Abstract
Blue light irradiation (BLI) is an FDA‐approved method for treating certain types of infections, like acne, and is becoming increasingly attractive as an antimicrobial strategy as the prevalence of antibiotic‐resistant “superbugs” rises. However, no study has delineated the effectiveness of BLI throughout different bacterial growth phases, especially in more BLI‐tolerant organisms such as Escherichia coli. While the vast majority of E. coli strains are nonpathogenic, several E. coli pathotypes exist that cause infection within and outside the gastrointestinal tract. Here, we compared the response of E. coli strains from five phylogenetic groups to BLI with a 455 nm wavelength (BLI 455), using colony‐forming unit and ATP measurement assays. Our results revealed that BLI 455 is not bactericidal, but can retard E. coli growth in a manner that is dependent on culture age and strain background. This observation is critical, given that bacteria on and within mammalian hosts are found in different phases of growth.
Keywords: E. coli, membranes, pathogenesis, persisters
1. Introduction
The steady increase in antibiotic resistance rates among bacteria has sparked a major research effort to identify new antibacterial and antivirulence therapies (Bebell & Muiru, 2014; Brooks & Brooks, 2014; Roca et al., 2015; Thabit, Crandon, & Nicolau, 2014; Uchil, Kohli, Katekhaye, & Swami, 2014). Phototherapy is among these alternative approaches and is used routinely to treat skin infections, like acne (Dai et al., 2012; Dong et al., 2016; Liu et al., 2016; Maisch et al., 2014; Opel et al., 2015; Pei, Inamadar, Adya, & Tsoukas, 2015; Piccolo et al., 2014). Phototherapy employs visible light in the 400–700 nm wavelength range to activate either exogenous (as in photodynamic therapy‐PDT) or endogenous photosensitizers (PSs) (Lambrechts, Demidova, Aalders, Hasan, & Hamblin, 2005; Maclean, Macgregor, Anderson, & Woolsey, 2008, 2009; Nussbaum, Lilge, & Mazzulli, 2002; Orenstein et al., 1997). Endogenous bacterial PSs, such as porphyrins and flavins, absorb light in the 400–500 nm blue light wavelength range and are suggested to respond to blue light irradiation (BLI) (Cieplik et al., 2014; Dai et al., 2012; Lubart, Lipovski, Nitzan, & Friedmann, 2011).
Previous reports suggested that irradiation with visible light without the use of exogenous PSs kills species like Acinetobacter baumannii, Aggregatibacter actinomycetemcomitans, Enterococcus faecalis, Escherichia coli, Porphyromonas gingivalis, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pyogenes by as much as 90% after exposure (Bumah, Masson‐Meyers, Cashin, & Enwemeka, 2013; Buonanno et al., 2013; Cieplik et al., 2014; Donnelly et al., 2009; Enwemeka, Williams, Enwemeka, Hollosi, & Yens, 2009; Gad, Zahra, Francis, Hasan, & Hamblin, 2004; Guffey & Wilborn, 2006a,b; Hamblin et al., 2005; Lipovsky, Nitzan, Gedanken, & Lubart, 2010; Lubart et al., 2011; Maisch et al., 2014; Nitzan, Malik, Kauffman, & Ehrenberg, 1997; Perni et al., 2009; Soukos et al., 2005; Zolfaghari et al., 2009). These studies included wavelengths ranging from 207 to 880 nm and energy doses ranging from 1 to 420 J/cm2. A lower wavelength corresponds to a higher energy per particle of light (photon). The energy dose is defined by the total energy delivered per irradiated area. At a constant energy dose, the number of photons increases as the wavelength increases. A. actinomycetemcomitans, E. coli, P. gingivalis, Porphyromonas intermedia, Porphyromonas nigrescens, Prevotella melaninogenica, S. aureus, and Streptococcus constellatus were reported to be killed using light at 455 nm (+/‐ 5 nm) without the use of exogenous PSs and 4–150 J/cm2 (Cieplik et al., 2014; Lipovsky et al., 2010; Soukos et al., 2005). However, in all studies, bacterial viability was measured only as a function of colony‐forming units (CFUs), therefore, not accounting for the potential formation of viable but nonculturable subpopulations.
Among the potential endogenous PSs harbored by bacteria, are porphyrin‐containing cytochromes, ubiquinones, and flavin‐containing enzymes, such as flavin adenine nucleotide (FAD). Cytochromes and flavin‐containing enzymes are integral components of the bacterial electron transport chain (ETC). For instance, the reduced form of FAD, FADH2, is utilized as an electron donor through an oxidative reaction resulting in FAD (Fig. S1a and (Cook, Greening, Hards, & Berney, 2014; Richter & Ludwig, 2009; Sazanov, 2014)). Cytochromes and ubiquinones, serve as both electron acceptors and mobile electron carriers in the ETC (Fig. S1a and (Aussel et al., 2014; Cook et al., 2014; McNeil & Fineran, 2013; Richter & Ludwig, 2009; Sazanov, 2014)). The aromatic rings within electron donors and carriers absorb photons from light, which makes them ideal endogenous PSs (Herzfeld, 1940). Flavins have absorption peaks at 380 and 450 nm, porphyrins around 400 nm, while ubiquinones have various peaks in the 230–500 nm range (Chandra et al., 2000; Land, Simic, & Swallow, 1971; Yagi, Ozawa, & Harada, 1959; Zenichowski, Gothe, & Saalfrank, 2007).
Previous studies indicated that light promotes PSs to an excited state, after which electrons (or energy) are transferred to molecules such as molecular oxygen, forming reactive oxygen species (ROS) (Fig. S1b and (Feuerstein, Ginsburg, Dayan, Veler, & Weiss, 2005; Liang et al., 2013; Lubart et al., 2011; Malik, Hanania, & Nitzan, 1990; Marugán, van Grieken, Pablos, & Sordo, 2010)). Increased ROS levels can cause damage to cellular proteins, lipids, and DNA (Farr & Kogoma, 1991; Feuerstein, Moreinos, & Steinberg, 2006; Liang et al., 2013; Lipovsky, Nitzan, Friedmann, & Lubart, 2009; Lipovsky et al., 2010).
To date, most studies focused on the antimicrobial effects of BLI were performed using bacteria in the exponential growth phase (Cieplik et al., 2014; Feuerstein et al., 2005; Guffey & Wilborn, 2006a,b; Lambrechts et al., 2005; Lipovsky et al., 2009, 2010; Nitzan, Gutterman, Malik, & Ehrenberg, 1992; Orenstein et al., 1997; Yang, Inokuchi, & Adler, 1995; Yin et al., 2015). Many elegant studies have now demonstrated that in the majority of infections, the inoculating bacteria are at extremely low concentrations (Dupont, Levine, & Hornick, 1989; Gonzalez, Lane, Wagner, & Weening, 2015; Griffin & Tauxe, 1991; Handley, Dube, & Revell, 2004; Kotloff, Losonsky, Nataro, & Wasserman, 1995; Lorange, Race, & Sebbane, 2005; Tacket, Binion, & Bostwick, 1992) and that bacteria are not always exponentially growing (Helaine & Holden, 2013). In many respects, stationary phase bacteria differ from their exponential phase counterparts (Anderl, Zahller, Roe, & Stewart, 2003; Cabeen, 2014; Kolter, Siegele, & Tormo, 1993; Mangan et al., 2006; Mouslim & Hughes, 2014; Navarro Llorens, Tormo, & Martínez‐García, 2010; Roop et al., 2003; Wang et al., 2014). For instance, E. coli, P. aeruginosa, and S. aureus cultures have a low, stable subpopulation of “persister” cells during lag and early‐exponential phases, and this subpopulation increases during mid‐exponential and stationary phases (Keren, Kaldalu, Spoering, Wang, & Lewis, 2004). Persister cells are phenotypically distinct from their genetically identical, active sister cells (Bigger, 1944; Keren, Shah, Spoering, Kaldalu, & Lewis, 2004; Keren, Kaldalu, et al., 2004) and their dormant state allows them to tolerate the presence of antibiotics (Bigger, 1944; Keren, Kaldalu, et al., 2004; Keren, Shah, et al., 2004). In addition to persister cells, there is an increasing body of literature demonstrating extensive population heterogeneity in bacterial communities during infection, which also changes as the culture ages (Balaban, Gerdes, Lewis, & McKinney, 2013; Bush et al., 2011; Darby, Venugopal, Ehrt, & Nathan, 2011; Floyd et al., 2015; Hisert et al., 2004; Lechner, Lewis, & Bertram, 2012; Moy et al., 2006; Smith, Nathan, & Peavy, 2005; Tian, Bryk, Itoh, Suematsu, & Nathan, 2005). Considering the dynamic flux in bacterial subpopulations during growth, successful translation of BLI into potential antibacterial applications requires an assessment of the bacterial response to BLI during different growth phases.
In this study, we evaluated the effects of BLI at a 455 nm wavelength (BLI455) without the use of exogenous PSs on the growth and viability of different E. coli strains from different phylogenetic groups. We performed our analyses during exponential, transition, and early stationary phases of growth to encompass responses to BLI that differ based on bacterial growth phase. We selected BLI455 for investigation, as this wavelength is within the range of FDA‐approved phototherapy devices, including “bili‐blankets” used to treat jaundiced neonates (Morris et al., 2013; Sherbiny, Youssef, Sherbini, El‐Behedy, & Sherief, 2015). Our results indicated that although BLI455 reduced the number of growing E. coli cells to varying degrees in a strain‐ and growth phase‐dependent manner, BLI did not significantly impact the viability of any strain. Rather, BLI treatment induced a viable but nonculturable state, which may influence chronic infection states in the hospital setting.
2. Experimental Procedures
2.1. Strains and constructs
The following E. coli strains were used: phylogenetic group A strains DH5α [laboratory‐adapted (Laboratories, 1986)] and MG1655 [K‐12 (Bachmann, 1996)]; group B1 strains E343 and E402 [nonpathogenic isolates (Rúgeles, Bai, Martínez, Vanegas, & Gómez‐Duarte, 2010)]; group B2 UPEC strains UTI89 (Mulvey et al., 1998) and EC958 (Totsika et al., 2011); group D enterotoxigenic (ETEC) strain E9034A (Levine et al., 1984); and group E enterohemorrhagic (EHEC) O157:H7 strain Sakai (Hayashi et al., 2001).
2.2. Culture conditions
Bacterial cultures were seeded in LB and incubated at 37°C while shaking overnight (Bertani, 1951). Aliquots of overnight cultures were normalized to an optical density at 600 nm (OD600) of 0.05 into LB subculture in 125 ml flasks. Subcultures were incubated at 37°C and shaken at 200 RPM for all analyses described.
2.3. Growth curves
Bacteria were inoculated as described in the section above. A 120‐μl aliquot was removed from each culture hourly. Of each aliquot, 100 μl were diluted 1:10 in fresh LB and the OD600 was recorded using a Thermo Scientific NanoDrop 2000 spectrophotometer. The remaining 20 μl were then serially diluted in LB for enumeration of colony‐forming units (CFUs). A multichannel pipette was used to spot eight different dilutions with five technical replicates per dilution. Incubation occurred overnight at room temperature (RT). Growth curve experiments were repeated at least three independent times, with a minimum of five technical replicates per biological repeat. Generation times were calculated using Equation (1), where G is generation time, t is the time interval, b is the CFU of bacteria at the end of the time interval, and B is the CFU of bacteria at the beginning of the time interval.
| (1) |
2.4. In vitro light delivery to E. coli
Cultures were set up as described above. Aliquots were obtained for irradiation and plating during exponential (t = 3 hr), transition (t = 5 or 6 hr depending on the strain), and stationary (t = 8 hr) growth phases. Aliquots were serially diluted as described above and 10 μl from one serial dilution (~101 to 102 cells) was spotted on solid LB agar and exposed to BLI455. BLI455 was carried out with a Thorlabs Mounted High‐Power 455 nm LED lamp and controlled by a high‐powered LED driver (Thorlabs DC2100). The light source was placed 10 mm ± 1 mm above the 10‐μl spots, to achieve a power flux output of ~520 mW/cm2. A total energy dose of 120 J/cm2 was delivered to each sample. Irradiated and nonirradiated controls were then incubated overnight at ambient temperature. CFUs were counted the following day. Experiments were performed with at least three biological replicates of three technical replicates.
2.5. Survival fraction determination
To determine the percent change in exposed versus unexposed CFUs, the CFUs postirradiation were enumerated and compared to the CFUs of corresponding, nonirradiated spots. The reductions were calculated using Equation (2).
| (2) |
2.6. Viability assay
Cell viability was determined using the CellTiter‐Glo Luminescent Cell Viability Assay kit (Promega). Two assays were performed: one to determine the overall differences in ATP of unexposed and exposed samples and the second to determine if differences in ATP amounts of unexposed and exposed samples were due to cell death (cell lysis) or inhibition of replication. For both assays, 50 μl of liquid culture was placed on a glass cover slip directly under the light source. The height between the light source and the sample was adjusted to deliver 120 J/cm2 for the increased irradiated area because of the increased volume and spread on the glass slide, compared to the spread on agar plates. After BLI455, the sample was transferred to a 96‐well plate and allowed to incubate for 30 min, allowing for at least one replication cycle to occur. After incubation in the first assay, triplicate samples of unexposed and exposed aliquots were diluted 10‐fold in LB and 50 μl of each diluted sample was transferred to a black, 96‐well plate (Costar). In the second assay, 200 μl of unexposed and exposed samples (previously diluted 10‐fold after the 30 min incubation) were collected in separate 1.5 ml plastic tubes for each strain. The plastic tubes were placed in a centrifuge for 2 min at 16,100g to pellet cells. After centrifugation, the supernatant was transferred to a new 1.5 ml plastic tube. The pellets were then resuspended in 200 μl of LB. Triplicate samples (50 μl each) of supernatant or resuspended pellet were added to individual wells in a black well, 96‐well plate (Costar) and quantified as follows. For both assays, an equal volume of CellTiter‐Glo substrate/buffer mix was added to each well and mixed thoroughly. After addition of the CellTiter‐Glo, the plates were allowed to shake orbitally for 2 min to stabilize the signal and then luminescence values of ATP were measured using a SpectraMax i3 (Molecular Devices). Luminescence was also determined for wells filled only with LB to subtract background luminescence due to the media. Experiments were performed with at least three biological replicates of three technical replicates.
2.7. Biofilm assay
Strains were grown exponentially in 3 ml LB and normalized to an OD600 of 1. Cultures were then diluted 200‐fold in fresh LB and used to seed biofilm plates. Biofilm assays in LB at RT were performed in 96‐well PVC plates as previously described (Pinkner et al., 2006) and quantitatively measured 24 hr post seeding, using crystal violet (O'Toole et al., 1999).
The effect of BLI on preformed colony biofilms was also evaluated. Overnight cultures were normalized to a starting OD600 of 0.05. Agar plates were spotted with 10 μl of bacterial inoculum. Plates were left to grow in the dark at RT for 3 days. After 3 days of growth, half of the plates were irradiated with BLI455 at 120 J/cm2. Plates were then placed in the dark at RT for two additional days. On day 5 post seeding, the diameters of unexposed and exposed biofilms were measured. This assay was repeated with three biological replicates of each strain.
2.8. Persister assay
Persister assays were performed using ofloxacin, as described in (Allison, Brynildsen, & Collins, 2011). For the control experiments, stationary phase cultures of WT UTI89 and a mutant with known reduced proton motive force production, UTI89ΔvisC (Conlon et al., 2016; Floyd et al., 2016) were subjected to 5 μg/ml ofloxacin for 4 hr. Samples were withdrawn for CFU enumeration prior to ofloxacin exposure. Susceptibility assays were repeated three times. The average percent survival from the three independent experiments is reported. For analysis of the BLI‐treated bacteria, the method for “In vitro light delivery to E. coli” was followed prior to the start of this assay. One colony was chosen from all technical and biological replicates of unexposed and exposed plates. Glass test tubes with 2 ml of LB were inoculated with one colony each from both the unexposed and exposed plates. Samples were placed into a shaking incubator at 37°C for 3 hr. After the 3‐hr incubation, two 750‐μl samples were aliquoted from glass test tubes and were placed in plastic 1.5 ml tubes. One of the 750‐μl aliquots was treated with ofloxacin (5 μg/ml). Plastic tubes were placed back into the shaking incubator for an additional 2‐hr incubation. After the second incubation, samples were centrifuged at 18,000g RPM for 2 min and washed in LB to reduce the carry‐over effects of ofloxacin. Aliquots of samples were serially diluted and plated for CFU enumeration. The average percent survival is reported.
3. Results
3.1. Strain‐ and growth phase‐specific responses to BLI455 for E. coli
Much of the previous work evaluating the efficacy of BLI in growth reduction of E. coli and other gram‐negative bacteria investigated only one strain from each bacterial species (Alves et al., 2015; Hanakova et al., 2014; Liang et al., 2013; Nussbaum et al., 2002; Popov et al., 2005; Tschowri, Busse, & Hengge, 2009; Tschowri, Lindenberg, & Hengge, 2012). While analyzing a single, model strain provides an initial basis for comparison, understanding how different strains within a species respond to BLI is essential to develop successful therapeutic approaches against them, especially given the strain heterogeneity within species like E. coli (Croxen & Finlay, 2010). E. coli strains belong to different phylogenetic groups, of which B2 and D harbor most pathogenic strains, while B1 mostly comprises nonpathogenic strains. We selected a range of strains from A, B1, B2, D, and E phylogenetic groups for our analyses. The different strains were first evaluated for growth rate differences to pinpoint exponential, transition, and stationary phase times for each one (Figure 1). These analyses revealed differences in the growth rates of these strains, with the nonpathogenic strain MG1655, a K‐12 derivative, exhibiting a generation time of ~18 min during exponential phase (Figure 1c). The uropathogenic E. coli (UPEC) isolates UTI89 and EC958, had nearly identical generation times (~22.6–22.8 min); while, the gastrointestinal E. coli strains exhibited slower generation times (Figure 1c).
Figure 1.
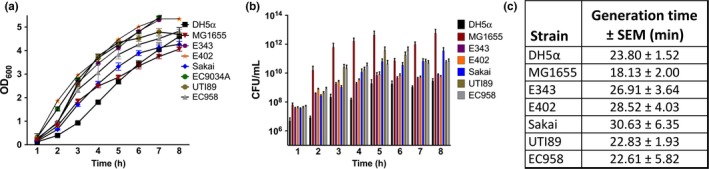
Growth rate heterogeneity exists between E. coli strains. Growth curves of E. coli strains ranging from nonpathogenic laboratory‐engineered strain DH5α; commensal strains MG1655, E343, and E402; enterohemorrhagic (EHEC) strain Sakai; and uropathogenic E. coli (UPEC) strains UTI89 (cystitis isolate) and EC958 (multi‐drug‐resistant isolate). Measuring the optical density alone can sometimes be misleading, depending on the surface factors of a particular strain; therefore, the growth curve is presented as (a) Optical density at 600 nm (OD 600) versus time and (b) colony‐forming units per milliliter (CFU/ml) versus time for the seven strains. (c) To account for the varying growth rates, the generation time (plus the standard error mean‐SEM) was measured between hours 2 and 4 for each strain. MG1655 has the fastest growth rate (lowest generation time); however, looking at OD600 alone, MG1655 appears to grow slower than every other strain tested. Growth curve experiments were repeated at least three times independently. Error bars represent the standard error mean
We then tested each strain for susceptibility to BLI455 at their corresponding exponential, transition, and stationary growth phases, using the workflow depicted in Fig. S2. Figure 2 depicts the obtained results as a function of exposure (Figure 2a) and growth phase (Figure 2b). Of all strains tested, only DH5α displayed ~1.5–2.5 logs of decrease in CFUs following BLI, and this reduction was conserved during all phases of growth (Figure 2). During stationary and transition phase, MG1655 exhibited statistically insignificant changes as a result of BLI455; though, nearly a 1‐log decrease in the amount of CFUs from the unexposed to the exposed samples was observed for this strain during exponential phase. The commensal E343 and E402 and multi‐drug‐resistant UPEC strain EC958 exhibited modest, though statistically insignificant, susceptibility to the effects of BLI455 during all growth phases (Figure 2). The enterotoxigenic (ETEC) E9034A was more susceptible to BLI455 in exponential phase compared to transition and stationary phases where CFUs became minimally reduced (Figure 2). UPEC strains UTI89 and EC958, and EHEC strain Sakai were most susceptible in stationary phase (Figure 2). These data indicate that there are strain‐dependent responses to BLI455.
Figure 2.
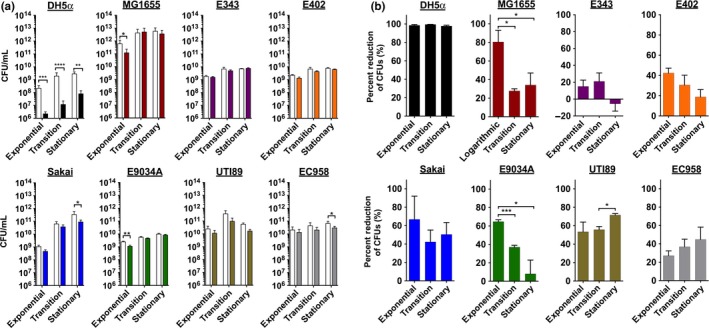
CFU reduction of different E. coli strains in response to blue light irradiation (BLI)455 at different growth phases. (a) Comparison of CFUs for unexposed (solid colored bars) and exposed (white bars) samples during exponential, transition, and stationary growth phases. All experiments were repeated a minimum of three times and analyzed via a Student t test. *, p < .05, **, p < .01; ***, p < .001; ****, p < .0001. (b) Comparison of percent reduction of CFUs with analyses of the changes over growth phases. The following strains are represented: phylogenetic group A strains DH5α (laboratory‐adapted) and MG1655 (K‐12); group B1 strains E343 and E402 (nonpathogenic isolates); group B2 UPEC strains UTI89 and EC958 (multi‐drug resistant); group D ETEC strain E9034A; and group E EHEC O157:H7 strain Sakai. Data represent the mean of three or more independent experiments. All experiments were repeated a minimum of three times and analyzed via One‐way anova. *, p < .05; ***, p < .001
3.2. BLI455 and 120 J/cm2 is not bactericidal against E. coli
The minimal reduction in CFUs observed in our analyses could be the result of bacterial cell lysis and/or altered bacterial growth, such as the formation of persister cells. Following a modified method of Allison et al., we assessed the effects of BLI on the formation of persister cells. We first analyzed the amount of persister cells formed by UPEC strain UTI89 (Fig. S3A). In contrast to numbers obtained for nonpathogenic E. coli (Allison et al., 2011), UTI89 forms close to 4% persister cells upon a 4 hr exposure to ofloxacin. However, this survival was minimally affected upon BLI treatment (Fig. S3B), indicating that under the conditions tested, the reduction in CFUs observed is not due to the formation of persister cells.
We next determined whether BLI treatment leads to another form of growth arrest, or whether it leads to bacterial cell lysis. In the case of bacterial cell lysis, membranes become compromised, resulting in release of ATP to the extracellular milieu. On the other hand, altered bacterial growth could result from a perturbation in proton flux across the inner membrane, which would lead to an overall reduction in ATP production via the ETC. We used an ATP quantitation assay to determine how BLI455 impacts total, intracellular and extracellular ATP levels (Fig. S4 and S5). To enable accurate ATP measurements, the sample volume irradiated for the ATP assays was 50 μl, compared to 10 μl used to quantify the ability of BLI455 to reduce bacterial growth. The percent reduction in CFUs in response to BLI455 was determined for both volumes, using a representative set of strains (Fig. S6).
First, the total ATP levels in bacterial samples were measured (Figure 3a). DH5α, which had the greatest overall reduction in CFU in response to BLI455 (Figure 2), exhibited no changes in the overall ATP levels during exponential and transition phase, but had approximately a 25% reduction in ATP levels at the stationary growth phase (Figure 3a). For MG1655, reduced ATP levels were observed in the exposed samples during the exponential growth phase and corresponded to the reduction in CFUs (Figure 2). The Sakai strain exhibited similar ATP levels between BLI and nonirradiated bacteria during exponential and transition phase and a modest reduction in ATP at stationary phase (Figure 3a). The ATP levels for strains UTI89 and EC958 were statistically insignificant from BLI455 (Figure 3a). These results suggested that BLI455 might induce altered ATP production or release in strains like DH5α, MG1655, and Sakai.
Figure 3.
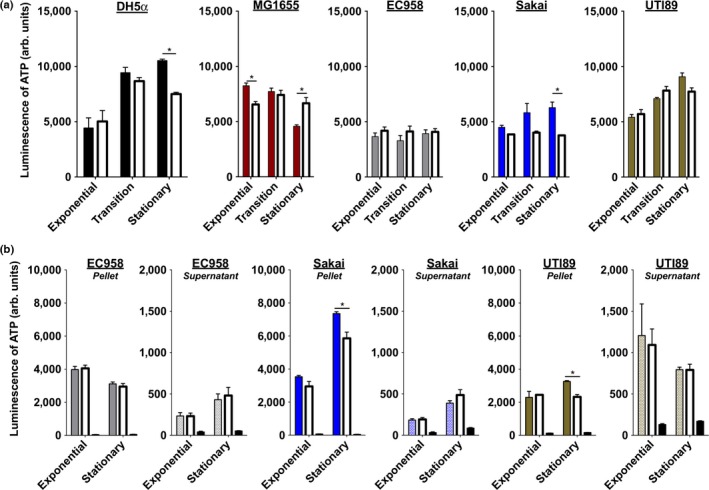
Blue light irradiation (BLI)455 is not completely bactericidal against E. coli. Viability assays, using an ATP release assay to measure ATP levels of unexposed and BLI 455‐treated cells. ATP levels were measured using Promega's CellTiter‐Glo Kit to lyse cells and quantify ATP via luminescence. (a) The differences in relative ATP levels of unexposed (solid colored bars) and exposed (white bars) samples. (b) The differences in relative ATP in the pellet (intact) and supernatant (lysed) of unexposed (colored bars) and exposed (white bars) samples. The third bars are the controls, in which no bacteria was added, just the detection reagent and LB media (black bars). To determine if the differences in ATP levels were due to cell death or inhibition of replication. ATP in the supernatant and ATP in the pellet for both unexposed and exposed samples were measured. EC958, Sakai, and UTI89 were chosen as three representative strains to evaluate whether BLI is bacteriostatic or bactericidal because EC958 ATP levels were constant among the unexposed and exposed samples; Sakai and UTI89 had differences in levels of unexposed and exposed samples at each growth phase. Experiments were repeated three times. Error bars represent the standard error mean. Statistical analysis was performed using an unpaired, two‐tailed Student's t test. *, p < .05
We next measured ATP released into the supernatant fraction (indicative of cell lysis), as well as ATP levels in cellular pellets for three representative strains: EC958, Sakai, and UTI89 (workflow is depicted in Fig. S4b). The ATP in the supernatant and cellular fractions was measured for both unexposed and exposed samples. EC958 was the least susceptible to BLI455 (Figure 2). Consistent with this observation and the insignificant changes in total ATP levels in response to irradiation (Figure 3a), we saw no significant changes in extracellular and intracellular levels of ATP between exposed and unexposed samples (Figure 3b). These observations validated that, for this particular E. coli strain, BLI455 is not effective at eliminating growth. For Sakai, there was no significant change in ATP levels during exponential phase between the unexposed and exposed supernatant fractions (Figure 3b), suggestive of no significant compromise to cellular membranes. Similarly, there were no significant changes in the ATP levels between the cellular fractions from exposed and unexposed cells from the exponential growth phase. This was in agreement with the modest reduction in CFUs observed during exposure in the exponential growth phase for strain Sakai (Figure 2). However, a significant reduction in intracellular ATP was observed for exposed fractions from the stationary growth phase, which was accompanied by a modest (but statistically insignificant) increase in the ATP levels in the corresponding supernatant fraction (Figure 3b). These data suggest that in the case of Sakai, BLI455 may exert some bactericidal effect (based on the modest increase in extracellular ATP), as well as bacteriostatic effects (based on the greater reduction in intracellular ATP that is not equivalent to the increase in extracellular ATP; note change in scale on the y‐axes between pellet and extracellular measurements). While strain UTI89 had greater reduction in CFUs compared to EC958 and Sakai in all growth phases (Figure 2), the differences in ATP of both intracellular and extracellular ATP mimicked the trends of Sakai (Figure 3b). There were no significant differences in ATP levels during exponential phase between the unexposed and exposed supernatant fractions (Figure 3b). As in Sakai, this suggests the cellular membranes are not significantly compromised. Similarly, in the exponential phase for the intracellular fraction, there were no significant changes in the ATP levels from exposed and unexposed cells (Figure 3b). Conversely, during the stationary phase, the ATP levels of the exposed cells were significantly lower than the levels in unexposed cells (Figure 3b). Combined with insignificant differences in the amount of lysed ATP in the supernatant, these findings suggest that BLI455 is bacteriostatic for UTI89. All together, the observed reductions in CFUs, with noncorresponding reductions in ATP levels suggest that BLI455 is either inhibiting replication of cells or drastically slowing the rate of replication. Slightly higher, but statistically insignificant, levels of ATP were observed in the supernatant of all tested strains in the stationary phase of growth (Figure 3b and S5). It is possible that some cell death is occurring, but the overall data suggest that the main mechanism of action is bacteriostatic and that the lower CFUs in exposed samples, as compared to unexposed samples, were not solely a result of cell death.
4. Discussion
In this study, we evaluated BLI455‐induced growth reduction and bactericidal activity during different growth stages of nonpathogenic, pathogenic, and multi‐drug‐resistant (extended spectrum beta‐lactamase producing) E. coli strains to determine intraspecies variation to a potential antimicrobial approach. We report that blue light‐mediated growth reduction of E. coli is minimal and also varies significantly as a function of strain background. Additionally, the bactericidal efficacy of BLI on all strains tested here demonstrated that BLI treatment at the wavelength and energy dose tested is mainly bacteriostatic. The same findings were observed with testing of BLI on preformed biofilms. The ability to inhibit outgrowth of preformed colony biofilms was also assessed by treating biofilms with BLI455 after 3 days of growth and measuring the biofilm diameters 48 hr after treatment. There were no differences in the size of the biofilms in unexposed and exposed samples of preformed biofilms with BLI455 with the same energy dose (120 J/cm2) used for treatment of planktonic cells (Fig. S7).
These findings suggest that BLI may be inducing a transient viable but nonculturable phenotype in a portion of the treated E. coli population and this effect leads to an apparent reduction in CFUs, but does not correspond to cell death. While BLI455 is used in numerous applications, including the treatment of jaundice in neonates, very few studies characterized the behavior of irradiated bacteria in a strain‐ and growth phase‐dependent manner. Numerous studies elegantly showed that there is extensive heterogeneity in bacterial populations, which changes as a function of external stimuli and growth phase (Anderl et al., 2003; Cabeen, 2014; Kolter et al., 1993; Mangan et al., 2006; Mouslim & Hughes, 2014; Navarro Llorens et al., 2010; Roop et al., 2003; Wang et al., 2014). In addition, bacteria that are commonly associated with humans and other vertebrate hosts are not typically growing exponentially, but are rather found in various growth phases, including extended stationary phase. Examining the bacterial responses in more physiologically relevant situations is essential, if we are to understand how to prevent or combat infections. The studies described in this work demonstrate that BLI treatment does not significantly impair E. coli viability and suggest that the use of BLI as an antimicrobial strategy may retard the growth of some strains, possibly including commensals, while conferring an advantage to pathogenic/opportunistic bacteria or bacteria found at a different phase of growth. For example, our findings demonstrated that UPEC strain EC958, a multi‐drug ST131 lineage E. coli (Totsika et al., 2011) and ETEC strain E9034A (Levine et al., 1984) were the least susceptible to the effects of BLI455; however, nonpathogenic E343 (Rúgeles et al., 2010) was also significantly resistant to the effects of BLI455. These findings have wide‐ranging ramifications in the way phototherapy may be used to treat or prevent infections.
The conventional model describing the BLI susceptibility mechanism is through damage from the generation of ROS, most notably singlet oxygen (Farr & Kogoma, 1991; Feuerstein et al., 2005, 2006; Liang et al., 2013; Lipovsky et al., 2009, 2010; Lubart et al., 2011; Malik et al., 1990; Marugán et al., 2010). Studies also indicate that low‐level stimulation of ROS can enhance proliferation of bacterial growth with the resulting daughter cells sometimes exhibiting altered replication rates (Dai et al., 2012; Lipovsky et al., 2009, 2010; Lubart, Lavi, Friedmann, & Rochkind, 2006; Lubart et al., 2011; Nussbaum et al., 2002). ROS generation could be a contributing factor in the reduced growth observed in response to BLI. Furthermore, lowering of the pH has been shown to protonate the cell membrane and “revive” bacteriostatic cells, suggesting that in a biological application and in the optimal environment, BLI‐treated bacteria may revert to a viable, replicating state (Darby et al., 2011; Hisert et al., 2004; Smith et al., 2005; Tian et al., 2005). Therefore, BLI455 treatment of E. coli (and possibly other gram‐negative bacteria) could lead to a viable but nonculturable state that can revive under appropriate conditions. This possibility raises questions with regard to long‐term consequences in cases when BLI is used on areas that teem with bacterial communities, such as the human skin. For example, BLI455 is routinely used to treat jaundiced neonates. What are the consequences of these treatments on the emerging skin microbiota? This question would be very interesting to pursue in a longitudinal fashion, especially in the context of prematurely born neonates and how BLI may transiently influence the skin microbiome.
In summary, our studies demonstrated a nonbactericidal effect of BLI on E. coli growth and demonstrated significant differences in intraspecies responses to BLI. Our studies also suggest that BLI induces a viable but nonculturable state in E. coli cells that may facilitate survival in the presence of BLI stress. However, transiently impairing the ability of E. coli to grow using BLI, may pose an attractive future means of preventing colonization on abiotic surfaces such as urinary catheters.
Conflict of Interest
None declared.
Supporting information
Acknowledgments
The authors thank members of the Hadjifrangiskou laboratory for critical evaluation of the manuscript and thoughtful discussions. A special thank you to Dr. Oscar Gómez‐Duarte for contributing B1 strains 343 and 402. This work was funded by academic professional development funds (to Maria Hadjifrangiskou); government support under and awarded by Department of Defense, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship (grant 32 CFR 168a to Courtney A. Mitchell (Abana)); support from the Department of Education through the Graduate Assistance in Areas of National Need (GAANN) Fellowship (grant P200A090323 to Courtney A. Mitchell (Abana)); the National Gem Consortium Fellowship (to Courtney A. Mitchell (Abana)). This work was supported in part by the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH in the form of VR19265 to JRB and funds through the Vanderbilt University School of Engineering (to Bridget R. Rogers). The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Abana CM, Brannon JR, Ebbott RA, et al. Characterization of blue light irradiation effects on pathogenic and nonpathogenic Escherichia coli . MicrobiologyOpen. 2017;6:e466 https://doi.org/10.1002/mbo3.466
Contributor Information
Bridget R. Rogers, Email: bridget.rogers@vanderbilt.edu
Maria Hadjifrangiskou, Email: maria.hadjifrangiskou@vanderbilt.edu.
References
- Allison, K. R. , Brynildsen, M. P. , & Collins, J. J. (2011). Metabolite‐enabled eradication of bacterial persisters by aminoglycosides. Nature, 473, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, E. , Esteves, A. C. , Correia, A. , Cunha, A. , Faustino, M. A. F. , Neves, M. G. P. M. S. , & Almeida, A . (2015). Protein profiles of Escherichia coli and Staphylococcus warneri are altered by photosensitization with cationic porphyrins. Photochemical & Photobiological Sciences, 14, 1169–1178. [DOI] [PubMed] [Google Scholar]
- Anderl, J. N. , Zahller, J. , Roe, F. , & Stewart, P. S. (2003). Role of nutrient limitation and stationary‐phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrobial Agents and Chemotherapy, 47, 1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aussel, L. , Pierrel, F. , Loiseau, L. , Lombard, M. , Fontecave, M. , & Barras, F. (2014). Biosynthesis and physiology of coenzyme Q in bacteria. Biochimica et Biophysica Acta (BBA) ‐ . Bioenergetics, 1837, 1004–1011. [DOI] [PubMed] [Google Scholar]
- Bachmann, B . (1996). Derivations and genotypes of some mutant derivatives of Escherichia coli K‐12 In Neidhart F. C. & Curtiss R. (eds.), Escherichia coli and Salmonella: Cellular and Molecular Biology (pp. 2460–2488). Washington, D.C.:ASM Press. [Google Scholar]
- Balaban, N. Q. , Gerdes, K. , Lewis, K. , & McKinney, J. D. (2013). A problem of persistence: Still more questions than answers? Nature Reviews Microbiology, 11, 587–591. [DOI] [PubMed] [Google Scholar]
- Bebell, L. M. , & Muiru, A. N. (2014). Antibiotic use and emerging resistance: How can resource‐limited countries turn the tide? Global Heart, 9, 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani, G . (1951). STUDIES ON LYSOGENESIS I. : The Mode of Phage Liberation by Lysogenic Escherichia coli. Journal of Bacteriology. 62, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger, J. W . (1944). Treatment of staphylococcal infections with penicillin by intermittent sterilisation. The Lancet, 244, 497–500. [Google Scholar]
- Brooks, B. D. , & Brooks, A. E. (2014). Therapeutic strategies to combat antibiotic resistance. Advanced Drug Delivery Reviews, 78, 14–27. [DOI] [PubMed] [Google Scholar]
- Bumah, V. V. , Masson‐Meyers, D. S. , Cashin, S. E. , & Enwemeka, C. S. (2013). Wavelength and bacterial density influence the bactericidal effect of blue light on methicillin‐resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, 31, 547–553. [DOI] [PubMed] [Google Scholar]
- Buonanno, M. , Randers‐Pehrson, G. , Bigelow, A. W. , Trivedi, S. , Lowy, F. D. , Spotnitz, H. M. , … Brenner, D. J. (2013). 207‐nm UV Light ‐ A Promising Tool for Safe Low‐Cost Reduction of Surgical Site Infections. I. In Vitro Studies. PLoS ONE, 8, e76968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, K. , Courvalin, P. , Dantas, G. , Davies, J. , Eisenstein, B. , Huovinen, P. , … Zgurskaya, H. I. (2011). Tackling antibiotic resistance. Nature Reviews. Microbiology, 9, 894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeen, M. T. (2014). Stationary phase‐specific virulence factor overproduction by a lasR mutant of Pseudomonas aeruginosa. PLoS ONE, 9, e88743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, R. , Tiwari, M. , Kaur, P. , Sharma, M. , Jain, R. , & Dass, S. (2000). Metalloporphyrins—Applications and clinical significance. Indian Journal of Clinical Biochemistry, 15, 183–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieplik, F. , Späth, A. , Leibl, C. , Gollmer, A. , Regensburger, J. , Tabenski, L. , … Schmalz, G. (2014). Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clinical Oral Investigations, 18, 1763–1769. [DOI] [PubMed] [Google Scholar]
- Conlon, B. P. , Rowe, S. E. , Gandt, A. B. , Nuxoll, A. S. , Donegan, N. P. , Zalis, E. A. , … Lewis, K. (2016). Persister formation in Staphylococcus aureus is associated with ATP depletion. Nature Microbiology, 1, 16051. [DOI] [PubMed] [Google Scholar]
- Cook, G. M. , Greening, C. , Hards, K. , & Berney, M . (2014). Chapter One ‐ Energetics of Pathogenic Bacteria and Opportunities for Drug Development In Robert K. P. (Ed.), Advances in Microbial Physiology (pp. 1–62). Cambridge, Massachusetts: Academic Press. [DOI] [PubMed] [Google Scholar]
- Croxen, M. A. , & Finlay, B. B. (2010). Molecular mechanisms of Escherichia coli pathogenicity. Nature Reviews Microbiology, 8, 26–38. [DOI] [PubMed] [Google Scholar]
- Dai, T. , Gupta, A. , Murray, C. K. , Vrahas, M. S. , Tegos, G. P. , & Hamblin, M. R. (2012). Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resistance Updates, 15, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby, C. M. , Venugopal, A. , Ehrt, S. , & Nathan, C. F . (2011). Mycobacterium tuberculosis gene Rv2136c is dispensable for acid resistance and virulence in mice. Tuberculosis (Edinburgh, Scotland), 91, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Zhou, G. , Chen, J. , Shen, L. , Jianxin, Z. , Xu, Q. , & Zhu, Y . (2016). A new LED device used for photodynamic therapy in treatment of moderate to severe acne vulgaris. Photodiagnosis and Photodynamic Therapy, 13, 188–195. [DOI] [PubMed] [Google Scholar]
- Donnelly, R. F. , Cassidy, C. M. , Loughlin, R. G. , Brown, A. , Tunney, M. M. , Jenkins, M. G. , & McCarron, P. A. (2009). Delivery of Methylene Blue and meso‐tetra (N‐methyl‐4‐pyridyl) porphine tetra tosylate from cross‐linked poly(vinyl alcohol) hydrogels: A potential means of photodynamic therapy of infected wounds. Journal of Photochemistry and Photobiology B: Biology, 96, 223–231. [DOI] [PubMed] [Google Scholar]
- Dupont, H. L. , Levine, M. M. , & Hornick, R. B . (1989). Inoculum size in shigellosis and implications for expected mode of transmission. The Journal of Infectious Diseases, 159, 1126–1128. [DOI] [PubMed] [Google Scholar]
- Enwemeka, C. S. , Williams, D. , Enwemeka, S. K. , Hollosi, S. , & Yens, D. (2009). Blue 470‐nm light kills Methicillin‐Resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, 27, 221–226. [DOI] [PubMed] [Google Scholar]
- Farr, S. B. , & Kogoma, T. (1991). Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiological Reviews, 55, 561–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein, O. , Ginsburg, I. , Dayan, E. , Veler, D. , & Weiss, E. I. (2005). Mechanism of Visible Light Phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochemistry and Photobiology, 81, 1186–1189. [DOI] [PubMed] [Google Scholar]
- Feuerstein, O. , Moreinos, D. , & Steinberg, D. (2006). Synergic antibacterial effect between visible light and hydrogen peroxide on Streptococcus mutans. Journal of Antimicrobial Chemotherapy, 57, 872–876. [DOI] [PubMed] [Google Scholar]
- Floyd, K. A. , Mitchell, C. A. , Eberly, A. R. , Colling, S. J. , Zhang, E. W. , Depas, W. , … Hadjifrangiskou, M. (2016). The UbiI (VisC) Aerobic Ubiquinone Synthase Is Required for Expression of Type 1 Pili, Biofilm Formation, and Pathogenesis in Uropathogenic Escherichia coli. Journal of Bacteriology, 198, 2662–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd, K. A. , Moore, J. L. , Eberly, A. R. , Good, J. A. D. , Shaffer, C. L. , Zaver, H. , … Hadjifrangiskou, M. (2015). Adhesive fiber stratification in uropathogenic Escherichia coli biofilms unveils oxygen‐mediated control of type 1 pili. PLoS Pathogens, 11, e1004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad, F. , Zahra, T. , Francis, K. P. , Hasan, T. , & Hamblin, M. R. (2004). Targeted photodynamic therapy of established soft‐tissue infections in mice. Photochemical & Photobiological Sciences, 3, 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, R. J. , Lane, M. C. , Wagner, N. J. , & Weening, E. H . (2015). Dissemination of a highly virulent pathogen: Tracking the early events that define infection. PLoS, 11, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, P. M. , & Tauxe, R. V . (1991). The epidemiology of infections caused by Escherichia coli O157: H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiologic Reviews, 13, 60–98. [DOI] [PubMed] [Google Scholar]
- Guffey, J. S. , & Wilborn, J. (2006a). Effects of combined 405‐nm and 880‐nm light on Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Photomedicine and Laser Surgery, 24, 680–683. [DOI] [PubMed] [Google Scholar]
- Guffey, J. S. , & Wilborn, J. (2006b). In vitro bactericidal effects of 405‐nm and 470‐nm blue light. Photomedicine and Laser Surgery, 24, 684–688. [DOI] [PubMed] [Google Scholar]
- Hamblin, M. R. , Viveiros, J. , Yang, C. , Ahmadi, A. , Ganz, R. A. , & Tolkoff, M. J. (2005). Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrobial Agents and Chemotherapy, 49, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakova, A. , Bogdanova, K. , Tomankova, K. , Pizova, K. , Malohlava, J. , Binder, S. , … Kolarova, H. (2014). The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiological Research, 169, 163–170. [DOI] [PubMed] [Google Scholar]
- Handley, S. A. , Dube, P. H. , & Revell, P. A . (2004). Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infection and Immunity, 72, 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, T. , Makino, K. , Ohnishi, M. , Kurokawa, K. , Ishii, K. , Yokoyama, K. , … Shinagawa, H. (2001). Complete genome sequence of enterohemorrhagic Eschelichia coli O157:H7 and genomic comparison with a laboratory strain K‐12. DNA Research, 8, 11–22. [DOI] [PubMed] [Google Scholar]
- Helaine, S. , & Holden, D. W. (2013). Heterogeneity of intracellular replication of bacterial pathogens. Current Opinion in Microbiology, 16, 184–191. [DOI] [PubMed] [Google Scholar]
- Herzfeld, K. F. (1940). Theory of light absorption in simple aromatic compounds. Proceedings of the American Philosophical Society, 82, 359–367. [Google Scholar]
- Hisert, K. B. , Kirksey, M. A. , Gomez, J. E. , Sousa, A. O. , Cox, J. S. , Jacobs, W. R. , … McKinney, J. D. (2004). Identification of mycobacterium tuberculosis counterimmune (cim) mutants in immunodeficient mice by differential screening. Infection and Immunity, 72, 5315–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren, I. , Kaldalu, N. , Spoering, A. , Wang, Y. , & Lewis, K . (2004). Persister cells and tolerance to antimicrobials. FEMS Microbiology, 230, 13–18. [DOI] [PubMed] [Google Scholar]
- Keren, I. , Shah, D. , Spoering, A. , Kaldalu, N. , & Lewis, K. (2004). Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. Journal of Bacteriology, 186, 8172–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter, R. , Siegele, D. A. , & Tormo, A. (1993). The stationary phase of the bacterial life cycle. Annual Review of Microbiology, 47, 855–874. [DOI] [PubMed] [Google Scholar]
- Kotloff, K. L. , Losonsky, G. A. , Nataro, J. P. , & Wasserman, S. S . (1995). Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli‐Shigella flexneri 2a vaccine strain EcSf2a‐2. Vaccine, 13, 495–502. [DOI] [PubMed] [Google Scholar]
- Laboratories, B. R. (1986). BRL pUC host: E. coli DH5α competent cells. Bethesda Research Laboratories Focus, 8, 9. [Google Scholar]
- Lambrechts, S. A. , Demidova, T. N. , Aalders, M. C. , Hasan, T. , & Hamblin, M. R. (2005). Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochemical & Photobiological Sciences, 4, 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land, E. J. , Simic, M. , & Swallow, A. J. (1971). Optical absorption spectrum of half‐reduced ubiquinone. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics, 226, 239–240. [DOI] [PubMed] [Google Scholar]
- Lechner, S. , Lewis, K. , & Bertram, R. (2012). Staphylococcus aureus persisters tolerant to bactericidal antibiotics. Journal of Molecular Microbiology and Biotechnology, 22, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, M. M. , Ristaino, P. , Marley, G. , Smyth, C. , Knutton, S. , Boedeker, E. , … Cheney, C. (1984). Coli surface antigens 1 and 3 of colonization factor antigen II‐positive enterotoxigenic Escherichia coli: Morphology, purification, and immune responses in humans. Infection and Immunity, 44, 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, J.‐Y. , Yuann, J.‐M. P. , Cheng, C.‐W. , Jian, H.‐L. , Lin, C.‐C. , & Chen, L.‐Y. (2013). Blue light induced free radicals from riboflavin on E. coli DNA damage. Journal of Photochemistry and Photobiology B: Biology, 119, 60–64. [DOI] [PubMed] [Google Scholar]
- Lipovsky, A. , Nitzan, Y. , Friedmann, H. , & Lubart, R. (2009). Sensitivity of Staphylococcus aureus strains to broadband visible light. Photochemistry and Photobiology, 85, 255–260. [DOI] [PubMed] [Google Scholar]
- Lipovsky, A. , Nitzan, Y. , Gedanken, A. , & Lubart, R. (2010). Visible light‐induced killing of bacteria as a function of wavelength: Implication for wound healing. Lasers in Surgery and Medicine, 42, 467–472. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Zeng, W. , Hu, D. , Jha, S. , Ge, Q. , Geng, S. , … Wang, X. (2016). The long‐term effect of 1550 nm erbium:Glass fractional laser in acne vulgaris. Lasers in Medical Science, 31, 453–457. [DOI] [PubMed] [Google Scholar]
- Lorange, E. A. , Race, B. L. , & Sebbane, F . (2005). Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. Journal of Infectious Diseases, 191, 1907–1912. [DOI] [PubMed] [Google Scholar]
- Lubart, R. , Lavi, R. , Friedmann, H. , & Rochkind, S. (2006). Photochemistry and photobiology of light absorption by living cells. Photomedicine and Laser Surgery, 24, 179–185. [DOI] [PubMed] [Google Scholar]
- Lubart, R. , Lipovski, A. , Nitzan, Y. , & Friedmann, H. (2011). A possible mechanism for the bactericidal effect of visible light. Laser Therapy, 20, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean, M. , Macgregor, S. J. , Anderson, J. G. , & Woolsey, G. (2008). High‐intensity narrow‐spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiology Letters, 285, 227–232. [DOI] [PubMed] [Google Scholar]
- Maclean, M. , Macgregor, S. J. , Anderson, J. G. , & Woolsey, G. (2009). Inactivation of bacterial pathogens following exposure to light from a 405‐nanometer light‐emitting diode array. Applied and Environment Microbiology, 75, 1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch, T. , Eichner, A. , Späth, A. , Gollmer, A. , König, B. , Regensburger, J. , & Bäumler, W. (2014). Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PLoS ONE, 9, e111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, Z. , Hanania, J. , & Nitzan, Y. (1990). New trends in photobiology bactericidal effects of photoactivated porphyrins — An alternative approach to antimicrobial drugs. Journal of Photochemistry and Photobiology B: Biology, 5, 281–293. [DOI] [PubMed] [Google Scholar]
- Mangan, M. W. , Lucchini, S. , Danino, V. , Cróinín, T. Ó. , Hinton, J. C. D. , & Dorman, C. J. (2006). The integration host factor (IHF) integrates stationary‐phase and virulence gene expression in Salmonella enterica serovar Typhimurium. Molecular Microbiology, 59, 1831–1847. [DOI] [PubMed] [Google Scholar]
- Marugán, J. , van Grieken, R. , Pablos, C. , & Sordo, C. (2010). Analogies and differences between photocatalytic oxidation of chemicals and photocatalytic inactivation of microorganisms. Water Research, 44, 789–796. [DOI] [PubMed] [Google Scholar]
- McNeil, M. B. , & Fineran, P. C. (2013). Prokaryotic assembly factors for the attachment of flavin to complex II. Biochimica et Biophysica Acta (BBA) ‐ . Bioenergetics, 1827, 637–647. [DOI] [PubMed] [Google Scholar]
- Morris, B. H. , Tyson, J. E. , Stevenson, D. K. , Oh, W. , Phelps, D. L. , O'Shea, T. M. , … Higgins, R. D. (2013). Efficacy of phototherapy devices and outcomes among extremely low birth weight infants: Multi‐center observational study. Journal of Perinatology: Official Journal of the California Perinatal Association, 33, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouslim, C. , & Hughes, K. T. (2014). The effect of cell growth phase on the regulatory cross‐talk between flagellar and Spi1 virulence gene expression. PLoS Pathogens, 10, e1003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy, T. I. , Ball, A. R. , Anklesaria, Z. , Casadei, G. , Lewis, K. , & Ausubel, F. M. (2006). Identification of novel antimicrobials using a live‐animal infection model. Proceedings of the National Academy of Sciences of the United States of America, 103, 10414–10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey, M. A. , Lopez‐Boado, Y. S. , Wilson, C. L. , Roth, R. , Parks, W. C. , Heuser, J. , & Hultgren, S. J. (1998). Induction and evasion of host defenses by type 1‐piliated uropathogenic Escherichia coli. Science, 282, 1494–1497. [DOI] [PubMed] [Google Scholar]
- Navarro Llorens, J. M. , Tormo, A. , & Martínez‐García, E . (2010). Stationary phase in gram‐negative bacteria. FEMS Microbiology Review, 34, 476–495. [DOI] [PubMed] [Google Scholar]
- Nitzan, Y. , Gutterman, M. , Malik, Z. , & Ehrenberg, B. (1992). Inactivation of Gram‐negative bacteria by photosensitized porphyrins. Photochemistry and Photobiology, 55, 89–96. [DOI] [PubMed] [Google Scholar]
- Nitzan, Y. , Malik, Z. , Kauffman, M. , & Ehrenberg, B . (1997). Induction of endogenic porphyrin production in bacteria and subsequent photoinactivation by various light sources. Proceedings of SPIE (society of photographic instrumentation engineers) Conference, 3191, pp. 89–94.
- Nussbaum, E. L. , Lilge, L. , & Mazzulli, T . (2002). Effects of 630‐, 660‐, 810‐, and 905‐nm laser irradiation delivering radiant exposure of 1‐50 J/cm2 on three species of bacteria in vitro. Journal of Clinical Laser Medicine & Surgery, 20, 325–333. [DOI] [PubMed] [Google Scholar]
- Opel, D. R. , Hagstrom, E. , Pace, A. K. , Sisto, K. , Hirano‐Ali, S. A. , Desai, S. , & Swan, J. (2015). Light‐emitting Diodes: A Brief Review and Clinical Experience. The Journal of Clinical and Aesthetic Dermatology, 8, 36–44. [PMC free article] [PubMed] [Google Scholar]
- Orenstein, A. , Klein, D. , Kopolovic, J. , Winkler, E. , Malik, Z. , Keller, N. , & Nitzan, Y. (1997). The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunology and Medical Microbiology, 19, 307–314. [DOI] [PubMed] [Google Scholar]
- O'Toole, G. A. , Pratt, L. A. , Watnick, P. I. , Newman, D. K. , Weaver, V. B. , & Kolter, R. (1999). Genetic approaches to study of biofilms. Methods in Enzymology, 310, 91–109. [DOI] [PubMed] [Google Scholar]
- Pei, S. , Inamadar, A. C. , Adya, K. A. , & Tsoukas, M. M. (2015). Light‐based therapies in acne treatment. Indian Dermatology Online Journal, 6, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perni, S. , Piccirillo, C. , Pratten, J. , Prokopovich, P. , Chrzanowski, W. , Parkin, I. P. , & Wilson, M. (2009). The antimicrobial properties of light‐activated polymers containing methylene blue and gold nanoparticles. Biomaterials, 30, 89–93. [DOI] [PubMed] [Google Scholar]
- Piccolo, D. , di Marcantonio, D. , Crisman, G. , Cannarozzo, G. , Sannino, M. , Chiricozzi, A. , & Chimenti, S. (2014). Unconventional use of intense pulsed light. BioMed Research International, 2014, 618206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkner, J. S. , Remaut, H. , Buelens, F. , Miller, E. , Aberg, V. , Pemberton, N. , … Almqvist, F. (2006). Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proceedings of the National Academy of Sciences of the United States of America, 103, 17897–17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov, D. E. , Tuchina, E. S. , Chernova, J. A. , Podshibyakin, D. , Rudik, D. V. , Samsonova, M. , … Tuchin, V. V . (2005). The affect of low‐coherent light on microbial colony forming ability and morphology of some gram‐positive and gram‐negative bacteria. SPIE Proceedings, 5771, 353–356. [Google Scholar]
- Richter, O.‐M. H. , & Ludwig, B. (2009). Electron transfer and energy transduction in the terminal part of the respiratory chain — Lessons from bacterial model systems. Biochimica et Biophysica Acta (BBA) ‐ Bioenergetics, 1787, 626–634. [DOI] [PubMed] [Google Scholar]
- Roca, I. , Akova, M. , Baquero, F. , Carlet, J. , Cavaleri, M. , Coenen, S. , … Vila, J. (2015). The global threat of antimicrobial resistance: Science for intervention. New Microbes and New Infections, 6, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop, R. M.II, Gee, J. M. , Robertson, G. T. , Richardson, J. M. , NG, W.‐L. , & Winkler, M. E . (2003). Brucella stationary‐phase gene expression and virulence. Annual Review of Microbiology, 57, 57–76. [DOI] [PubMed] [Google Scholar]
- Rúgeles, L. C. , Bai, J. , Martínez, A. J. , Vanegas, M. C. , & Gómez‐Duarte, O. G. (2010). Molecular characterization of diarrheagenic Escherichia coli strains from stools samples and food products in Colombia. International Journal of Food Microbiology, 138, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazanov, L. (2014). The mechanism of coupling between electron transfer and proton translocation in respiratory complex I. Journal of Bioenergetics and Biomembranes, 46, 247–253. [DOI] [PubMed] [Google Scholar]
- Sherbiny, H. S. , Youssef, D. M. , Sherbini, A. S. , El‐Behedy, R. , & Sherief, L. M. (2015). High‐intensity light‐emitting diode vs fluorescent tubes for intensive phototherapy in neonates. Paediatrics and International Child Health, (2046905515Y), 0000000006. [DOI] [PubMed] [Google Scholar]
- Smith, I. , Nathan, C. , & Peavy, H. H. (2005). Progress and new directions in genetics of Tuberculosis: An NHLBI working group report. American Journal of Respiratory and Critical Care Medicine, 172, 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukos, N. S. , Som, S. , Abernethy, A. D. , Ruggiero, K. , Dunham, J. , Lee, C. , … Goodson, J. M. (2005). Phototargeting oral black‐pigmented bacteria. Antimicrobial Agents and Chemotherapy, 49, 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket, C. O. , Binion, S. B. , & Bostwick, E . (1992). Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. The American Journal of Tropical Medicine and Hygiene, 47, 276–283. [DOI] [PubMed] [Google Scholar]
- Thabit, A. K. , Crandon, J. L. , & Nicolau, D. P. (2014). Antimicrobial resistance: Impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opinion on Pharmacotherapy, 16, 159–177. [DOI] [PubMed] [Google Scholar]
- Tian, J. , Bryk, R. , Itoh, M. , Suematsu, M. , & Nathan, C. (2005). Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: Identification of α‐ketoglutarate decarboxylase. Proceedings of the National Academy of Sciences of the United States of America, 102, 10670–10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsika, M. , Beatson, S. A. , Sarkar, S. , Phan, M.‐D. , Petty, N. K. , Bachmann, N. , … Schembri, M. A. (2011). Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: Genome analysis and virulence mechanisms. PLoS ONE, 6, e26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschowri, N. , Busse, S. , & Hengge, R. (2009). The BLUF‐EAL protein YcgF acts as a direct anti‐repressor in a blue‐light response of Escherichia coli. Genes & Development, 23, 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschowri, N. , Lindenberg, S. , & Hengge, R. (2012). Molecular function and potential evolution of the biofilm‐modulating blue light‐signalling pathway of Escherichia coli. Molecular Microbiology, 85, 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchil, R. R. , Kohli, G. S. , Katekhaye, V. M. , & Swami, O. C . (2014). Strategies to combat antimicrobial resistance. Journal of Clinical and Diagnostic Research, 8, ME01–ME04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.‐H. , Singh, R. , Benoit, M. , Keyhan, M. , Sylvester, M. , Hsieh, M. , … Matin, A. C. (2014). Sigma S‐dependent antioxidant defense protects stationary‐phase Escherichia coli against the bactericidal antibiotic gentamicin. Antimicrobial Agents and Chemotherapy, 58, 5964–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi, K. , Ozawa, T. , & Harada, M. (1959). Change of absorption spectrum of flavin adenine dinucleotide by its binding with both D‐amino acid oxidase apo‐protein and benzoate. Nature, 184, 1938–1939. [DOI] [PubMed] [Google Scholar]
- Yang, H. , Inokuchi, H. , & Adler, J. (1995). Phototaxis away from blue light by an Escherichia coli mutant accumulating protoporphyrin IX. Proceedings of the National Academy of Sciences of the United States of America, 92, 7332–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, R. , Wang, M. , Huang, Y.‐Y. , Landi, G. , Vecchio, D. , Chiang, L. Y. , & Hamblin, M. R. (2015). Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: Oxygen‐independent photokilling in presence of azide and new mechanistic insights. Free Radical Biology and Medicine, 79, 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenichowski, K. , Gothe, M. , & Saalfrank, P. (2007). Exciting flavins: Absorption spectra and spin–orbit coupling in light–oxygen–voltage (LOV) domains. Journal of Photochemistry and Photobiology A: Chemistry, 190, 290–300. [Google Scholar]
- Zolfaghari, P. S. , Packer, S. , Singer, M. , Nair, S. P. , Bennett, J. , Street, C. , & Wilson, M. (2009). In vivo killing of Staphylococcus aureus using a light‐activated antimicrobial agent. BMC Microbiology, 9, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


