Abstract
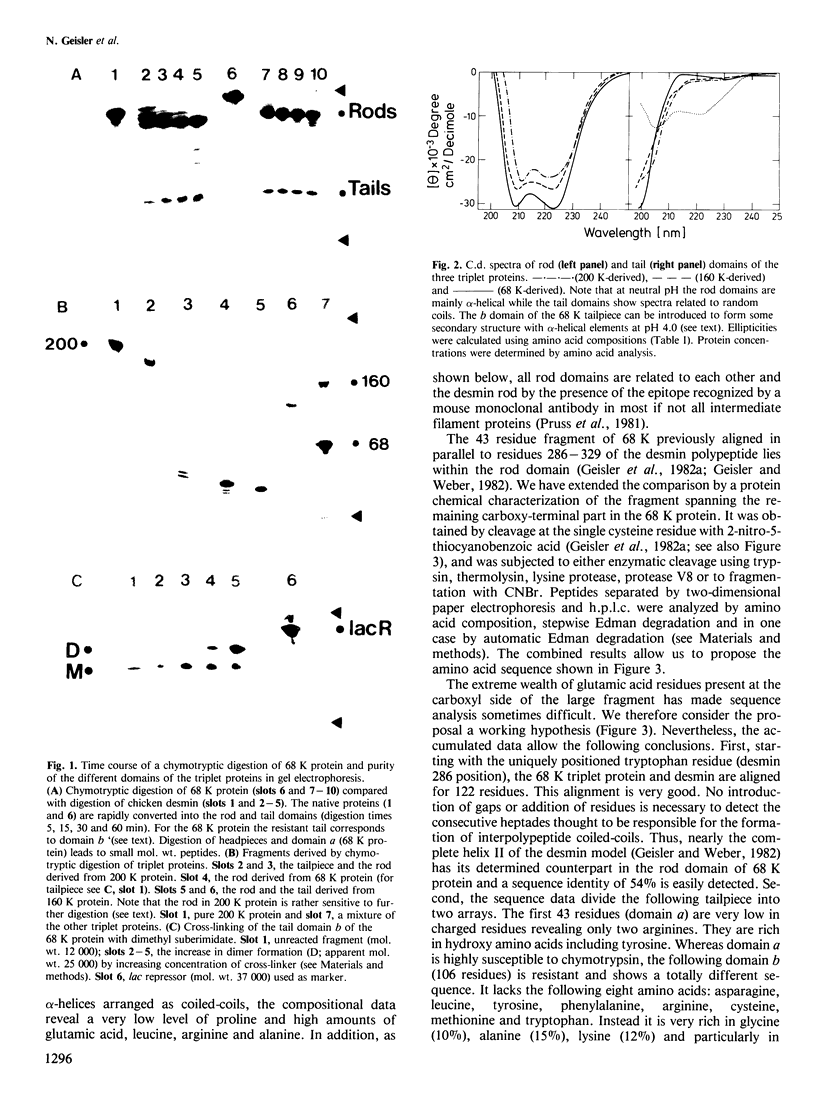
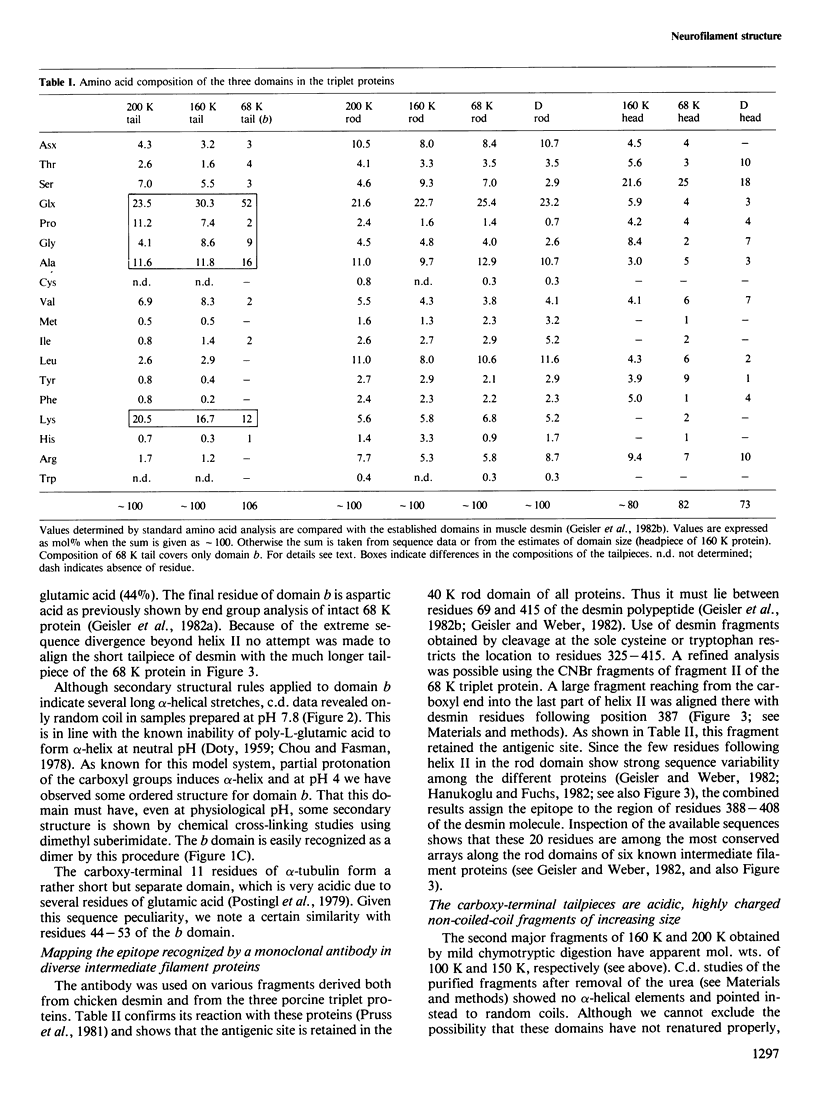
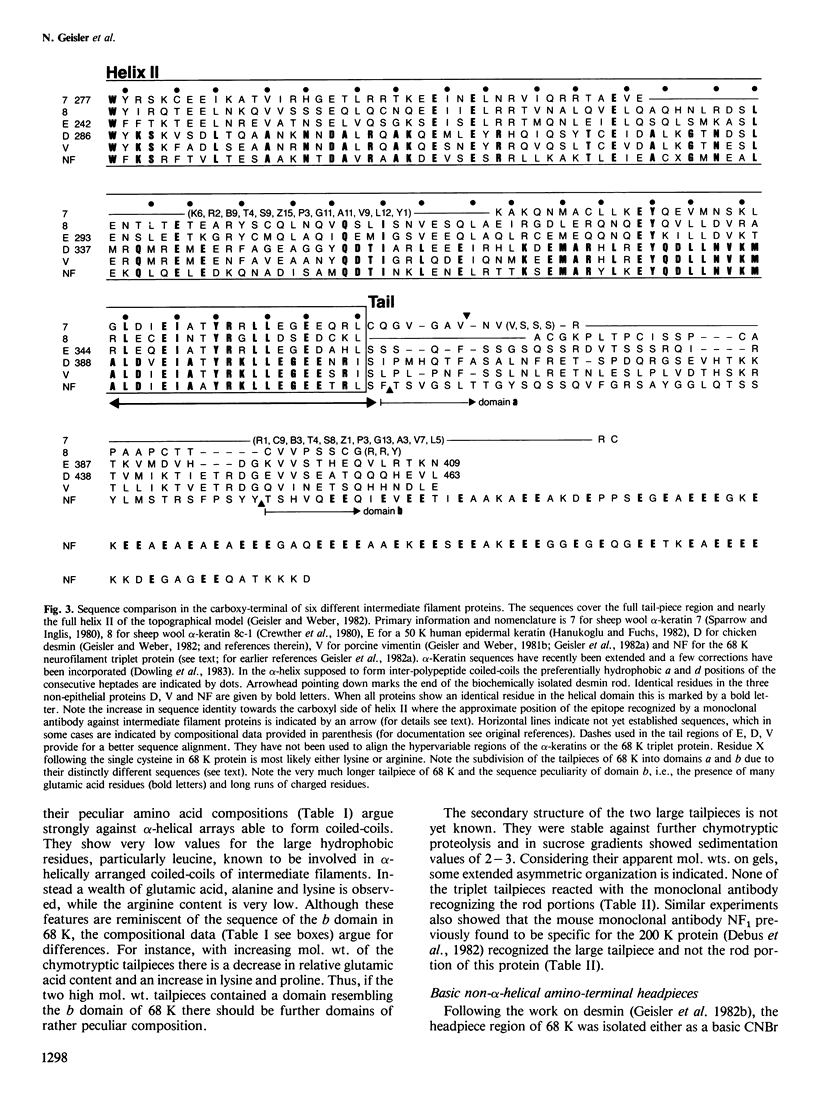
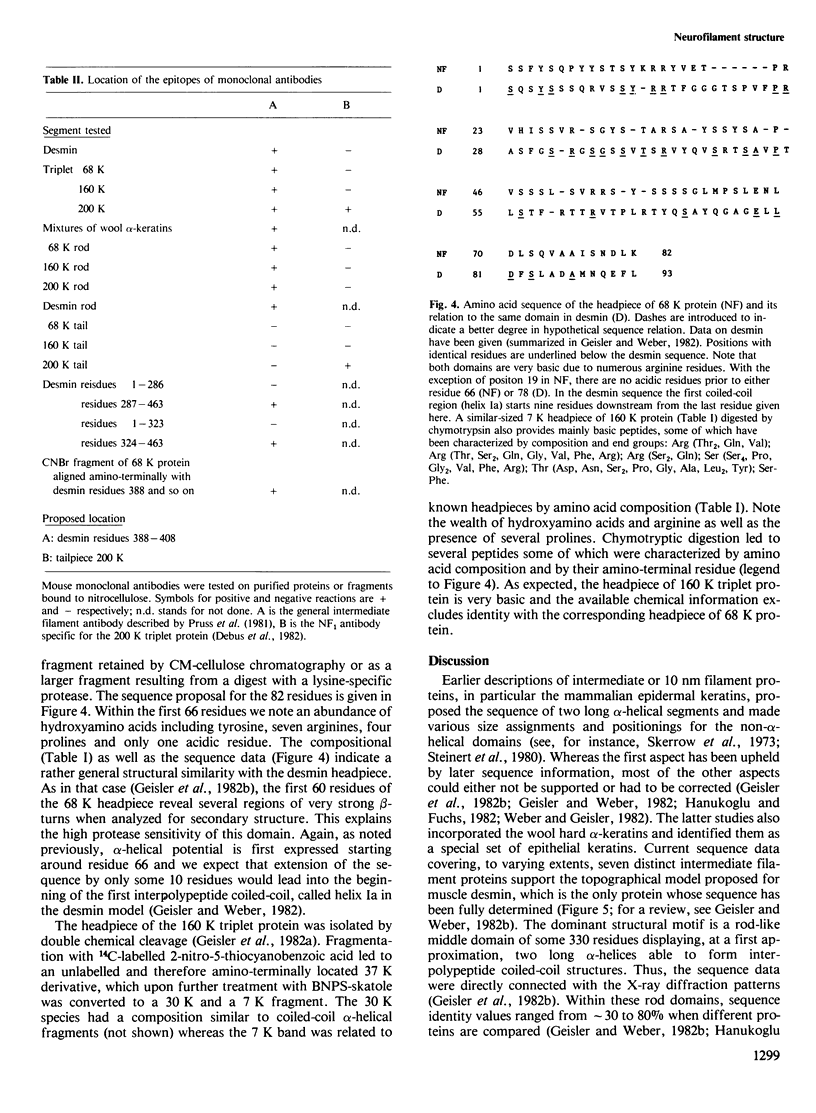
Mammalian neurofilament triplet proteins (68 K, 160 K and 200 K) have been correlated by a biochemical, immunological and protein chemical study. The 160 K and 200 K triplet proteins are intermediate filament proteins in their own right, since they reveal the alpha-helical coiled-coil rod domain analyzed in detail for the 68 K protein. Triplet proteins display two distinct arrays. Their amino-terminal region built analogously to non-neuronal intermediate filament proteins should allow a co-polymerization process via the interaction of coiled-coil domains. The extra mass of all triplet proteins is allocated to carboxy-terminally located extensions of increasing size and unique amino acid sequences. These may provide highly charged scaffolds suitable for interactions with other neuronal components. Such a domain of 68 K reveals, in sequence analysis, 47 glutamic acids within 106 residues. The epitope recognized by a monoclonal antibody reacting probably with all intermediate filament proteins has been mapped. It is located within the last 20 residues of the rods, where six distinct intermediate filament proteins point to a consensus sequence.
Full text
PDF
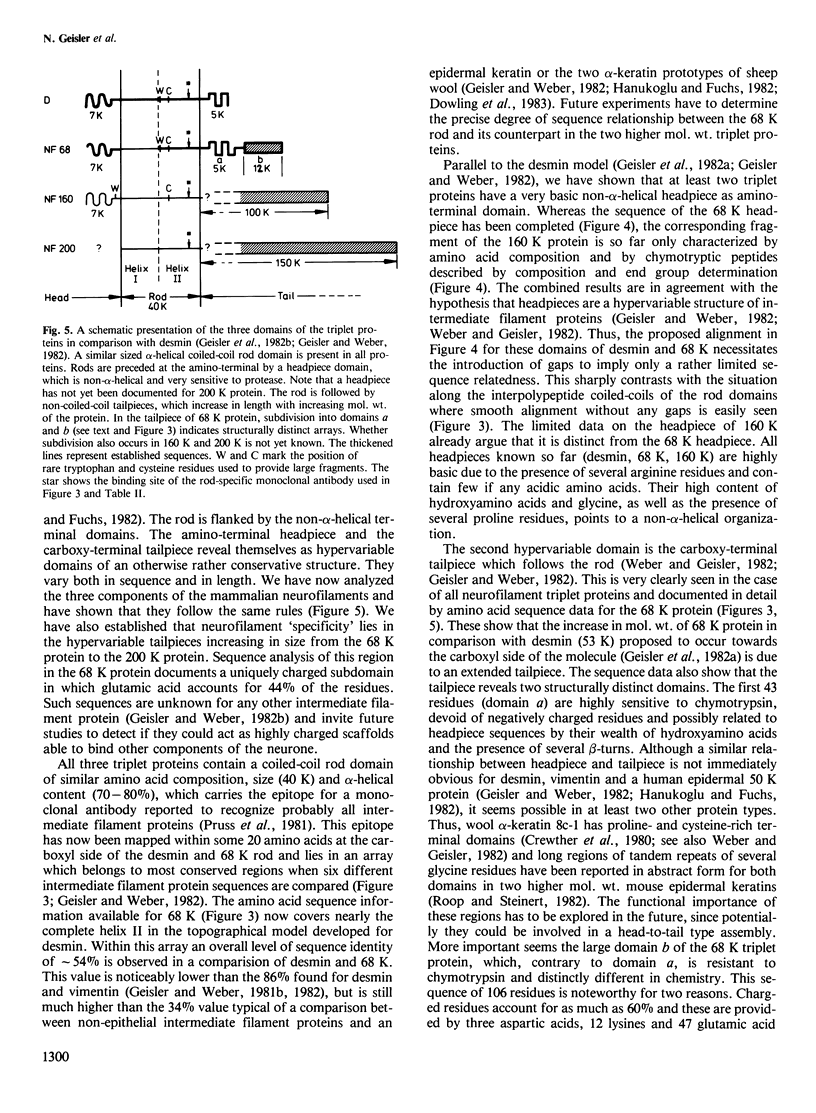


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Anderton B. H. Intermediate filaments: a family of homologous structures. J Muscle Res Cell Motil. 1981 Jun;2(2):141–166. doi: 10.1007/BF00711866. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Debus E., Flügge G., Weber K., Osborn M. A monoclonal antibody specific for the 200 K polypeptide of the neurofilament triplet. EMBO J. 1982;1(1):41–45. doi: 10.1002/j.1460-2075.1982.tb01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacourte A., Filliatreau G., Boutteau F., Biserte G., Schrevel J. Study of the 10-nm-filament fraction isolated during the standard microtubule preparation. Biochem J. 1980 Nov 1;191(2):543–546. doi: 10.1042/bj1910543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling L. M., Parry D. A., Sparrow L. G. Structural homology between hard alpha-keratin and the intermediate filament proteins desmin and vimentin. Biosci Rep. 1983 Jan;3(1):73–78. doi: 10.1007/BF01121573. [DOI] [PubMed] [Google Scholar]
- Eagles P. A., Gilbert D. S., Maggs A. The location of phosphorylation sites and Ca2+-dependent proteolytic cleavage sites on the major neurofilament polypeptides from Myxicola infundibulum. Biochem J. 1981 Oct 1;199(1):101–111. doi: 10.1042/bj1990101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagles P. A., Gilbert D. S., Maggs A. The polypeptide composition of axoplasm and of neurofilaments from the marine worm Myxicola infundibulum. Biochem J. 1981 Oct 1;199(1):89–100. doi: 10.1042/bj1990089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetti P., Autilio Gambetti L., Papasozomenos S. C. Bodian's silver method stains neurofilament polypeptides. Science. 1981 Sep 25;213(4515):1521–1522. doi: 10.1126/science.6169146. [DOI] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Weber K. Proteinchemical characterization of three structurally distinct domains along the protofilament unit of desmin 10 nm filaments. Cell. 1982 Aug;30(1):277–286. doi: 10.1016/0092-8674(82)90033-2. [DOI] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. Related amino acid sequences in neurofilaments and non-neural intermediate filaments. Nature. 1982 Apr 1;296(5856):448–450. doi: 10.1038/296448a0. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: desmin and vimentin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4120–4123. doi: 10.1073/pnas.78.7.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. Self-assembly in Vitro of the 68,000 molecular weight component of the mammalian neurofilament triplet proteins into intermediate-sized filaments. J Mol Biol. 1981 Sep 25;151(3):565–571. doi: 10.1016/0022-2836(81)90011-5. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Henderson D., Geisler N., Weber K. A periodic ultrastructure in intermediate filaments. J Mol Biol. 1982 Feb 25;155(2):173–176. doi: 10.1016/0022-2836(82)90444-2. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975 Aug;66(2):351–366. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lee V., Wu H. L., Schlaepfer W. W. Monoclonal antibodies recognize individual neurofilament triplet proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6089–6092. doi: 10.1073/pnas.79.19.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem R. K., Hutchison S. B. Purification of individual components of the neurofilament triplet: filament assembly from the 70 000-dalton subunit. Biochemistry. 1982 Jun 22;21(13):3221–3226. doi: 10.1021/bi00256a029. [DOI] [PubMed] [Google Scholar]
- Liem R. K., Yen S. H., Salomon G. D., Shelanski M. L. Intermediate filaments in nervous tissues. J Cell Biol. 1978 Dec;79(3):637–645. doi: 10.1083/jcb.79.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micko S., Schlaepfer W. W. Protein composition of axons and myelin from rat and human peripheral nerves. J Neurochem. 1978 May;30(5):1041–1049. doi: 10.1111/j.1471-4159.1978.tb12397.x. [DOI] [PubMed] [Google Scholar]
- Milam L., Erickson H. P. Visualization of a 21-nm axial periodicity in shadowed keratin filaments and neurofilaments. J Cell Biol. 1982 Sep;94(3):592–596. doi: 10.1083/jcb.94.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Intermediate filaments: cell-type-specific markers in differentiation and pathology. Cell. 1982 Dec;31(2 Pt 1):303–306. doi: 10.1016/0092-8674(82)90122-2. [DOI] [PubMed] [Google Scholar]
- Ponstingl H., Little M., Krauhs E., Kempf T. Carboxy-terminal amino acid sequence of alpha-tubulin from porcine brain. Nature. 1979 Nov 22;282(5737):423–425. doi: 10.1038/282423a0. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Sharp G. A., Shaw G., Weber K. Immunoelectronmicroscopical localization of the three neurofilament triplet proteins along neurofilaments of cultured dorsal root ganglion neurones. Exp Cell Res. 1982 Feb;137(2):403–413. doi: 10.1016/0014-4827(82)90042-8. [DOI] [PubMed] [Google Scholar]
- Shaw G., Weber K. The distribution of the neurofilament triplet proteins within individual neurones. Exp Cell Res. 1981 Nov;136(1):119–125. doi: 10.1016/0014-4827(81)90043-4. [DOI] [PubMed] [Google Scholar]
- Skerrow D., Matoltsy A. G., Matoltsy M. N. Isolation and characterization of the helical regions of epidermal prekeratin. J Biol Chem. 1973 Jul 10;248(13):4820–4826. [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Goldman R. D. Intermediate filaments of baby hamster kidney (BHK-21) cells and bovine epidermal keratinocytes have similar ultrastructures and subunit domain structures. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4534–4538. doi: 10.1073/pnas.77.8.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Geisler N. The structural relation between intermediate filament proteins in living cells and the alpha-keratins of sheep wool. EMBO J. 1982;1(10):1155–1160. doi: 10.1002/j.1460-2075.1982.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard M., Simon C. Antibody decoration of neurofilaments. J Cell Biol. 1981 May;89(2):198–205. doi: 10.1083/jcb.89.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]



