covariate in sensitivity analyses and (2) by re-estimating all models keeping only those whose PC1 scores indicated European ancestry (see Cleveland et al., 2015 for details).
Results
Alcohol use by age
Table 1 shows rates of alcohol misuse measures (drinking, drunkenness, and the alcohol misuse composite) by assessment wave. As expected, alcohol misuse rates increased with age, as 7.2% and 0.9% of adolescents engaged in drinking and drunkenness, respectively, at wave 1 (M age =11.8 years), with these estimates increasing to 46.0% and 31.8%, respectively, at wave 8. Retention rates for past-month drinking and drunkenness assessments in the gPROSPER sample were high, averaging 84% across waves and ranging from 68.6% at wave 8 to 92.6% at wave 2. Logistic multilevel models predicting retention (non-missingness) in the alcohol misuse composite by wave revealed a significant decrease in retention from waves 2 to 8 (b=−0.36, SE=0.01, p<.001). Retention over time differed between control and intervention schools (intervention x wave interaction p<.001), with adolescents in control schools showing steeper decreases in retention over time (b=−0.41, SE=0.02, p<.001) compared to adolescents in intervention schools (b=−0.31, SE=0.02, p<.001).
Table 1.
Rates of alcohol use measures by wave
| Wave | M Age (SD)a | Nb | Retention (% of Wave 1 N) | % | Alcohol Use Count (Range 0–2) | |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
||
| Drinking | Drunkenness | M (SD) | ||||
|
|
||||||
| 1 | 11.8 (0.4) | 1874 | -- | 7.2% | 0.9% | 0.08 (0.30) |
| 2 | 12.2 (0.4) | 1735 | 92.6% | 8.8% | 1.2% | 0.10 (0.34) |
| 3 | 13.2 (0.4) | 1734 | 92.5% | 14.8% | 2.9% | 0.18 (0.45) |
| 4 | 14.3 (0.4) | 1713 | 91.4% | 22.4% | 8.4% | 0.31 (0.61) |
| 5 | 15.2 (0.4) | 1693 | 90.3% | 31.6% | 15.7% | 0.47 (0.75) |
| 6 | 16.2 (0.4) | 1516 | 80.9% | 34.3% | 21.5% | 0.56 (0.82) |
| 7 | 17.2 (0.4) | 1378 | 73.5% | 39.2% | 26.5% | 0.66 (0.87) |
| 8 | 18.1 (0.4) | 1286 | 68.6% | 46.0% | 31.8% | 0.78 (0.90) |
M ages (and SDs) at each wave are calculated among adolescents in the analytic sample (those successfully genotyped on GABRA2 rs279845).
N is based on the number of participants in the analytic sample who provided valid data for either drinking or drunkenness at each wave.
GABRA2 x Intervention Effect on Alcohol Misuse as a Continuous Function of Age
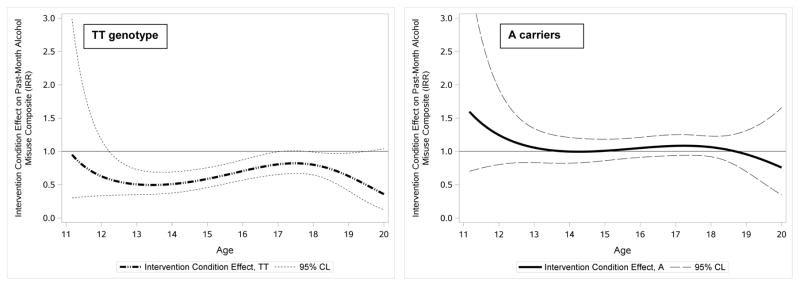
We identified a significant G x I interaction from ages 12–18. Figure 2 illustrates this interaction by showing the simple intervention condition effects for adolescents with the TT genotype versus A allele carriers. The left panel displays the intervention condition effect for adolescents with the TT genotype and shows that random assignment to the intervention condition significantly reduced alcohol misuse for these youth from ages 12.5 to 17, with the intervention effect peaking around age 13.5. The right panel shows the intervention condition effect for adolescents carrying the A allele and reveals that the intervention did not appear to significantly reduce alcohol misuse for these youth at any age. The confidence intervals of intervention effect sizes for TT genotype and A carrier youth did not overlap between the ages of 13–16, suggesting that differences in the intervention effect by GABRA2 genotype were most pronounced during this age period.
Figure 2. Age-varying GxI: Treatment condition reduced alcohol misuse for adolescents with the TT genotype but not for A allele carriers.
TVEM-estimated treatment condition simple effect sizes (IRRs, bolded lines) and 95% confidence limits (dashed lines) are presented as a continuous function of age for adolescents with the GABRA2 TT genotype (left panel), and for adolescents carrying the A allele (right panel). The flat lines in both panels at IRR=1 are reference lines against which to compare the age-varying regression coefficients. Effects are declared significant at all ages where their 95% confidence intervals do not include IRR=1. The difference in intervention condition effects on alcohol misuse between TT genotype and A allele carriers was most pronounced between ages 13 and 16, as the confidence intervals between the two groups do not overlap during these ages.
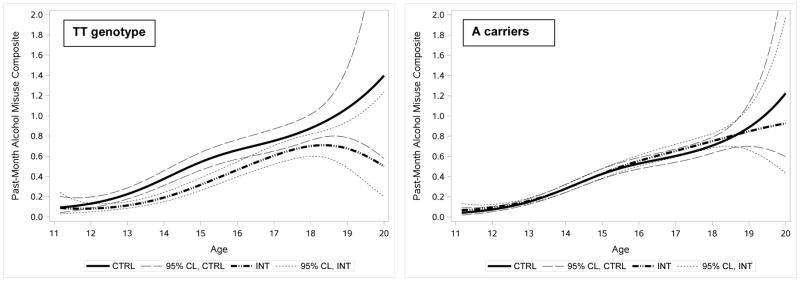
Figure 3 shows the age-varying G x I interaction in a different way: by showing the estimated age-varying alcohol misuse means by intervention condition, presented separately by GABRA2 genotype. The left panel shows predicted age-varying alcohol misuse means for TT genotype adolescents in the control versus intervention conditions. For both intervention and control TT adolescents, alcohol misuse increased with age. However, estimates for intervention and control TT adolescents do not overlap between ages 13 and 16, suggesting that the intervention reduced alcohol misuse for TT youth most strongly during these ages. The right panel shows age-varying alcohol misuse estimates by control versus intervention conditions for youth with GABRA2 A genotypes, and shows that the developmental course does not differ between control and intervention A carriers, indicating no intervention effect for these youth. Additional comparisons (not shown in Figure 3) revealed that among control adolescents, TT genotype youth showed significantly higher levels of alcohol misuse than A carriers from ages 11.5 to 18. In contrast, among intervention adolescents, TT genotype youth showed lower levels of alcohol use compared to A allele carriers from ages 13–16.
Figure 3. Age-varying alcohol use levels by intervention status and GABRA2 genotype.
TVEM estimated alcohol use means (and 95% confidence limits) for control and intervention adolescents are shown as a continuous function of age, presented separately for adolescents with the GABRA2 rs279845 TT genotype (left panel) and adolescents carrying the A allele of GABRA2 rs279845 (right panel). Confidence intervals for alcohol misuse levels did not overlap between TT control and TT intervention youth from ages 13–16, suggesting that random assignment to the intervention condition reduced alcohol misuse for TT youth most strongly during these ages.
Sensitivity Analyses
We found no differences in model effects when controlling for the PC1 scale or when we restricted models to only those with PC1-identified European ancestry; that is, we found little evidence for population stratification in either case. Additionally, given the correlation between GABRA2 genotype and gender, we also ran TVEM analyses controlling for Gender, Gender x GABRA2 TT, and Gender x Intervention age-varying interaction effects. Our pattern of results was replicated, suggesting that our G x I results are robust to gender differences.
Discussion
We tested the age-varying interaction between GABRA2 (SNP rs279845) genotype and random assignment to the PROSPER preventive interventions predicting alcohol misuse across adolescence in a large sample of rural youth. We found an age-varying GABRA2 x Intervention that was significant from ages 12–18. For youth with the TT genotype, the intervention significantly reduced alcohol misuse whereas no significant reduction was found for A allele carriers at any age. The difference in intervention effects by GABRA2 genotype was most pronounced from ages 13–16, when confidence intervals of group-specific functions did not overlap. In the control group, TT genotype youth showed higher levels of alcohol misuse throughout adolescence, whereas in the intervention group, TT youth showed lower levels of early adolescent misuse (ages 13–16) compared to A carriers. Taken together, these results suggest that preventive interventions targeting goal-setting, decision-making, peer group affiliation, parent-child relationships, and family functioning can reduce the risk of alcohol misuse among adolescents with the GABRA2 TT genotype, especially during early to mid-adolescence, a crucial window for the prevention of future alcohol abuse or dependence. Moreover, these results provide strong evidence for gene-environment interaction related to the GABRA2 gene because the intervention “environment” was randomly assigned, thus breaking the genotype-environment correlation that is often present in tests of gene-environment interaction where the environment is naturally occurring (van IJzendoorn & Bakermans-Kranenburg, 2015).
Our study builds on and extends the work of prior GxI studies by providing evidence of G x I interaction involving GABRA2 that changes with development and appears strongest for early-to-mid adolescent alcohol misuse. Our results suggest the possibility that the TT genotype is associated with differential susceptibility to environmental effects on alcohol misuse given that (a) control adolescents with the TT genotype showed higher levels of alcohol misuse throughout adolescence, whereas in the intervention group, TT youth showed lower levels of early to mid-adolescent alcohol misuse; and (b) the intervention reduced alcohol misuse for adolescents with the TT genotype but not for adolescents carrying the A allele, which suggests that adolescents with the TT genotype were significantly responsive to the intervention as would be expected if the TT genotype is indeed associated with differential susceptibility.
We identified the TT genotype as the risk genotype for alcohol misuse among control adolescents, as TT youth showed higher levels of alcohol misuse in the control group than youth with A alleles. This finding corresponds with previous research showing the major allele (T) of GABRA2 rs279845 as the “risk” allele, associated with externalizing psychopathology trajectory in adolescence (ages 12–22; Dick et al., 2009; Dick et al., 2011) and alcohol dependence in adults (ages 18 and older; Edenberg et al., 2004). Other studies, however, have found risk associations with the minor allele (A); these associations include trajectories of drunkenness frequency from adolescence to young adulthood (ages 14–25; Dick et al., 2014), alcohol dependence in adulthood (mean age 43 years; Fehr et al., 2006; Lind et al., 2008), and more negative subjective effects of alcohol administered to moderate drinking young adults in a laboratory setting (mean age 24 years; Uhart et al., 2013). The causes of inconsistency in the GABRA2 rs279845 risk allele identifications across studies are not entirely clear from the extant literature and warrant future research. We note that a number of factors may be contributing, including differences in the outcomes assessed, the developmental period at which gene-behavior associations were tested, and differences in whether GABRA2 genotype was coded based on reverse versus forward DNA strand across studies, as the A and T alleles are complements of one another and this information is not always reported. Because the precise biological action of the rs279845 SNP is not currently well known, however, these suggested causes remain speculative until future research is able to provide support.
Limitations of this study include the following. First, our reliance on a single genetic marker provides an incomplete picture of how GABRA2 genetic risk interacts with preventive environments across adolescence. Gene-behavior associations are likely to involve “many genes of small effect”, and our chosen GABRA2 SNP is but one small piece of the puzzle. Second, although our genetic associations appeared robust to third variables, such as ancestry and gender, we cannot conclude that the GABRA2 gene shows a causal effect on alcohol use or responsivity to intervention, as this effect may be due to GABRA2–associated behavioral traits (i.e., externalizing problems) or other genetic markers in high linkage disequilibrium with the TT genotype of GABRA2 rs279845. Third, the PROSPER project was delivered in communities of predominantly White adolescents in rural areas; it is thus unclear whether our pattern of findings would hold for adolescents of other racial/ethnic backgrounds as well as for adolescents in urban areas. Fourth, although previous findings support the validity of adolescent self-reports of drinking and drunkenness (Smith, McCarthy, & Goldman, 1995), our alcohol use measures were based on youth self-reports, which may be subject to social desirability bias. Fifth, the multicomponent nature of the intervention limits our ability to determine which components were most versus least effective on average, by genotype, or by other pre-existing characteristics. However, both the family- and school-focused intervention components have been previously established as evidence-based interventions (Spoth et al., 2011), providing some assurance that both components are contributing to reduction in alcohol misuse.
Our findings have implications for research and prevention. For research, this study provides additional weight to the body of evidence identifying GABRA2 gene variants as moderators of environmental – and intervention – effects on externalizing behavior and alcohol misuse. Our findings underscore the need for more research into the mechanisms that explain associations between GABRA2 variants, environmental responsiveness, and externalizing behaviors. For prevention, our findings suggest that family- and youth-focused interventions such as those delivered via the PROSPER project may have differential effects based on adolescents’ genotypes, and these differential effects may vary with age. Previous findings on PROSPER project effects have shown that the intervention appears more effective in reducing substance use outcomes for youth at behavioral risk (Spoth et al., 2007; Spoth et al., 2013). Our results add to this work by showing that genetic risk may also moderate PROSPER project effects, as youth with the GABRA2 TT genotype, who were at higher risk in the control group, saw greater intervention-led reductions in alcohol misuse compared to youth with A genotypes, especially during early-to-mid-adolescence (ages 13–16), an important age period for the prevention of alcohol use disorders. The non-significant intervention effects across all of adolescence for GABRA2 A carriers, who were at lower risk for alcohol use in the control condition, may suggest the need for alternate or additional intervention content to reduce early and later adolescent drinking among these youth. Overall, our findings support the notion that the preventive intervention effectiveness may vary by genetic risk and age, and emphasize the need for continued research into how tailoring intervention content may help reduce the burden of alcohol use disorders in adolescence and adulthood for as many individuals as possible.
Acknowledgments
The authors would like to thank Kerry Hair, Dr. Deborah Grove and Ashley Price of the Penn State Genomics Core Facility, and Amanda Griffin of Penn State, Shirley Huck, Cathy Owen, Debra Bahr, and Anthony Connor of the Behavioral Research Services at Iowa State University Survey, and Rob Schofield and Dean Stankowski of the Penn State University Survey Research. PROSPER and gPROSPER projects were supported by grant numbers DA013709 and DA030389 from the National Institute on Drug Abuse (NIDA), respectively. Preparation of this article was supported by NIDA awards P50 DA010075 and P50 DA039838. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Funding. This research was supported by the National Institute on Drug Abuse (NIDA) NIH Grant #’s DA030389 and DA013709. Michael A. Russell is supported by grant numbers P50-DA010075 and P50-DA039838 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Conflict of Interest. The authors declare that they have no conflict of interest.
Ethical Approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent. Informed consent was obtained from all individual participants included in the study.
References
- Albert D, Belsky DW, Crowley DM, Bates JE, Pettit GS, Lansford JE, … Dodge KA. Developmental mediation of genetic variation in response to the Fast Track prevention program. Development and Psychopathology. 2015;27:81–95. doi: 10.1017/S095457941400131X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SR, Philibert RA, Chen Yf, Murry VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene × environment hypotheses tested via a randomized prevention design. Child Development. 2009;80:645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Chen Y-f, Beach SR, Kogan SM, Yu T, DiClemente RJ, … Philibert RA. Differential sensitivity to prevention programming: A dopaminergic polymorphism-enhanced prevention effect on protective parenting and adolescent substance use. Health Psychology. 2014;33:182–191. doi: 10.1037/a0031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Yf, Beach SR. Differential susceptibility to prevention: GABAergic, dopaminergic, and multilocus effects. Journal of Child Psychology and Psychiatry. 2013;54:863–871. doi: 10.1111/jcpp.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Chassin L, Sher KJ, Hussong A, Curran P. The developmental psychopathology of alcohol use and alcohol disorders: Research achievements and future directions. Development and Psychopathology. 2013;25:1567–1584. doi: 10.1017/S0954579413000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland HH, Schlomer GL, Vandenbergh DJ, Feinberg M, Greenberg M, Spoth R, … Hair KL. The conditioning of intervention effects on early adolescent alcohol use by maternal involvement and dopamine receptor D4 (DRD4) and serotonin transporter linked polymorphic region (5-HTTLPR) genetic variants. Development and Psychopathology. 2015;27:51–67. doi: 10.1017/S0954579414001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland HH, Schlomer GL, Vandenbergh DJ, Wiebe RP. Gene × intervention designs: Promising step toward understanding etiology and building better preventive interventions. Criminology & Public Policy (in press) [Google Scholar]
- Cleveland HH, Schlomer GL, Vandenbergh DJ, Wolf PSA, Feinberg M, Greenberg M, … Redmond C. Genetic and intervention effects on patterns of alcohol use across early and late adolescence (under review) [Google Scholar]
- Costello EJ, Eaves L, Sullivan P, Kennedy M, Conway K, Adkins DE, … van den Oord E. Genes, environments, and developmental research: Methods for a multi-site study of early substance abuse. Twin Research and Human Genetics. 2013;16:505–515. doi: 10.1017/thg.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neuroscience & Biobehavioral Reviews. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Latendresse S, Porjesz B, Schuckit M, Rangaswamy M, … Agrawal A. How phenotype and developmental stage affect the genes we find: GABRA2 and impulsivity. Twin Research and Human Genetics. 2013;16:661–669. doi: 10.1017/thg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, … Almasy L. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behavior Genetics. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- Dick DM, Cho SB, Latendresse SJ, Aliev F, Nurnberger JI, Edenberg HJ, … Bucholz K. Genetic influences on alcohol use across stages of development: GABRA2 and longitudinal trajectories of drunkenness from adolescence to young adulthood. Addiction Biology. 2014;19:1055–1064. doi: 10.1111/adb.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Latendresse SJ, Lansford JE, Budde JP, Goate A, Dodge KA, … Bates JE. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Archives of General Psychiatry. 2009;66:649–657. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Latendresse SJ, Lansford JE, Budde JP, Goate A, Dodge KA, … Bates JE. Errors in table and results in: Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Archives of General Psychiatry. 2011;68:980–980. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, … Jones K. Variations in GABRA2, encoding the α2 subunit of the GABA-A receptor, are associated with alcohol dependence and with brain oscillations. The American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA A receptors in the development of alcoholism. Pharmacology Biochemistry and Behavior. 2008;90:95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, … Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatric Genetics. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Duncan CE, Webster MJ, Elashoff M, Weickert CS. Developmental co-regulation of the β and γ GABA A receptor subunits with distinct α subunits in the human dorsolateral prefrontal cortex. International Journal of Developmental Neuroscience. 2010;28:513–519. doi: 10.1016/j.ijdevneu.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 1975–2013: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Kendler KS. A conceptual overview of gene-environment interaction and correlation in a developmental context. In: Kendler KS, Jaffee SR, Romer D, editors. The dynamic genome and mental health: The role of genes and environments in youth development. New York: Oxford University Press; 2011. pp. 5–28. [Google Scholar]
- Kilb W. Development of the GABAergic system from birth to adolescence. The Neuroscientist. 2012;18:613–630. doi: 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA-A receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Annual research review: Developmental considerations of gene by environment interactions. Journal of Child Psychology and Psychiatry. 2011;52:429–441. doi: 10.1111/j.1469-7610.2011.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YI, van de Geijn B, Raj A, Knowles DA, Petti AA, Golan D, … Pritchard JK. RNA splicing is a primary link between genetic variation and disease. Science. 2016;352:600–604. doi: 10.1126/science.aad9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, Agrawal A, Montgomery GW, Heath AC, Martin NG, Whitfield JB. The role of GABRA2 in alcohol dependence, smoking, and illicit drug use in an Australian population sample. Alcoholism: Clinical and Experimental Research. 2008;32:1721–1731. doi: 10.1111/j.1530-0277.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean PJ, Farb DH, Russek SJ. Mapping of the α 4 subunit gene (GABRA4) to human chromosome 4 defines an α 2—α 4—β 1—γ 1 gene cluster: Further evidence that modern GABA-A receptor gene clusters are derived from an ancestral cluster. Genomics. 1995;26:580–586. doi: 10.1016/0888-7543(95)80178-o. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology: Concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Perspectives on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- NIAAA. Underage drinking: A major public health challenge. 2003 Retrieved from http://pubs.niaaa.nih.gov/publications/aa59.htm.
- Onori N, Turchi C, Solito G, Gesuita R, Buscemi L, Tagliabracci A. GABRA2 and alcohol use disorders: No evidence of an association in an Italian case–control study. Alcoholism: Clinical and Experimental Research. 2010;34:659–668. doi: 10.1111/j.1530-0277.2009.01135.x. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Stallings MC, Crowley TJ, Gelhorn HL, McQueen MB, Ehringer MA. Test of association between GABRA2 (SNP rs279871) and adolescent conduct/alcohol use disorders utilizing a sample of clinic referred youth with serious substance and conduct problems, controls and available first degree relatives. Drug and Alcohol Dependence. 2010;106:199–203. doi: 10.1016/j.drugalcdep.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg JE, Maggs JL. A developmental perspective on alcohol use and heavy drinking during adolescence and the transition to young adulthood. Journal of Studies on Alcohol, Supplement. 2002:54–70. doi: 10.15288/jsas.2002.s14.54. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G. Distribution of the major γ-aminobutyric acid A receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. Journal of Comparative Neurology. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- Smith GT, McCarthy DM, Goldman MS. Self-reported drinking and alcohol-related problems among early adolescents: Dimensionality and validity over 24 months. Journal of Studies on Alcohol. 1995;56:383–394. doi: 10.15288/jsa.1995.56.383. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss U, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-a2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. Journal of Psychiatric Research. 2008;42:184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Spoth R. Opportunities to meet challenges in rural prevention research: Findings from an evolving community-university partnership model. The Journal of Rural Health. 2007;23:42–54. doi: 10.1111/j.1748-0361.2007.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Greenberg M, Bierman K, Redmond C. PROSPER community–university partnership model for public education systems: Capacity-building for evidence-based, competence-building prevention. Prevention Science. 2004;5:31–39. doi: 10.1023/b:prev.0000013979.52796.8b. [DOI] [PubMed] [Google Scholar]
- Spoth R, Greenberg M, Turrisi R. Preventive interventions addressing underage drinking: State of the evidence and steps toward public health impact. Pediatrics. 2008;121:S311–S336. doi: 10.1542/peds.2007-2243E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Clair S, Shin C, Greenberg M, Feinberg M. Preventing substance misuse through community–university partnerships: Randomized controlled trial outcomes 4½ years past baseline. American Journal of Preventive Medicine. 2011;40:440–447. doi: 10.1016/j.amepre.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Shin C, Azevedo K. Brief family intervention effects on adolescent substance initiation: School-level growth curve analyses 6 years following baseline. Journal of Consulting and Clinical Psychology. 2004;72:535–542. doi: 10.1037/0022-006X.72.3.535. [DOI] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Shin C, Greenberg M, Clair S, Feinberg M. Substance-use outcomes at 18 months past baseline: The PROSPER community–university partnership trial. American Journal of Preventive Medicine. 2007;32:395–402. doi: 10.1016/j.amepre.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Shin C, Greenberg M, Feinberg M, Schainker L. PROSPER community–university partnership delivery system effects on substance misuse through 6 1/2 years past baseline from a cluster randomized controlled intervention trial. Preventive Medicine. 2013;56:190–196. doi: 10.1016/j.ypmed.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Shiyko MP, Li R, Li Y, Dierker L. A time-varying effect model for intensive longitudinal data. Psychological Methods. 2012;17:61–77. doi: 10.1037/a0025814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, Zucker RA. Rule breaking mediates the developmental association between GABRA2 and adolescent substance abuse. Journal of Child Psychology and Psychiatry. 2014;55:1372–1379. doi: 10.1111/jcpp.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Villafuerte S, Heitzeg MM, Burmeister M, Zucker RA. Susceptibility effects of GABA receptor subunit alpha-2 (GABRA2) variants and parental monitoring on externalizing behavior trajectories: Risk and protection conveyed by the minor allele. Development and Psychopathology. 2016;28:15–26. doi: 10.1017/S0954579415000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Weerts EM, McCaul ME, Guo X, Yan X, Kranzler HR, … Wand GS. GABRA2 markers moderate the subjective effects of alcohol. Addiction Biology. 2013;18:357–369. doi: 10.1111/j.1369-1600.2012.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn MH, Bakermans-Kranenburg MJ. Genetic differential susceptibility on trial: Meta-analytic support from randomized controlled experiments. Development and Psychopathology. 2015;27:151–162. doi: 10.1017/S0954579414001369. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, … Dahl RE. Transitions into underage and problem drinking: Developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121:S273–S289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]