Abstract
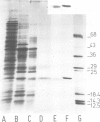
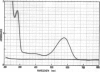
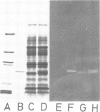
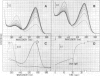
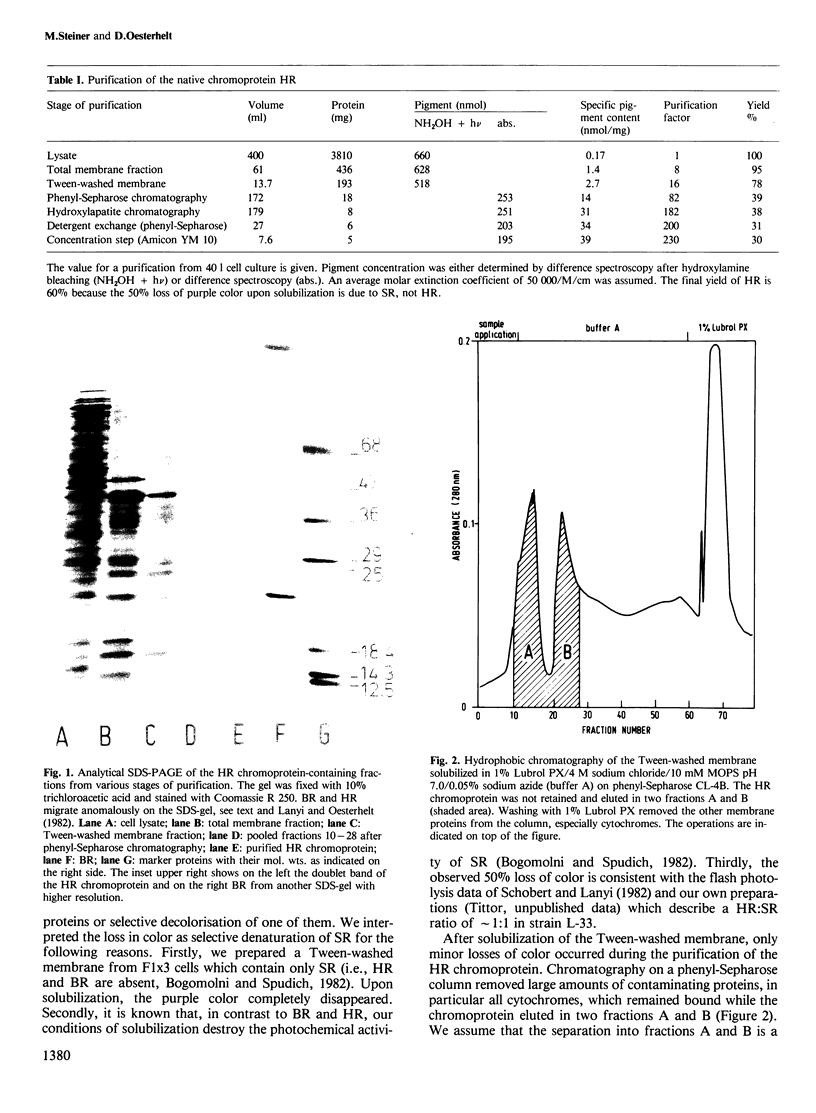
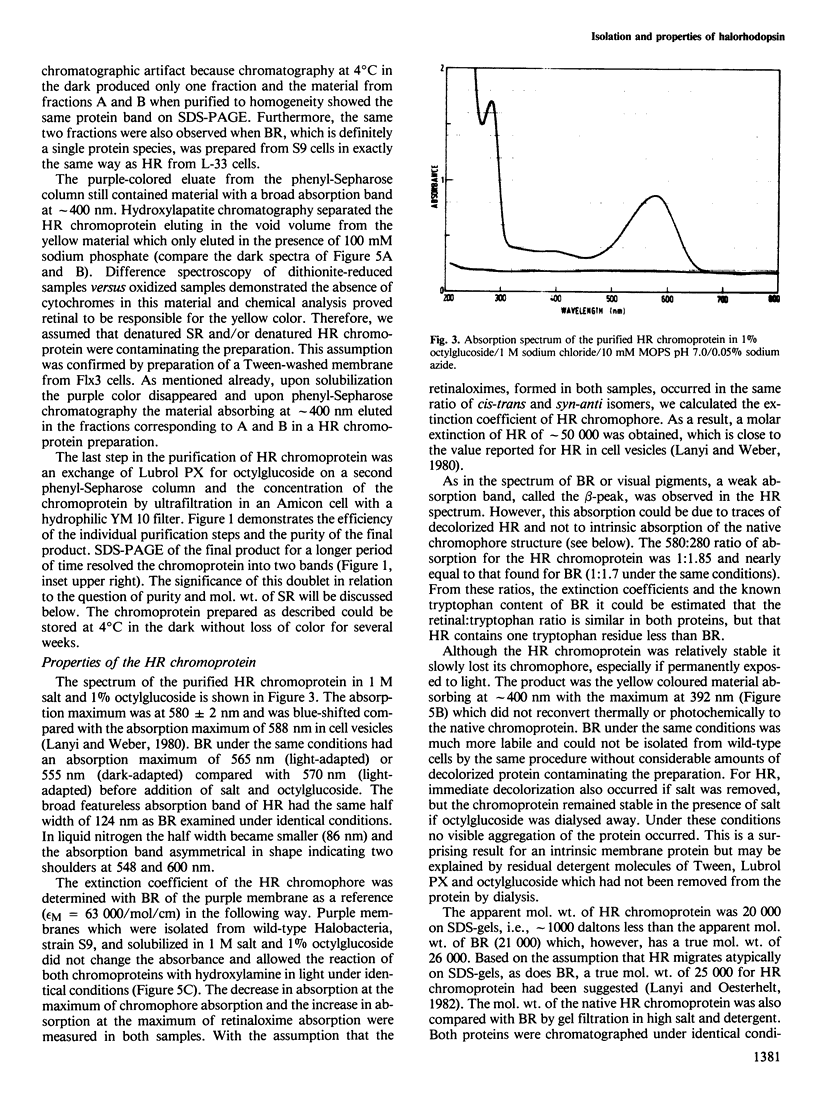
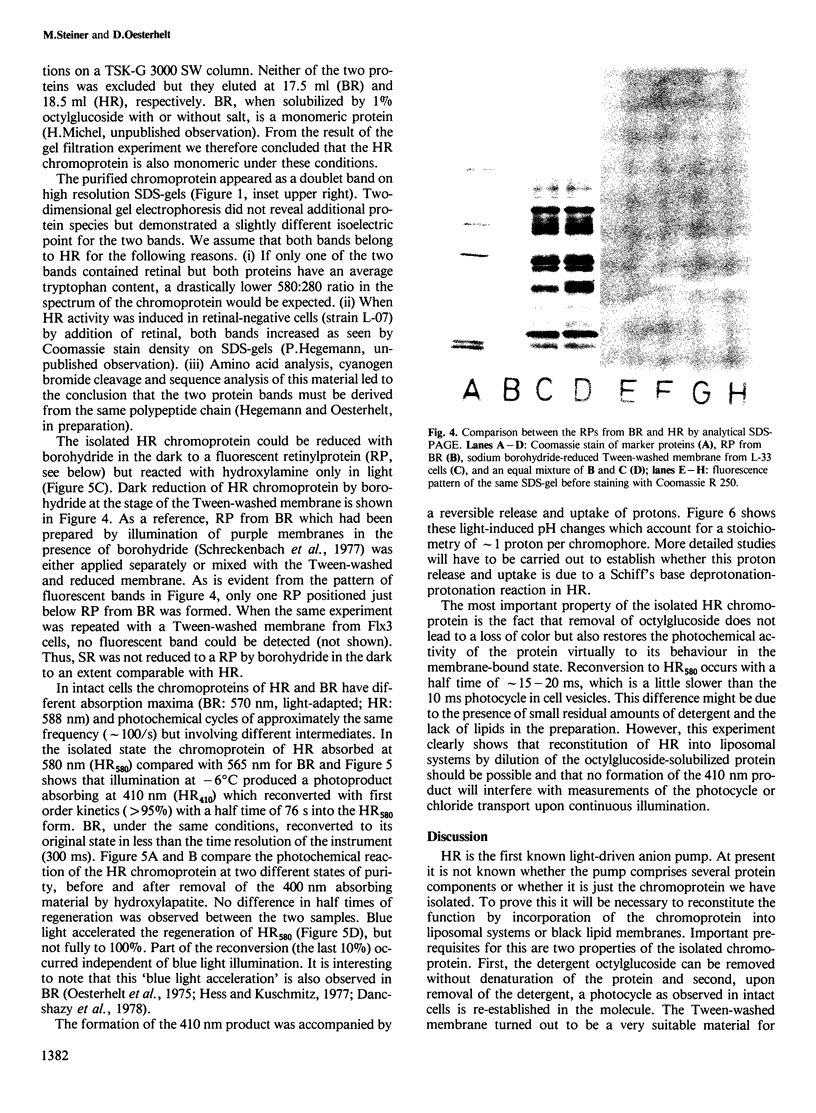
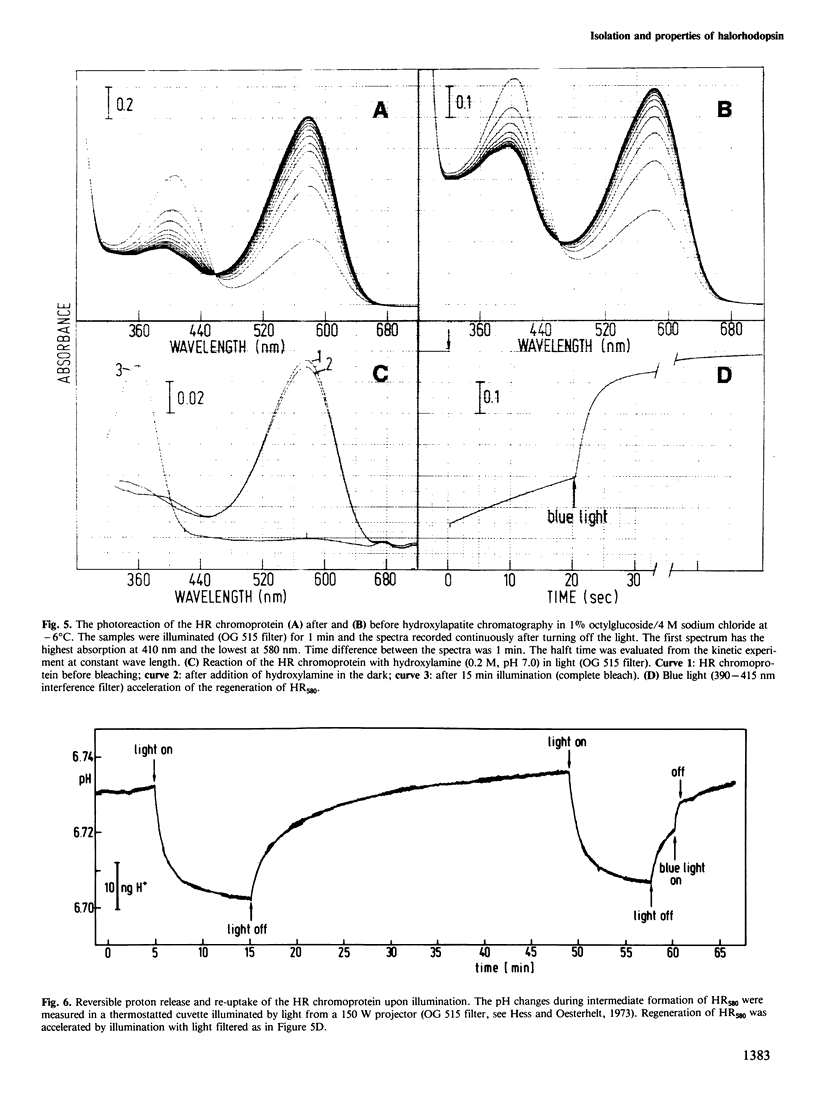
The native chromoprotein of the light-driven chloride pump halorhodopsin (HR) was isolated from Halobacterium halobium strain L-33 which lacks bacteriorhodopsin but contains 'slow cycling rhodopsin-like pigment' (SR). A membrane fraction was prepared in low salt and dissolved in a high salt medium by the detergents Lubrol PX or octylglucoside. These conditions destroyed the chromophore of SR but not the HR pigment. Chromatography on phenyl-Sepharose and hydroxylapatite produced, in 60% yield, a 230-fold enriched monomeric chromoprotein with an apparent mol. wt. of 20,000. The chromoprotein was stable in 1 M NaCl and 1% octylglucoside and remained stable upon removal of detergent. It reacted with borohydride in the dark and with hydroxylamine in the light. The absorption maximum of the light-adapted state is at 580 + 2 nm and its molar extinction approximately 50,000/M/cm. Upon illumination in the presence of detergent it was converted into a 410 nm absorbing species with concomitant release of protons. A thermal reconversion to the 580 nm species occurred with a half time of 76 s at -6 degrees C. Blue light absorbed by the photoproduct accelerated the re-conversion as well as the re-uptake of protons. Removal of the detergent prevented the light-induced formation of the 410 nm species. Under these conditions a photochemical behaviour similar to that in intact cells and cell vesicles, i.e., a photocycle in the 10-20 ms range was observed. These findings form the basis for functional reconstitution of HR.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancsházy Z., Drachev L. A., Ormos P., Nagy K., Skulachev V. P. Kinetics of the blue light-induced inhibition of photoelectric activity of bacteriorhodopsin. FEBS Lett. 1978 Dec 1;96(1):59–63. doi: 10.1016/0014-5793(78)81062-x. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Engström P. Multiple forms of methyl-accepting chemotaxis proteins distinguished by a factor in addition to multiple methylation. J Bacteriol. 1981 Jan;145(1):35–42. doi: 10.1128/jb.145.1.35-42.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann P., Steiner M., Oesterhelt D. Isolation and characterization of the retinal-binding component of halorhodopsin. EMBO J. 1982;1(10):1177–1183. doi: 10.1002/j.1460-2075.1982.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. The photochemical reaction of the 412 nm chromophore of bacteriorhodopsin. FEBS Lett. 1977 Feb 15;74(1):29–24. doi: 10.1016/0014-5793(77)80743-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Oesterhelt D. Identification of the retinal-binding protein in halorhodopsin. J Biol Chem. 1982 Mar 10;257(5):2674–2677. [PubMed] [Google Scholar]
- Lanyi J. K., Weber H. J. Spectrophotometric identification of the pigment associated with light-driven primary sodium translocation in Halobacterium halobium. J Biol Chem. 1980 Jan 10;255(1):243–250. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. V. Analysis of the proteins synthesized in ultraviolet light-irradiated Escherichia coli following infection with the bacteriophages lambdadrifd 18 and lambdadfus-3. Mol Gen Genet. 1976 Mar 30;144(3):339–343. doi: 10.1007/BF00341733. [DOI] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982 Sep 10;257(17):10306–10313. [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Bogomolni R. A., Spudich J. L. Genetic and biochemical resolution of the chromophoric polypeptide of halorhodopsin. Biochem Biophys Res Commun. 1983 Apr 15;112(1):332–338. doi: 10.1016/0006-291x(83)91835-1. [DOI] [PubMed] [Google Scholar]
- Wieland F., Dompert W., Bernhardt G., Sumper M. Halobacterial glycoprotein saccharides contain covalently linked sulphate. FEBS Lett. 1980 Oct 20;120(1):110–114. doi: 10.1016/0014-5793(80)81058-1. [DOI] [PubMed] [Google Scholar]