Abstract
Macrophage accumulation is a critical step during development of chronic inflammation, initiating progression of many devastating diseases. Leukocyte-specific integrin αDβ2 (CD11d/CD18) is dramatically upregulated on macrophages at inflammatory sites. Previously we found that CD11d overexpression on cell surfaces inhibits in vitro cell migration due to excessive adhesion. Here we have investigated how inflammation-mediated CD11d upregulation contributes to macrophage retention at inflammatory sites during atherogenesis.
Atherosclerosis was evaluated in CD11d−/−/ApoE−/− mice after 16 weeks on a Western diet. CD11d deficiency led to a marked reduction in lipid deposition in aortas and isolated macrophages. Macrophage numbers in aortic sinuses of CD11d−/− mice were reduced without affecting their apoptosis and proliferation. Adoptive transfer of fluorescently-labeled wild-type and CD11d−/− monocytes into ApoE−/− mice demonstrated similar recruitment from circulation, but reduced accumulation of CD11d−/− macrophages within the aortas.
Furthermore, CD11d expression was significantly upregulated on macrophages in atherosclerotic lesions and M1 macrophages in vitro. Interestingly, expression of the related ligand-sharing integrin CD11b was not altered. This difference defines their distinct roles in the regulation of macrophage migration. CD11d-deficient M1 macrophages demonstrated improved migration in a three-dimensional fibrin matrix and during resolution of peritoneal inflammation, while migration of CD11b−/− M1 macrophages was not affected.
These results prove the contribution of high densities of CD11d to macrophage arrest during atherogenesis. Since high expression of CD11d was detected in several inflammation-dependent diseases, we suggest that CD11d/CD18 upregulation on pro-inflammatory macrophages may represent a common mechanism for macrophage retention at inflammatory sites, thereby promoting chronic inflammation and disease development.
Keywords: Integrin αDβ2, CD11d/CD18, inflammation, atherosclerosis, M1 macrophages
Introduction
Low-grade chronic inflammation underlies the development of a variety of devastating diseases such as atherosclerosis, obesity, diabetes, arthritis and others (1–3). The development of chronic inflammation is characterized by excessive accumulation of macrophages at inflammatory sites(4). The recruitment and accumulation of monocytes/macrophages at sites of inflammation depends on macrophage adhesive receptors. Prominent among the leukocyte adhesion receptors are the four members of the integrin β2 subfamily: αLβ2 (CD11a/CD18, LFA-1), αMβ2 (CD11b/CD18, Mac-1), αxβ2 (CD11c/CD18, p150, 95) and αDβ2 (CD11d/CD18). Surprisingly, in comparison to other β2 integrins, there is limited information on the function of CD11d/CD18. There are only a few publications that demonstrate the effect of CD11d on the development of the inflammatory response, and these have been studied in models of spinal cord injury (5–7), lethal systemic infections (8) and malaria (9). However, the molecular mechanism of CD11d function is still not understood.
Ligand recognition, followed by specific intracellular signaling, is a critical step that determines integrin-mediated cellular responses. In a previous study, we found that CD11d/CD18 is a multiligand receptor that shares many extracellular matrix ligands (10) with its well characterized subfamily member CD11b/CD18. However, CD11b and CD11d have a different distribution on different subsets of leukocytes.
Integrin CD11d/CD18 has been shown to have low to moderate expression on circulating leukocytes or on splenic red pulp macrophages, but significantly upregulates on inflammatory macrophages. Namely, the increased expression of CD11d was detected in human atherosclerotic lesions by immunostaining of aorta sections (11), on human macrophage foam cells differentiated in vitro by measuring mRNA levels on isolated cells (12) and in white adipose tissue during metabolic syndrome by detecting mRNA levels in rat and human samples (13).
Such expression patterns point to the potential role of this receptor in the development of chronic inflammation. In contrast, the CD11b level is moderate in atherosclerotic lesions or inflamed adipose tissue, but manifests at a high expression on activated neutrophils and several subsets of resident macrophages. Clearly, this difference in the expression patterns reflects its distinct functions during inflammation.
Among many of the described functions, the contribution of β2 integrins to leukocyte migration is fundamental. It has been shown that adhesive receptors such as β2 integrins can promote or inhibit cell migration depending on the microenvironment. Mathematical models and experimental approaches imply that cell migration exhibits a bell-shaped response to cell-substratum adhesiveness, generating a maximum speed at the intermediate values of adhesive strength(14–16). Consequently, a very low cell-substratum adhesiveness cannot support cell migration, while a very high cell-substratum adhesiveness generates cell arrest and prevents cell migration. The adhesiveness depends upon the three elements including ligand concentration, integrin affinity and integrin density (14). The last is the most important in the inflamed extravascular space, which is rich with pro-inflammatory mediators that activate integrins, and with ligands deposited in the extracellular matrix during inflammation.
We have demonstrated that a moderate density of αMβ2- and αDβ2-transfected cells support migration, but high expression of any of either of these integrins significantly impedes cell motility (17,18). Therefore, the upregulation of CD11b and CD11d on specific subsets of inflammatory leukocytes suggests its potential contribution to cell arrest, revealing the importance of CD11b for regulation of neutrophil migration and CD11d for macrophage migration. Of note, αMβ2-mediated neutrophil arrest was recently demonstrated to be important during transendothelial migration (19).
The retention of macrophages at the inflammatory site is a critical step for the accumulation of macrophages and generation of pathophysiological outcomes, which are implicated in the release of pro-inflammatory mediators, new leukocyte recruitment and tissue damage. The importance of a mechanism of macrophage retention is an important subject that has a strong therapeutic potential.
We hypothesize that high expression of CD11d/CD18 on macrophages at the site of inflammation increases cell-substratum adhesiveness which causes macrophage retention and promotes the development of chronic inflammation. The present study is focused on testing this hypothesis using atherosclerosis as a pathophysiologically relevant model.
Materials and Method
Reagents and antibodies
Reagents were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human and mouse IFNγ, IL-13, MCP-1 and LPS were purchased from Invitrogen Corporation (Carlsbad, CA). Anti-human CD11d mAb (clone 240I) and anti-mouse CD11d mAb (clone 205C) were generously provided by Eli Lilly Corporation (Indianapolis, IN). Polyclonal antibody against the CD11d I-domain was described previously (10). The antibody recognizes both human and mouse αD I-domains and has no cross-reactivity with recombinant human and mouse αM, αX and αL I-domains. The antibody was isolated from rabbit serum by affinity chromatography using αDI-domain-Sepharose. Anti-CD11d antibodies were fluorescently labeled with Alexa 488 using a kit from Invitrogen Corporation. Mouse FITC-, APC- and PE- conjugated anti-CD11b mAb (clone M1/70), Ly6C and F4/80 mAbs were from eBioscience (San Diego, CA). The mAb 44a directed against the human αM integrin (CD11b) subunit was purified from the conditioned media of the hybridoma cell line obtained from American Type Culture Collection (ATCC, Manassas, VA) using protein A agarose (GE Healthcare, Piscataway, NJ). The expression of inflammatory cytokines in mouse blood was evaluated using Mouse Inflammation antibody array C1 (RayBiotech, Inc.) and ELISA Kits (Boster Ltd., Pleasanton, CA).
Animals and Diet
C57BL/6J mice were bought from Jackson laboratory (Bar Harbor, ME). Generation of CD11d-deficient mice (B6.129S7-Itgadtm1BII/J) was described before (20). The resulting chimeric animals were crossed to C57BL/6 mice, and then backcrossed to the same for 12 generations. For our experiments, CD11d−/− mice were crossbred with ApoE−/− mice (strain- B6.129P2-Apoetm1Unc/J) to obtain littermate CD11d−/−/ApoE−/− mice and CD11d+/+/ApoE−/− mice were used as controls (define as ApoE−/− in the paper). Atherosclerosis was induced by feeding 4-week old CD11d−/−/ApoE−/− mice and ApoE−/− control with a Western diet containing 0.2% cholesterol and 42% fat (TD88137, Harlan Teklad) for 16 weeks. All procedures were performed according to animal protocols approved by the Cleveland Clinic and East Tennessee State University IACUC.
Atherosclerosis lesion analysis
After 16 weeks on a Western diet mice were sacrificed by pentobarbital overdose and perfused through the heart with 10 mL PBS. For aortic sinus analysis, serial cryosections of 10-μm thickness were taken from the region of the proximal aorta through the aortic sinus and stained with Oil Red O and hematoxylin. For aortic tree analysis, the entire aorta was removed, from the heart, extending 5–10 mm after bifurcation of the iliac arteries and including the subclavian right and left common carotid arteries. It was then dissected free of fat and evaluated for lesion development by en face Oil Red O staining followed by morphometry of scanned images using Image-Pro Plus software. The levels of total cholesterol and triglycerides were evaluated using an ABBOTT Architect CI-8200 instrument.
Aorta digestion and flow cytometry analysis
Aortas were isolated from ApoE−/− mice at 20 weeks of age after being fed a Western diet, and digested as described before (21). Briefly, aortas were digested with a mix of enzymes: collagenase type XI (125 U/ml), Hyaluronidase (60 U/ml), DNase I (60 U/ml) and collagenase type I (450 U/ml) at 37°C for 60 min. After digestion the samples were filtered through a 70μm mesh cell strainer. Isolated cells were preincubated with FC-block solution and subjected to multicolor flow cytometry. The samples were analyzed for expression of CD11d and CD11b on cells positive for the macrophage marker F4/80 using a LCRII flow cytometer (Becton Dickinson & Co., Mountain View, CA).
Flow cytometry analysis was also performed to assess the expression of CD11d, CD11b and scavenger receptors on monocyte-derived human macrophages, mouse peripheral blood monocytes, mouse peritoneal macrophages and bone marrow-derived monocyte progenitors. Cells were harvested and pre-incubated with 4% normal goat serum for 30 min at 4°C, then 2×106 cells were incubated with specific antibody for 30 min at 4°C. Non-conjugated antibodies required additional incubation with Alexa 488 (568 or 633)-donkey anti-mouse IgG (at a 1:2000 dilution) for 30 min at 4°C. Finally, the cells were washed and analyzed using a FACScan™ (Becton Dickinson).
Immunofluorescence staining of aortic sinuses
Cryosections (10 μm) of aortic sinus were warmed up to room temperature for 30 minutes prior to immunofluorescence staining. Tissue sections were fixed in ice-cold acetone for 10 minutes followed by permeabilizing with 0.2% Tween-20 for 10 min to increase the signal of intracellular binding sites. Tissue sections were washed in PBS and incubated with SuperBlock (PBS) Blocking buffer (Thermo Scientific, Rockford, IL, USA) for 45 min to block nonspecific binding. Tissues were then incubated at 4°C overnight with the primary antibody (1:200 dilution of rat anti-mouse Mac3 or 1:500 dilution of rat anti-mouse Ly-6G). After washing several times with PBS, the sections were incubated with Alexa Fluor 488-conjugated donkey anti-rat IgG (1:1000 dilution) for 1 hour at room temperature. The sections were subsequently washed and mounted with mounting media containing DAPI (Life Technologies Corporation, Grand Island, NY, USA) and sealed. The tissue sections were examined with an Olympus BX41 Microscope and fluorescence images were captured using the Optronics MagnaFire SP CCD camera (Olympus America Inc., Center Valley, PA, USA). The captured images were analyzed by using digital image analysis software (AxioVision 4.7.2; Carl Zeiss, Germany). Control sections without primary antibody were also performed at the same time.
Isolation of human monocytes
Human peripheral blood monocytes were isolated and purified from whole blood as described previously (22). Briefly, PBS-diluted blood was layered over a Ficoll-Paque density solution and centrifuged. The mononuclear cell layer was collected and contaminating platelets were removed by centrifugation through bovine calf serum (BCS) after overlaying the serum with the mononuclear cells. Monocytes were isolated from the platelet-free mononuclear cells by adherence to flasks precoated with BCS and containing DMEM and 10% BCS. The flasks were incubated for 2 h at 37°C in 10% CO2. Non-adherent cells were removed by washing with DMEM. Adherent cells were detached with PBS containing 5 mM EDTA. The monocytes were collected, washed three times and incubated at 37°C in 10% CO2 for at least 2h before their use in experiments. The purity of the monocytes was ~90% which was confirmed for each experiment by flow cytometry analysis with anti-CD14 mAb. These studies complied with all relevant federal guidelines and institutional policies regarding the use of human subjects.
Generation of classically activated (M1) and alternatively activated (M2) mouse and human macrophages
Peritoneal macrophages from 8–12 week old mice (WT and CD11d−/−, n = 3 mice per group) were harvested by lavage of the peritoneal cavity with 5 ml of sterile PBS 3 days after intraperitoneal (IP) injection of 4% thioglycollate (TG; 0.5ml). The cells were washed twice with PBS and resuspended in complete RPMI media. The cell suspension was transferred into 100mm petri dishes and incubated for 2h at 37°C in humidified air containing 5% CO2 atmosphere. Nonadherent cells were washed out with RPMI media, and the adherent macrophages were replenished with RPMI media. The macrophages were differentiated to M1 phenotype by treatment with recombinant mouse interferon-γ (IFN-γ) (100 U/ml, Thermo Fisher) for 4 days. Medium with IFN-γ was changed every 2 days or as required. The M1 phenotype macrophages from WT and CD11d−/− were labeled with red fluorescent marker PKH26 and green fluorescent marker PKH67, respectively, according to the manufacturer’s instructions (Sigma-Aldrich). The fluorescently-labeled cells were dissociated from the plates using 5mM EDTA in PBS and used for the experiments thereafter.
Isolated human monocytes were plated in 6-well plates for 5 days in the presence of IFNγ (100 U/ml) to generate an M1 phenotype or with IL-4 (2nM) to generate an M2 phenotype as described before (23). After incubations cells were collected using PBS supplemented with 5 mM EDTA for further experiments.
Adoptive transfer of wild type and CD11d−/− monocytes to the ApoE-deficient mice
Monocyte progenitors, which are an established source for the adoptive transfer experiments, were isolated as described previously (24). Briefly, bone marrow cells were aspirated from mice femurs. Red cells were lysed by cold water. T cells and B cells were removed by negative magnetic cell sorting using anti-CD90 and anti-CD45R immunomagnetic beads (Miltenyi Biotec). Isolated cells were tested by flow cytometry. Cell purity was evaluated using antibody against CD11b and reached about 90%. The expression of CD11d corresponded to CD11d density on blood monocytes (Suppl. Fig. 1).
Isolated wild type monocyte progenitors were labeled with a near-infrared fluorescent dye VivoTrack 680 (PerkinElmer) and CD11d-deficient monocytes were labeled with another near-infrared fluorescent dye, DiR (1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindotri-carbocyanine Iodide) (PerkinElmer). VivoTrack 680 (e×675/em720) and DiR (e×745/em800) fluorescent dyes are not overlapping and can be detected simultaneously in the tissue. For the labeling, 100μg ViVoTrack 680 was dissolved in 600μl of PBS and mixed with 30×106 cells resuspended in 1 ml of PBS for 15 min incubation at room temperature. DiR was dissolved in DMSO (2.5 mM) and added to 30×106 cells for 10 μM final concentration. Cells were incubated with DiR solution for 30 min at 37°C. After labeling cells were washed 3 times in PBS supplemented with 1% FBS. After final washing, cells were resuspended in Hanks′ balanced salt solution and counted.
An equal amount of labeled wild type and Cd11d−/− cells were mixed and 2.5×106 cells per group were injected in the tail vein of recipient ApoE−/− mice that had been fed a Western diet for 20 weeks and thus had significant atherosclerosis. Mice were sacrificed after 3 days, aortas and other organs isolated and analyzed using an IVIC Spectrum CT Imaging system (PerkinElmer). The intensity of fluorescent signals was normalized to the number of fluorescently labeled cells incubated in vitro during the experiment.
Adoptive transfer in the resolution of peritoneal inflammation
Fluorescently-labeled WT (red PKH26 dye) and CD11d−/− or CD11b−/− (green PKH67 dye) M1-activated macrophages were mixed in a 1:1 ratio and further injected intraperitoneally into wildtype mice with no inflammation or 4 days after TG induced inflammation. The equal ratio of red and green macrophages before the injection was verified by sample cytospin preparation followed by fluorescence microscope analysis (EVOS, Thermo Fisher Scientific, Waltham, MA USA). 3 days later, peritoneal macrophages were harvested with 5 ml PBS supplemented with 5 mM EDTA as described above. Peritoneal cells were cytospun and the percentages of red and green fluorescent macrophages were assessed by fluorescence microscopy using at least 9 fields of view per sample. The PKH26 and PKH67 dyes were switched in one experiment to verify the effect of dye on cell migration. We did not detect any difference between two dyes. The quantification of the data was analyzed by using Image Analysis Software (EVOS, Thermo Fisher).
Monocyte emigration from the circulation during peritoneal inflammation
Fluorescently-labeled WT (red PKH26 dye) and CD11d−/− (green PKH67 dye) 5–10 ×106 monocyte progenitors isolated as described above were injected into tail veins of C57BL/6 recipient mice (n=3, each group). Blood samples (after time intervals 1hr, 24hr, 48hr and 72hr) were collected and cytospun to further evaluate the percentage of red and green fluorescent cells under fluorescence microscope (EVOS, Thermo Fisher). The quantification of the data was analyzed by using Image Analysis Software (EVOS, Thermo Fisher). The equal ratio of red and green monocytes before the injection was verified by sample cytospin preparation followed by fluorescence microscope analysis.
Migration of macrophages in 3D fibrin gel
WT and CD11d−/− or WT and CD11b−/− peritoneal macrophages activated to M1 phenotype as described above were labeled with PKH26 red fluorescent dye and PKH67 green fluorescent dye, respectively. Cell migration assay was performed for 24 hours at 37°C in 5% CO2 in a sterile condition. An equal number of WT and CD11d−/− macrophages was evaluated by cytospin of mixed cells before the experiment and at the starting point before migration. Labeled WT (1.5×106) and CD11d−/− (1.5×106) activated macrophages were plated on the membranes of transwell inserts with a pore size of 8 μm and 6.5 mm in diameter (Costar, Corning, NY) precoated with fibrinogen (Fg). Fibrin gel (100 μl/sample) was made by 0.75mg/ml Fg containing 1% FBS and 1% P/S and activated by 0.5 U/ml thrombin. 30 nM of MCP-1 was added on the top of the gel to initiate the migration. Migrating cells were detected by Leica Confocal microscope (Leica-TCS SP8) and the results were analyzed by IMARIS 8.0 software.
Foam cell formation
For assessment of in vivo foam cell formation, CD11d−/−/ApoE−/− mice and ApoE−/− mice were fed the Western diet for 16 weeks following by IP injection with 1 ml of 4% thioglycollate. 72 hours later mice were sacrificed and cells from the peritoneal cavity were collected by lavage with 5 ml of cold PBS. Cells were plated on glass cover slips and non-adherent cells were washed out after 2 hours, which further selected for macrophages. Foam cell formation was evaluated by Oil Red O staining. The intensity of staining was quantified using computer-assisted image analysis software Image-Pro Plus (Media Cybernetics, Bethesda, MD).
Apoptosis and proliferation
Wild type and CD11d−/− peritoneal macrophages were isolated at 96 hours after IP injection of 4% thioglycollate and plated in 96-well or 6-well plates. After 2 hours, non-adherent cells were washed out. Cells were incubated in RPMI-1640 media, supplemented with 10% FBS and 1% pen/strep. Macrophage apoptosis was assessed after 24 hours incubation in media and after an additional 18 hours incubation in the presence of 15 μg/ml OxLDL using the Annexin V assay (Invitrogen). The assay detected the earliest events in apoptosis — the externalization of phosphatidylserine, which was assessed by binding of fluorescently labeled Annexin V. Late apoptosis was evaluated by cell staining with propidium iodide. Macrophage proliferation was evaluated after 5 days of culture in the presence of 50 μg/ml OxLDL or 60 ng/ml GM-CSF using CyQUANT® Direct proliferation assay kit (Invitrogen).
Quantitative RT-PCR
Cellular mRNA was extracted from macrophages using the Qiagen Oligotex mRNA Midi Kit. mRNA was reverse transcribed with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA) and real-time quantitative PCR was performed using SYBR Green Supermix (Bio-Rad) on an MyIQ2 two color real-time PCR detection system (Bio-Rad), with the thermal cycler conditions suggested by the manufacturer. The sequences of the CD11d primers were: sense 5′-GGAACCGAATCAAGGTCAAGTA-3′ and antisense 5′-ATCCATTGAGAGAGCTGAGCTG-3′. The sequences of the CD11b primers were: sense 5′-AAACCACAGTCCCGCAGAGA-3′ and antisense 5′-CGTGTTCACCAGCTGGCTTA-3′. GAPDH or 5S rRNA were used as an internal control (Ambion/Life Technologies, Grand Island, NY).
Statistical analysis
Statistical analyses were performed using Student’s t-test or Student’s paired t-tests where indicated in the text using SigmaPlot 13. A value of p<0.05 was considered significant.
Results
1. Integrin CD11d deficiency reduces the development of atherosclerosis
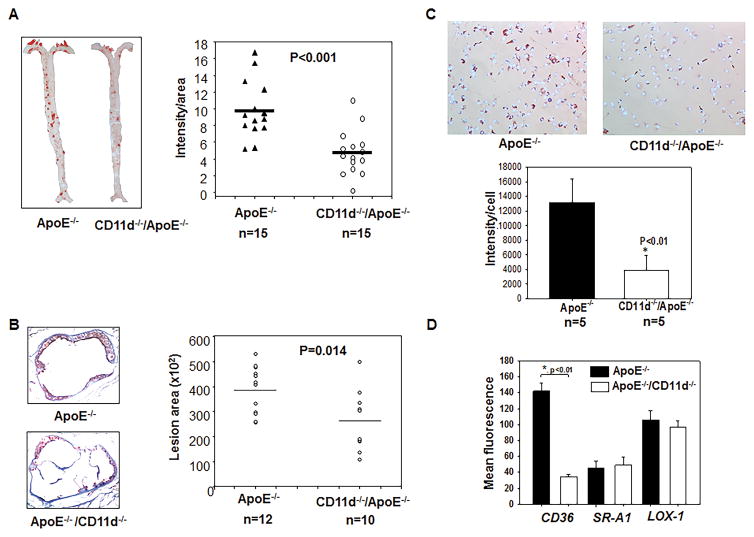
CD11d−/−/ApoE−/− and littermate CD11d+/+/ApoE−/− male mice were placed on a Western diet for 16 weeks to evaluate the potential role of CD11d in atherogenesis. The en face analysis of the total aorta area by Oil Red O staining revealed a 53% decrease in lesion area in CD11d−/−/ApoE−/− mice (p<0.001) (Fig. 1A). The total lesion area across sections of the aortic sinus was also assessed and found to be decreased by 35% (p=0.014) (Fig. 1B). Plasma lipid analysis demonstrated a slight decrease in the level of total cholesterol (22%, p=0.035) and low density lipoproteins (24%, p=0.015) and non-significant changes in the levels of triglycerides and high density lipoproteins in CD11d−/−/ApoE−/− mice (Supplemental Fig. II-A). Foam cell formation was evaluated using thioglycollate–induced peritoneal macrophages isolated from CD11d−/−/ApoE−/− mice and showed ~3-fold less lipid accumulation (p<0.01) compare to CD11d+/+/ApoE−/− control (Fig. 1C).
Fig. 1. Integrin CD11d deficiency decreases the development of atherosclerosis and reduces macrophage foam cell formation in mice.
CD11d−/−/ApoE−/− and ApoE−/− mice were fed a western diet for 16 weeks, then entire aortas (A) and aorta sinuses (B) and peritoneal macrophages (C) were isolated and stained with Oil Red O. Five slides were analyzed from each mouse. The staining was analyzed with Image-Pro Plus software. Statistical analysis was performed using Student’s t-test. (* P<0.01). D. Scavenger receptor expression was evaluated by flow cytometry on peritoneal macrophages isolated from CD11d−/−/ApoE−/− (white bars) and ApoE−/− (black bars) mice using anti-CD36, anti-SR-A1 and anti-LOX-1 antibodies. The data represent the mean + SEM from 3 mice of each group.
These data establish integrin CD11d as a pro-atherogenic receptor and raise a question regarding the mechanisms of CD11d function. We tested several potential mechanisms.
2. The reduced expression of CD36 mediates decreased foam cell formation
Since CD11d deficiency led to diminished oxidized lipid uptake, we tested the expression of major scavenger receptors in CD11d−/−/ApoE−/− macrophages. Flow cytometry analysis of isolated peritoneal macrophages revealed 3-fold reduced expression of CD36 in CD11d-deficient macrophages, while the levels of SRA1 and LOX remained the same (Fig. 1D). Therefore, the decreased expression of CD36 is involved in the diminished foam cell formation in CD11d−/−/ApoE−/− mice. However, in this study our major intent was to seek the common mechanism that may lay at the base of CD11d function in different chronic inflammatory diseases. The mechanism of CD11d-mediated foam cell formation is a separate project that is currently in progress.
3. CD11d deficiency attenuates the expression of several pro-inflammatory cytokines in mice
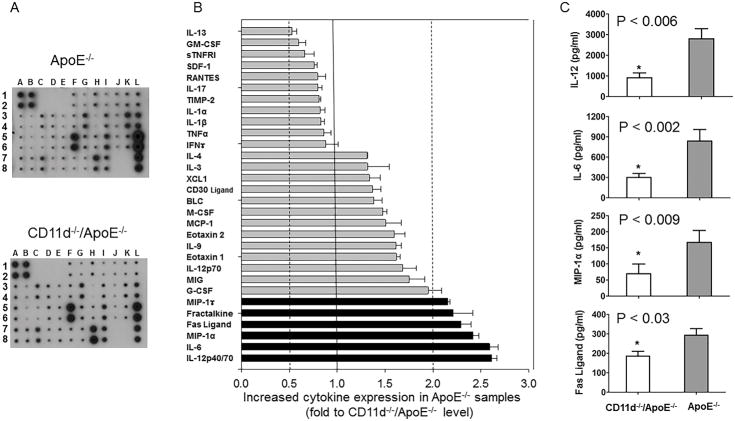
Modulation of cytokine balance during inflammation is a powerful regulator of the inflammatory response. To evaluate the potential contribution of CD11d to the production of pro-inflammatory cytokines and chemokines, we compared plasma samples isolated from CD11d−/−/ApoE−/− and CD11d+/+/ApoE−/− mice after 16 weeks of Western diet. The pooled plasma samples from 5 mice per group were evaluated using a Mouse Inflammation antibody array (RayBiotech, Inc.) (Fig. 2A). We focused only on the results that represent at least a two-fold change between cytokine/chemokine production in ApoE−/− and CD11d−/−/ApoE−/− mice. While only IL-13 expression demonstrated 2-fold upregulation in CD11d−/−/ApoE−/− mice, the concentrations of five cytokines, IL-6, IL-12, Fas ligand, Fractalkine and MIP-1, were decreased more than 2-fold in the CD11d−/−/ApoE−/− samples (Fig. 2B). To verify these results we tested the level of four cytokines, IL-6, IL-12, Fas ligand and MIP-1α using ELISA Kits (Boster Ltd., Pleasanton, CA) (Fig. 2C). Our data detected statistically significant downregulation of all tested cytokines in CD11d−/−/ApoE−/− and demonstrated a strong correlation between two assays. It has been shown that these cytokines are involved in the development of atherosclerosis, therefore their reduced expression indicates a potential mechanisms of CD11d in atherogenesis. On the other hand, upregulation of IL-13 in CD11d−/−/ApoE−/− mice may have atheroprotective functions (25). We used these results as a lead for our further study.
Fig. 2.
Inflammatory cytokine production in the plasma of CD11d−/−/ApoE−/− and ApoE−/− mice after 16 weeks on a western diet. A. The pooled plasma samples from five CD11d−/−/ApoE−/− and five ApoE−/− mice were evaluated in duplicates using the Mouse Inflammation antibody array C1 (RayBiotech, Inc. Norcross, GA) following the manual. The membrane identification chart is shown in the Supplemental materials (Supplemental Table 1). B. The ratio of cytokine expressions ApoE−/− versus CD11d−/−/ApoE−/− was calculated and plotted. Cytokines that demonstrate no difference in the level of expression between two groups, such as I-TAC, sTNRFII, TIMP-1, TECK, Leptin, KC, LIX, TCA-3, IL-2, were not plotted to simplify presentation. C. The levels of expression of IL-12, IL-6, Mip-1α and FAS ligand were verified using ELISA Kits (Boster Ltd., Pleasanton, CA). The data represent the mean + SEM from 6 mice of each group. Statistical analysis was performed using Student’s t-test.
4. CD11d deficiency reduced neutrophil accumulation in the atherosclerotic lesions
We found reduced expression of MIP-1α (CCL3) in the blood of CD11d−/−/ApoE−/− mice. MIP-1α is produced by macrophages and is responsible for the recruitment and activation of polymorphonuclear leukocytes during inflammation. Recent data demonstrate the role of neutrophils in atherosclerotic lesion formation (26). Particularly, it has been shown that leukocyte-specific MIP-1α deficiency inhibits atherosclerotic lesion development by affecting neutrophil accumulation. (27). Based on these observations, we tested neutrophil accumulation in the atherosclerotic lesions of CD11d/ApoE double-deficient mice and found a reduced number of neutrophils in comparison with the CD11d+/+/ApoE−/− control. (Supplemental Fig. II-B). Therefore, CD11d participates in neutrophil accumulation, which apparently has an effect on atherogenesis.
5. Integrin CD11d is upregulated on M1 macrophages in vitro and in atherosclerotic lesions
Despite a detected role of CD11d in neutrophil accumulation, the role of CD11d in macrophage function seems to be more significant. This assumption is based on the critical role of macrophages in atherogenesis and on the dominant expression of CD11d on macrophages. Moreover, most of identified cytokines are expressed by macrophages and related to macrophage functions.
Various studies have highlighted IL-6 as an upstream inflammatory cytokine that plays an important role in the development of atherosclerosis (28). The high level of IL-6 found in such conditions has multiple functions, including activation of endothelial cells, increased coagulation, and promotion of lymphocyte proliferation and differentiation. Fractalkine is a chemokine which is involved in macrophage recruitment during inflammation (29). IL-12 contributes to atherosclerosis by mediating the differentiation of naïve T cells into Th1 cells. It stimulates the production of IFNγ and TNFα from T cells and natural killer cells, and also reduces IL-4 mediated suppression of IFNγ (30). Therefore, IL-12 is involved in classical, pro-inflammatory activation of macrophages (M1). In contrast, IL-13, which is upregulated in CD11d−/−/ApoE−/− mice, is responsible for the alternative macrophage activation (31).
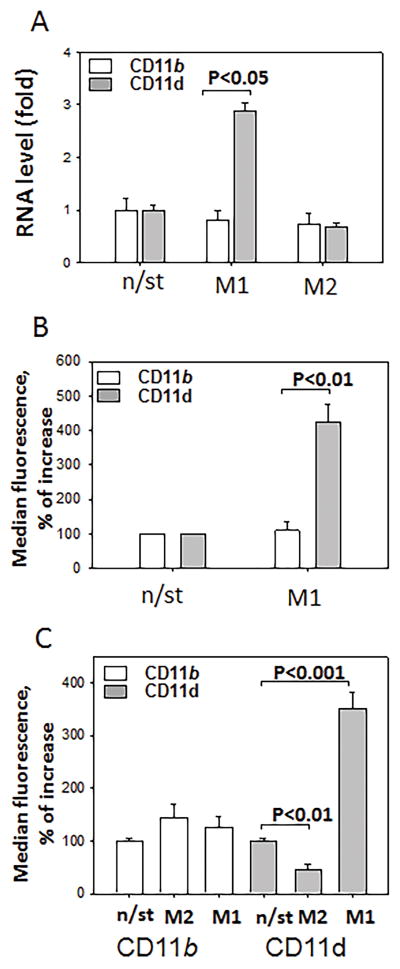
Taken together these data indicate a link between pro-inflammatory macrophages and CD11d. Accordingly, we tested the regulation of CD11d expression on M1 and M2 macrophages and compared its expression with its related integrin CD11b. Peritoneal macrophages were incubated 3 days in the presence of 100 U/ml IFNγ (M1) or 2 nM IL-4 (M2) and integrin expression was evaluated by q-PCR. Macrophage polarization was verified by q-PCR using markers for M1 (iNOS) and M2 (Arg1) subsets (Data not shown). CD11d was significantly upregulated on mouse peritoneal macrophages activated to the M1 phenotype (Fig. 3A), while the density of CD11b remained relatively unchanged. To confirm this result, CD11d and CD11b expressions on M1 macrophages were verified by FACS (Fig. 3B). To demonstrate similar pattern of expression for human CD11d, we also tested M1 and M2 activation of human monocyte-derived macrophages using 100 U/ml IFNγ and 2 nM IL-13. We found that the generation of the M1 phenotype significantly upregulated CD11d expression, while the M2 phenotype markedly decreased the CD11d level. In contrast, the expression of CD11b was not affected by macrophage polarization (Fig. 3C). These data clearly demonstrate the association of CD11d with pro-inflammatory M1 macrophages in both mice and humans.
Fig. 3. Integrin CD11d is upregulated on M1 macrophages in vitro.
Mouse peritoneal macrophages, isolated after the intraperitoneal injection of 4% thioglycollate, were plated and stimulated with 100 U/ml IFNγ or 2 nM IL-4 for 3 days. After incubation integrin expression was evaluated by real-time quantitative PCR (A) and by FACS (B) (n=5). C. Human primary monocytes were stimulated with IFNγ (M1) or IL-13 (M2) for 5 days and integrin expression was evaluated by FACS. Mean fluorescence values are plotted based on 5 independent experiments.
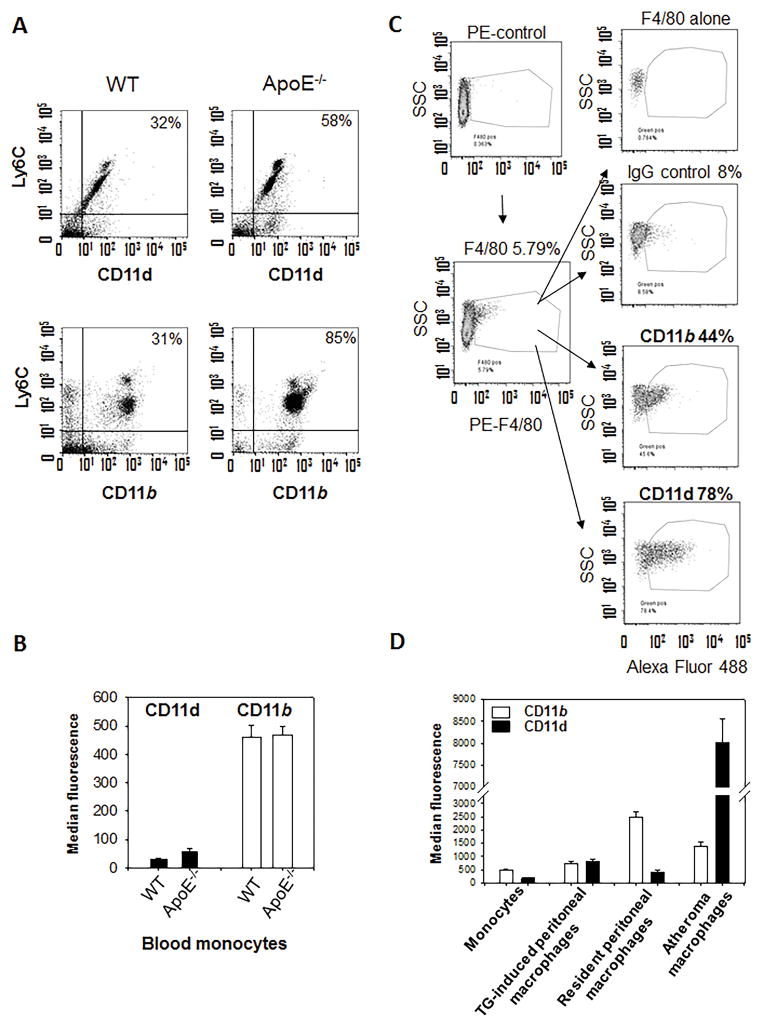
Since CD11d expression is regulated by inflammation we tested how inflammation, and particularly atherosclerosis, modify CD11d expression on monocytes in circulation. For this purpose, we compare CD11d and CD11b expression on Ly6C positive monocytes in WT mice on normal diet and ApoE−/− mice after 16 weeks on western diet (Fig. 4A). Monocytes from ApoE-deficient mice are characterized by much stronger expression of Ly6C (Ly6Chigh), which corresponds to the profile of inflammatory monocytes. Chronic inflammation increased percentage of both CD11d positive and CD11b positive monocytes in circulation. The density of CD11b on monocytes was not changed in ApoE−/− mice (Fig. 4B) and the detected level of expression (461±42 MFI) was in the range of previously published data (11,23). The density of CD11d was increased 1.7 fold (Mean fluorescence 32.2±1 (WT) versus 57.6±8, P=0.11) that markedly lower than CD11d expression on macrophages.
Fig. 4. Expression of CD11d on monocytes in circulation and on macrophages in atherosclerotic lesions during the development of atherogenesis.
A. Integrins CD11d and CD11b expression on monocytes in circulation of WT mice on normal diet and ApoE−/− mice 16 weeks on the western diet. The enriched fraction of blood monocytes was double stained with anti-Ly6C antibody (APC) and anti-CD11d (or anti-CD11b) antibodies (FITC). B. Data were evaluated using FlowJo software. C. Integrins CD11d and CD11b expression in atherosclerotic lesions. Aortas of ApoE−/− mice were isolated, digested and subjected to multi-color FACS with macrophage marker mAb F4/80 and integrin specific antibodies. Data are from a representative experiment of three with similar results. D. CD11d and CD11b expression on murine circulating monocytes; thioglycollate-induced peritoneal, resident peritoneal and atheroma macrophages. Data were plotted as the mean ± SEM.
The progression of chronic inflammation depends on excessive accumulation of M1 macrophages in the subendothelial space during atherogenesis (32). We sought to test whether CD11d expression was elevated on macrophages in atherosclerotic lesions. Flow cytometry of digested mouse atherosclerotic aortas identified a high expression of CD11d on macrophages in the lesion and confirmed a moderate expression of CD11b (33) (Fig. 4C). Remarkably, this pattern of expression is specific to chronic inflammation, since the level of CD11d is similar or lower on other subsets of monocytes/macrophages compared to CD11b (Fig. 4D) (17). Therefore, CD11d is upregulated on M1 macrophages and within the site of inflammation, while CD11b expression is not regulated by macrophage activation and is expressed at moderate levels at inflammatory sites.
These data demonstrate a dramatic difference in CD11d expression on monocytes and macrophages during inflammation and suggest a potential regulatory role of CD11d in migration/accumulation of M1 macrophages in tissue.
6. CD11d deficiency reduced macrophage accumulation in atherosclerotic lesions and does not have effects on macrophage apoptosis or proliferation
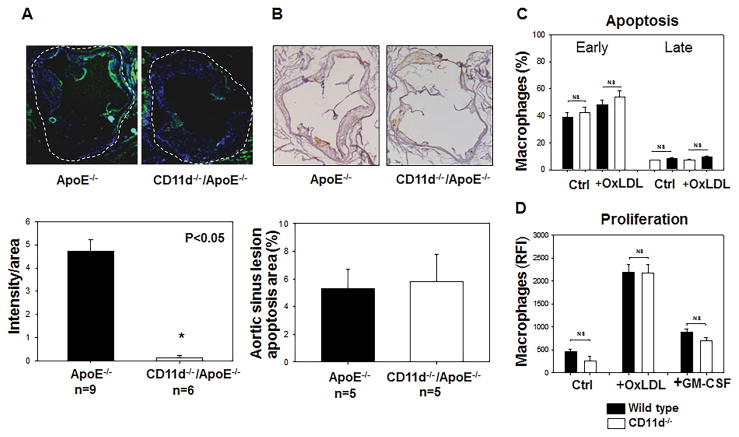
The analysis of aorta sinuses with anti-macrophage Mac-3 antibody demonstrated an attenuated accumulation of macrophages in the atherosclerotic lesions of CD11d−/−/ApoE−/− mice that cannot be explained by reduced level of cholesterol in circulation or decreased foam cell formation and indicates a potential role of CD11d in macrophage migration (Fig. 5A). However, the recent data demonstrate a significant role of apoptosis and proliferation on macrophage accumulations in the atherosclerotic lesions as well as on development of atherosclerosis. Our cytokine assay screening detected a reduced concentration of FAS ligand in CD11d−/−/ApoE−/− mice that indicates potential role of CD11d in apoptosis. The aorta root samples from ApoE−/− and CD11d−/−/ApoE−/− mice were stained with ApopTag peroxidase in Situ apoptosis kit (EMD Millipore), but demonstrated no difference between control and experimental groups (Fig. 5B). In addition, to test the effect of CD11d-deficiency on macrophage apoptosis and proliferation, WT and CD11d−/− peritoneal macrophages were isolated and incubated with oxidized lipids and GM-CSF for 5 days. There was no effect of CD11d-deficiency on the macrophage number and survival using Annexin V apoptosis assay (Fig. 5C) and CyQuant direct proliferation assay (Fig. 5D).
Fig. 5. Macrophage accumulation, apoptosis and proliferation in the mouse aortic sinus.
A. Macrophage staining in the aortic sinus from ApoE−/− and Apo−/−/CD11d−/− mice: Upper panel. Representative images of a cross section of the aortic sinus stained with Mac3 (40× magnification). The intima of vessel wall is surrounded by dash line. Lower panel, the graph represents the quantification of the surface area positive for Mac3. The data represent the mean + SEM of Mac3 positive areas in 6 sections of each group. *p<0.05. Integrin CD11d-deficiency does not affect macrophage apoptosis (B, C) or proliferation (D). B. Apoptosis was evaluated on aorta sinuses isolated from ApoE−/− and CD11d−/−/ApoE−/− mice using ApopTag peroxidase in Situ apoptosis kit. C. Peritoneal macrophages were isolated from WT and CD11d−/− mice and incubated in vitro in different conditions. Macrophage apoptosis was assessed after 24 hours incubation on plates and an additional 18 hours incubation in the presence of 15 mg/ml OxLDL using Annexin V assay. D. Macrophage proliferation was evaluated after 5 days in culture in the presence of 50 mg/ml OxLDL or 60 ng/ml GM-CSF using CyQUANT® Direct proliferation assay kit. Black bars – wild type macrophages, open bars – CD11d-deficient macrophages. Data were plotted as the mean ± SEM. Statistical analysis was performed using Student’s t-test.
Therefore, despite reduced concentration of Fas ligand we did not find changes in the apoptosis of CD11d−/−/ApoE−/− mice. These results are in agreement with published data that overexpression of Fas ligand during development of atherosclerosis increased lesion progression, but did not affect cell apoptosis. Rather, Fas ligand-mediated atherogenesis relates to increased lesion cellularity (34). Hence, CD11d-mediated macrophage accumulation most likely depends on the regulation of macrophage migration.
7. CD11d regulates the accumulation of pro-inflammatory macrophages during inflammation
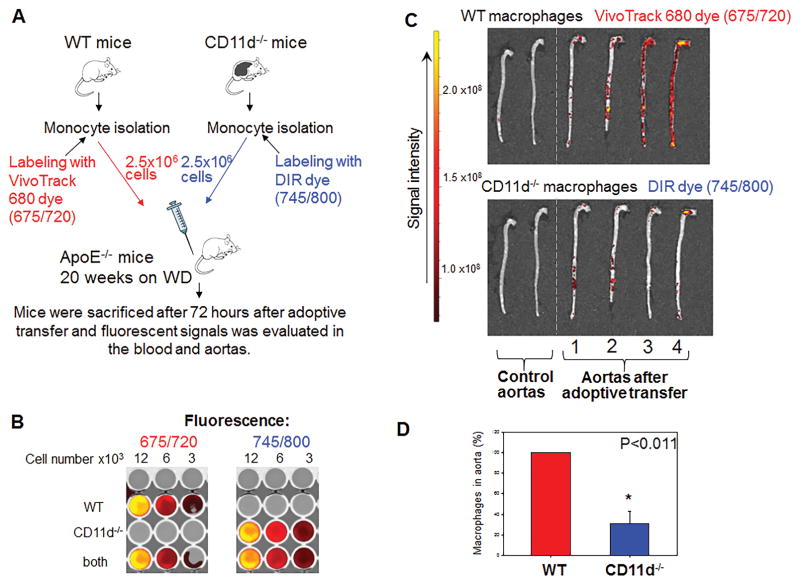
To clarify the contribution of integrin CD11d to the macrophage accumulation at the site of inflammation, we compared the migration of adoptively transferred wild type and CD11d-deficient monocytes within ApoE−/− mice (Fig. 6A). Wild type monocytes were labeled with Vivotrack 680 dye (675/720 nm) and CD11d−/− monocytes were labeled with DIR (1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindotri-carbocyanine Iodide) dye (745/800 nm) according to manufacturer instructions (both dyes are from PerkinElmer). Equal numbers of labeled WT monocytes and CD11d−/− monocytes were mixed and injected into the tail vein of ApoE-deficient mice, which were kept on a Western diet for 20 weeks. The input ratio, levels of labeling and overlapping of signal were assessed in vitro by plating monocytes in different concentrations in a 96-well plate. We also verified that the spectra of these two NIR dyes did not overlap after labeling (Fig. 6B).
Fig. 6. Tracking the migration of adoptively transferred fluorescently-labeled monocytes to the atherosclerotic lesions of ApoE-deficient mice.
A. WT and CD11d-deficient monocytes were labeled with VivoTrack680 and DIR near infrared fluorescent dyes, correspondingly. An equal number of labeled WT and CD11d−/− cells was injected into the same ApoE−/− mouse. After 72 hours aortas from donor mice were isolated and fluorescence was measured using IVIC Spectrum CT Imaging system. B. The intensity of fluorescent signals and potential overlapping of dyes were normalized to the number of fluorescently labeled cells incubated in vitro by plating labeled monocytes in different concentrations in a 96-well plate. C. Two control aortas (no fluorescent cells injected) and four experimental aortas were evaluated using IVIC Spectrum CT Imaging system. D. The calculated result is based on the ratio between WT and CD11d−/− macrophages in atherosclerotic aortas. Data were plotted as the mean ± SEM. Statistical analysis was performed using Student’s t-test.
After 72 hours, mice were euthanized and their aortas were isolated. The ApoE−/− mice without adoptive transfer were evaluated as a negative control. Aortas were examined using an IVIS Spectrum CT Imaging system (PerkinElmer) (Fig. 6C). The detected signals were adjusted to the cell number based on in vitro dilution (Fig. 6B), and accumulations of WT and CD11d−/− macrophages in the atherosclerotic lesions were compared. We detected variable accumulation of macrophages along individual aortas. Apparently, the total level of accumulation depends on lesion development (which varies from mouse to mouse). However, it is not critical for our experiments, since the same number of premixed WT and CD11d−/− monocytes in one syringe are injected into each particular mouse. We determined a ratio between WT and CD11d−/− macrophages in each individual experimental mouse. Our data demonstrated that the number of CD11d-deficient macrophages is reduced on average by 70% in comparison to WT macrophages (Fig. 6D). The analysis of blood samples revealed no fluorescence signal at 72 hours after adoptive transfer, which confirms that both groups of monocytes completely migrated from circulation to the tissue. Our results clearly demonstrate a contribution of CD11d to macrophage accumulation at the site of inflammation.
There are two potential mechanisms that can explain this effect: 1) decreased monocyte recruitment during inflammation or 2) decreased macrophage retention at the site of inflammation.
8. CD11d deficiency does not affect monocyte recruitment from circulation
Numerous publications have demonstrated that leukocyte transmigration from the blood stream to the subendothelial space is mediated by three integrins - α4β1 (CD49d/CD29), αLβ2 (CD11a/CD18) and αMβ2 (CD11b/CD18), (35–37). Based on the low expression of CD11d on monocytes its role in this process might be minimal, and moreover the analysis of blood samples of ApoE-deficient mice after monocyte adoptive transfer did not reveal differential recruitment of WT and CD11d−/− macrophages.
Nevertheless, we tested the effect of CD11d deficiency on emigration of monocytes from the circulation during inflammation using the model of peritoneal inflammation. Equal numbers of WT and CD11d-deficient monocytes labeled with red PKH26 and green PKH67 fluorescent dyes, correspondingly, were injected to the tail vein of mice at 1 hour after thioglycollate-induced peritoneal inflammation. Blood samples were taken at 1 h, 24 h, 48 h and 72 h. The numbers of labeled cells in blood stream were dramatically reduced after 24 hours. We found only a few cells in circulation, mostly CD11d−/−, which corresponds to 0.02% of the total number of injected cells (Data not shown). The PKH26 and PKH67 dyes were switched in one experiment to verify the effect of dye on cell migration. We did not detect any difference between two dyes. Therefore, we suggest that CD11d is not significantly involved in monocyte recruitment, but rather participates in macrophage retention at the site of inflammation. This hypothesis is supported by our previous observations that a high expression of CD11d/CD18 prevents cell motility (17).
9. CD11d deficiency enhances the migration of M1 macrophages in vitro and in vivo
To test this hypothesis, we evaluated the effect of CD11d deficiency on the migratory properties of M1-activated macrophages, since the expression of CD11d is upregulated during M1 macrophage maturation and the accumulation of pro-inflammatory macrophages leads to the development of chronic inflammation. As a control, we evaluated the effect of CD11b deficiency on M1 macrophage migration.
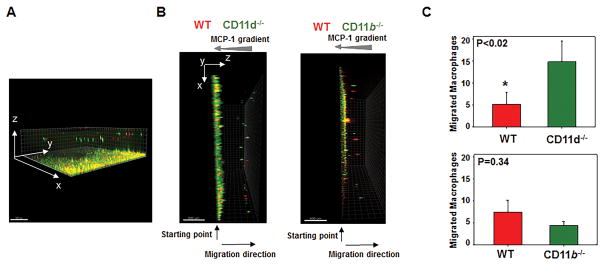
First, we tested the migration of M1 macrophages in vitro using 3D fibrin gel. Fibrin is a well characterized ligand for several integrins including CD11d and CD11b (10,38). For this purpose, WT and CD11d−/− macrophages were activated to M1 phenotype in vitro and labeled with red PKH24 (WT) and green PKH67 (CD11d−/−) fluorescent dyes, mixed together and coated on 3D fibrin gel. An equal number of WT and integrin-deficient- macrophages was evaluated by cytospin of mixed cells before the experiment and at the starting point before migration (Supplemental Fig. III). The migration was stimulated by a gradient of MCP-1 (Fig. 7). It has been shown that M1 activated macrophages have weak migratory abilities (39). In our experiments we also detected very slow migration of M1-activated WT macrophages; however, the migration was significantly enhanced in CD11d-deficient macrophages, demonstrating ~3-fold excess of migrating green CD11d−/− cells compared to red WT control (Fig. 7B, left panel). In contrast to this result, CD11b−/− macrophages (green cells; Fig. 7B, right panel) revealed 1.6 fold less migration (not-statistically significant difference) to compare with WT control. To exclude the effect of a particular dye on cell motility we performed one experiment with WT macrophages labeled with PKH67 and CD11d−/− macrophages labeled with PKH24. We did not observe any differences among the experiments.
Fig. 7. CD11d-deficiency improves the migration of pro-inflammatory M1-activated macrophages in 3-D fibrin gels.
Migration of fluorescently labeled WT and CD11d-deficient (or CD11b-deficient) M1 macrophages in 3D fibrin toward an MCP-1 gradient (A, B). A. The example of 3-D view of migrated macrophages generated by collecting Z-stack images through fibrin matrix. B. Side view of generated 3-D images. WT and CD11d−/− peritoneal macrophages (B, left panel) or WT and CD11b−/− peritoneal macrophages (B, right panel) were activated in vitro for 3 days with IFNγ, labeled with PKH26 and PKH67 fluorescent dyes, respectively, and plated on 3D polymerized fibrin in transwell inserts. Migration of macrophages was stimulated by 30 nM MCP-1 added to the top of the gel. After 24 hours migrating cells were detected by a Leica Confocal microscope (Leica-TCS SP8) and the results were analyzed by IMARIS 8.0 software (C). Statistical analyses were performed using Student’s paired t-tests (n=4 per group). Scale bar – 500 μM.
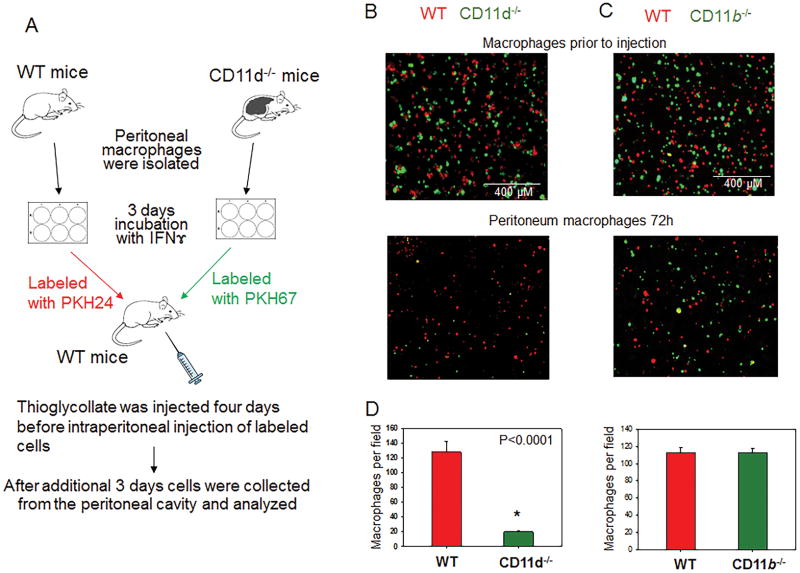
For another approach, we tested the efflux of M1 macrophages from the peritoneal cavity during the resolution of peritoneal inflammation. It has been shown that sterile peritoneal inflammation, mediated by thioglycollate, leads to macrophage accumulation in the peritoneal cavity. The resolution of inflammation that manifested in macrophage efflux from the peritoneal cavity started at day four after thioglycollate injection (40,41). An equal amount of labeled WT and CD11d−/− M1 macrophages, which were activated in vitro to M1 phenotype, were mixed and injected into the inflamed peritoneal cavity of WT mice (Fig. 8A). After 72 hours, the number of CD11d−/− macrophages in the peritoneal cavity was 6-fold less compared with WT control, which demonstrates increased emigration of CD11d−/− macrophages from the site of inflammation (Fig. 8B–D, left panels). The similar experiment performed with WT and CD11b−/− macrophages did not detect a significant difference (Fig. 8B–D, right panels), which concurs with our in vitro migration assay results.
Fig. 8. CD11d-deficiency improves the efflux of pro-inflammatory M1-activated macrophages from the peritoneal cavity.
A. Peritoneal macrophages were isolated from WT and integrin-deficient mice at 3 days after injection of thioglycollate (TG) and labeled with PKH26 and PKH67 fluorescent dyes. Labeled WT and CD11d−/− or WT and CD11b−/− macrophages were mixed in a 1:1 ratio and further injected intraperitoneally into WT mice at 4 days after TG induced inflammation. The equal ratio of red and green macrophages before the injection was verified by sample cytospin preparation (B). 3 days later, peritoneal macrophages were harvested, cytospun and the percentages of red and green fluorescent macrophages were assessed by fluorescence microscopy using at least 9 fields of view per sample (n=6) (C). The quantification of the data was analyzed by using Image Analysis Software (EVOS, Thermo Fisher) (D). Statistical analysis was performed using Student’s paired t-tests.
Apparently, the moderate expression of CD11b on M1 macrophages (Fig. 3) is not involved in macrophage retention. Therefore, in contrast to CD11b-knockout, CD11d-deficient macrophages have improved migratory properties in the model of resolution of peritoneal inflammation and in 3D fibrin gel in vitro. Taken together, these data demonstrate that integrin CD11d is a pro-atherogenic receptor that upregulates on M1 macrophages and is involved in the retention of pro-inflammatory macrophages in the atherosclerotic lesions.
Discussion
Integrin αDβ2 (CD11d/CD18) is the most recently discovered leukocyte integrin and there is limited understanding of its expression specificity and its functions during inflammation. CD11d has a unique expression pattern among integrins. Namely, CD11d has low (11) to moderate (47) density on leukocytes in circulation and resident macrophages, but is dramatically upregulated on macrophages at the inflammatory site (11,13), which suggests a specific role for this receptor during inflammation. Recent studies demonstrate the increased interest in the potential contribution of CD11d to the immune response. It has been shown that anti-CD11d antibody reduced the inflammation (5–7) and macrophage accumulation (42,43) after spinal cord injury and CD11d-deficiency has a protective effect during P. berghei malaria infection (9). In contrast, the survival of CD11d−/− mice after Salmonella typhimurium sepsis was reduced (8). Despite different outcomes, these results demonstrate that CD11d alters the systemic inflammatory response during acute inflammation. The goal of our study was to evaluate the contribution and understand the mechanism of CD11d function during the development of chronic inflammatory diseases focusing on the model of atherosclerosis.
Atherosclerosis is a chronic inflammatory disease, which is manifested in macrophage accumulation and oxidized lipid deposition at the inflamed vascular wall. In our project we found a decreased development of atherosclerosis and significantly reduced inflammatory component in CD11d−/−/ApoE−/− mice after 16 weeks on a Western diet. First, we found a significantly attenuated number of macrophages in the atherosclerotic lesions of these mice. Second, we defined that CD11d has a high expression on macrophages in atherosclerotic lesions of ApoE−/− mice and CD11d is upregulated during classical macrophage activation (M1 phenotype), which is associated with pro-inflammatory development. Third, we revealed that during adoptive transfer of WT and CD11d−/− monocytes to ApoE−/− mice, despite similar recruitment from the circulation, CD11d−/− macrophages demonstrate significantly reduced accumulation in atherosclerotic lesions. And fourth, we found that high expression of CD11d inhibits the migration of M1 macrophages in vitro in 3D fibrin gel and in vivo during macrophage emigration from the peritoneal cavity after sterile peritoneal inflammation. Taken together these data demonstrated the contribution of CD11d to the development of chronic inflammation via regulation of macrophage migration.
In addition, we detected decreased foam cell formation and reduced expression of CD36 on CD11d−/− macrophages, (Fig. 1). These data showed the effect of CD11d on oxidized lipid uptake. However, in this paper, we focused on the CD11d-mediated regulation of macrophage migration, the mechanism that can be involved in the development of different chronic inflammatory diseases. CD11d-mediated foam cell formation and a detailed signaling pathway for CD36 expression is the subject of separate investigation, which is currently in progress.
The role of other β2 integrins in the development of atherosclerosis has been evaluated and demonstrated a complexity of integrin functions. Total β2 deficiency, which suppresses expression of αLβ2 (CD11a/CD18), αMβ2 (CD11b/CD18), αXβ2 (CD11c/CD18) and αDβ2 (CD11d/CD18) integrins, had a protective effect (34% atherosclerotic lesion reduction) during early atherosclerosis; however, after 10 weeks on a western diet, β2 was involved in the progression of the disease (36%) (44). The detailed analysis of α subunits, which are associated with β2 integrin, revealed its different contribution to atherogenesis. CD11a has a proatherogenic effect, since injection of anti-CD11a monoclonal antibodies decreased by 31% macrophage adherence and their emigration into the intima of hypercholesterolemic rats (45). CD11c also demonstrated a proatherogenic effect, but the outcome is mediated by increased expression of CD11c on blood monocytes during hypercholesterolemia, which promotes integrin α4β1-mediated firm adhesion to and transmigration via endothelium during monocyte recruitment to the atherosclerotic lesions (46). In contrast, CD11b deficiency on the LDLR−/− background had no effect on the development of atherosclerosis (47).
Taken together, these results demonstrate the diverse mechanisms and often opposite roles of different integrins during atherogenesis. So far, the major integrin contribution to the development of atherosclerosis was demonstrated to be the recruitment of leukocytes (48) and modification of oxidized lipid uptake (23,46,49). Yet, the effect of integrins on macrophage retention during atherogenesis or other chronic inflammatory diseases was not suggested or evaluated.
Our conclusion regarding the contribution of CD11d to the retention of macrophages is based on the significant upregulation of CD11d on M1 macrophages in vitro and on macrophages in atherosclerotic lesions. Notably, the increased transcript for CD11d was previously detected in white adipose tissue during severe obesity (13) and in the lung during malaria-associated acute respiratory distress syndrome (9). Interestingly, both phenotypes are characterized by increased macrophage accumulation at the inflammatory site (50,51). However, the authors did not draw parallels between CD11d high expression and macrophage accumulation.
A high expression of integrins on the cell surface promotes a specific outcome to cell motility. According to the prevailing hypothesis, optimal cell migration requires a moderate level of integrin expression on the cell surface, whereas high expression mediates strong adhesion and arrests migration (14,15). In our previous studies, we found that low to moderate density of either integrin CD11b or CD11d promoted cell migration, while high expression inhibited cell motility (17,18). In the current study, in vitro, we demonstrated that migration of CD11d-deficient M1 macrophages through 3-D fibrin matrix is improved compared to WT control (Fig. 7). In vivo, we showed that M1 activated CD11d−/− macrophages exit from the peritoneal cavity faster than WT control during the resolution of peritoneal inflammation. The critical role of CD11d upregulation on M1 macrophages was demonstrated by the comparable analysis of CD11d−/− and CD11b−/− macrophages migration. CD11b and CD11d have a similar ligand binding properties (10), but different expression on M1 macrophages (Fig. 3). The analysis of in vivo and in vitro migration of CD11d−/− and CD11b−/− M1 macrophages reveals a dramatic difference in the motility of macrophages with a high or moderate levels of integrin expression that concurs with the model of cell migration (14,15) and our previous studies (17,18). Notably, it has been shown that natural emigration of macrophages from the peritoneal cavity is mediated by integrin α4β1 (41) or to some extent CD11b/CD18 (52). Since the acute peritoneal inflammation did not lead to the strong M1 and M2 activation of macrophages, we activated macrophages in vitro to the M1 phenotype and injected macrophages into the inflamed peritoneal cavity, creating an artificial model of resolution. CD11b is a well-recognized migratory receptor. However, apparently its contribution to migration is diminished in M1-activated macrophages, which is characterized by high expression of CD11d.
Most importantly, we detected diminished accumulation of fluorescently labeled adoptively transferred CD11d−/− macrophages, despite similar recruitment of WT and CD11d−/− monocytes from the circulation. We, and others, have demonstrated the expression of CD11d on leukocytes in circulation (23,53). VCAM-1 has been shown as a ligand for CD11d (54), that demonstrates a potential role of this integrin in monocyte adhesion to endothelium. However, the critical role of other integrins, such as CD11b/CD18, αLβ2 and α4β1 in the transmigration from bloodstream to subendothelial space has been well documented (55,56). Particularly, numerous experimental data demonstrated that α4β1 is a critical receptor for endothelial VCAM-1 during different inflammatory diseases including atherosclerosis (35–37). In addition, our previous data demonstrated that CD11d - VCAM-1 interaction has relatively low affinity (10), which attenuates its potential role in leukocyte recruitment from the circulation. Most likely, in CD11d-deficient mice, the contribution of CD11d to monocyte adhesion to the endothelium is compensated by α4β1 function. In agreement with this conclusion, our data showed no difference in the emigration of fluorescently labeled WT and CD11d−/− monocytes from the circulation of ApoE−/− mice during atherogenesis and WT mice during peritonitis. Therefore, the reduced accumulation of CD11d−/− macrophages in atherosclerotic lesions as well as improved migration of CD11d−/− M1 macrophages in vitro and in vivo clearly indicates the contribution of CD11d to macrophage arrest and to their retention at the site of inflammation.
The activation of macrophages toward the M1 phenotype is considered to be a critical component of pro-inflammatory development, which results in tissue damage, secretion of inflammatory mediators and additional recruitment of inflammatory cells from circulation. The accumulation of M1-like macrophages was detected at the site of inflammation during the progression of many chronic inflammatory diseases including atherosclerosis, diabetes, obesity, macular degeneration and others (50,57–59). Since CD11d high expression was detected in inflammatory tissues in several pathophysiological conditions (9,11,12,13,53) we suggest that the upregulation of CD11d on M1 macrophages can be a common mechanism that encourages retention of these pro-inflammatory macrophages at the site of inflammation, thereby promoting chronic inflammation and disease development. Consequently, the blocking of CD11d-mediated adhesion during atherogenesis or other inflammation-related pathologies is a new promising strategy for the resolution of chronic inflammation and the reversal of the progression of disease.
Supplementary Material
Acknowledgments
Sources of funding: These studies were supported by AHA 14GRNT20410074 and NIH grant DK102020 (V.P.Y).
Footnotes
Disclosures: None.
Reference List
- 1.Tobias P, Curtiss LK. Thematic review series: The immune system and atherogenesis. Paying the price for pathogen protection: toll receptors in atherogenesis. J Lipid Res. 2005;46:404–411. doi: 10.1194/jlr.R400015-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 3.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian S, Chait A. The effect of dietary cholesterol on macrophage accumulation in adipose tissue: implications for systemic inflammation and atherosclerosis. Curr Opin Lipidol. 2009;20:39–44. doi: 10.1097/mol.0b013e32831bef8b. [DOI] [PubMed] [Google Scholar]
- 5.Weaver LC, Bao F, Dekaban GA, Hryciw T, Shultz SR, Cain DP, Brown A. CD11d integrin blockade reduces the systemic inflammatory response syndrome after traumatic brain injury in rats. Exp Neurol. 2015;271:409–422. doi: 10.1016/j.expneurol.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao F, Brown A, Dekaban GA, Omana V, Weaver LC. CD11d integrin blockade reduces the systemic inflammatory response syndrome after spinal cord injury. Exp Neurol. 2011;231:272–283. doi: 10.1016/j.expneurol.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki Y, Bunting M, Stafforini DM, Harris ES, McIntyre TM, Prescott SM, Frutuoso VS, Amendoeira FC, de Oliveira ND, Vieira-de-Abreu A, Weyrich AS, Castro-Faria-Neto HC, Zimmerman GA. Integrin {alpha}D 2 Is Dynamically Expressed by Inflamed Macrophages and Alters the Natural History of Lethal Systemic Infections. J Immunol. 2008;180:590–600. doi: 10.4049/jimmunol.180.1.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Azevedo-Quintanilha IG, Vieira-de-Abreu A, Ferreira AC, Nascimento DO, Siqueira AM, Campbell RA, Teixeira Ferreira TP, Gutierrez TM, Ribeiro GM, PMES, Carvalho AR, Bozza PT, Zimmerman GA, Castro-Faria-Neto HC. Integrin alphaDbeta2 (CD11d/CD18) mediates experimental malaria-associated acute respiratory distress syndrome (MA-ARDS) Malar J. 2016;15:393. doi: 10.1186/s12936-016-1447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakubenko VP, Yadav SP, Ugarova TP. Integrin alphaDbeta2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties. Blood. 2006;107:1643–1650. doi: 10.1182/blood-2005-06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Vieren M, Le Trong H, St John T, Staunton DE, Gallatin WM. A novel leukointegrin, αdβ2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 12.Noti JD. Expression of the myeloid-specific leukocyte integrin gene CD11d during macrophage foam cell differentiation and exposure to lipoproteins. International Journal of Molecular Medicine. 2002;10:721–727. [PubMed] [Google Scholar]
- 13.Thomas AP, Dunn TN, Oort PJ, Grino M, Adams SH. Inflammatory phenotyping identifies CD11d as a gene markedly induced in white adipose tissue in obese rodents and women. J Nutr. 2011;141:1172–1180. doi: 10.3945/jn.110.127068. [DOI] [PubMed] [Google Scholar]
- 14.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 15.DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yakubenko VP, Belevych N, Mishchuk D, Schurin A, Lam SC, Ugarova TP. The role of integrin alpha D beta2 (CD11d/CD18) in monocyte/macrophage migration. Exp Cell Res. 2008;314:2569–2578. doi: 10.1016/j.yexcr.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lishko VK, V, Yakubenko P, Ugarova TP. The interplay between Integrins αMβ2 and α5β1 during cell migration to fibronectin. Exp Cell Res. 2003;283:116–126. doi: 10.1016/s0014-4827(02)00024-1. [DOI] [PubMed] [Google Scholar]
- 19.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, Barth CJ, Lugo G, Donnelly M, Nayer A, Moita LF, Schurer S, Traver D, Ruiz P, Vazquez-Padron RI, Ley K, Reiser J, Gupta V. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci Signal. 2011;4:ra57. doi: 10.1126/scisignal.2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Rodgers JR, Perrard XY, Perrard JL, Prince JE, Abe Y, Davis BK, Dietsch G, Smith CW, Ballantyne CM. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol. 2004;173:297–306. doi: 10.4049/jimmunol.173.1.297. [DOI] [PubMed] [Google Scholar]
- 21.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy B, Cathcart MK. Induction of 15-lipoxygenase expression by IL-13 requires tyrosine phosphorylation of Jak2 and Tyk2 in human monocytes. J Biol Chem. 1998;273:32023–32029. doi: 10.1074/jbc.273.48.32023. [DOI] [PubMed] [Google Scholar]
- 23.Yakubenko VP, Bhattacharjee A, Pluskota E, Cathcart MK. αMβ2 integrin activation prevents alternative activation of human and murine macrophages and impedes foam cell formation. Circ Res. 2011;108:544–554. doi: 10.1161/CIRCRESAHA.110.231803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Manivannan A, Dawson R, Crane IJ, Mack M, Sharp P, Liversidge J. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:6915–6923. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 27.de Jager SC, Bot I, Kraaijeveld AO, Korporaal SJ, Bot M, van Santbrink PJ, van Berkel TJ, Kuiper J, Biessen EA. Leukocyte-specific CCL3 deficiency inhibits atherosclerotic lesion development by affecting neutrophil accumulation. Arterioscler Thromb Vasc Biol. 2013;33:e75–e83. doi: 10.1161/ATVBAHA.112.300857. [DOI] [PubMed] [Google Scholar]
- 28.Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions. 1994;42:159–162. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- 29.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyemura K, Demer LL, Castle SC, Jullien D, Berliner JA, Gately MK, Warrier RR, Pham N, Fogelman AM, Modlin RL. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 32.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS ONE. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray JL, Shankar R. Down regulation of CD11b and CD18 expression in atherosclerotic lesion- derived macrophages. Am Surg. 1995;61:674–679. [PubMed] [Google Scholar]
- 34.Schneider DB, Vassalli G, Wen S, Driscoll RM, Sassani AB, DeYoung MB, Linnemann R, Virmani R, Dichek DA. Expression of Fas ligand in arteries of hypercholesterolemic rabbits accelerates atherosclerotic lesion formation. Arterioscler Thromb Vasc Biol. 2000;20:298–308. doi: 10.1161/01.atv.20.2.298. [DOI] [PubMed] [Google Scholar]
- 35.Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K. Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ Res. 1999;84:1237–1244. doi: 10.1161/01.res.84.11.1237. [DOI] [PubMed] [Google Scholar]
- 36.Issekutz AC, Issekutz TB. Monocyte migration to arthritis in the rat utilizes both CD11/CD18 and very late activation antigen 4 integrin mechanisms. J Exp Med. 1995;181:1197–1203. doi: 10.1084/jem.181.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, Jung S, Littman DR, Ley K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 2001;108:1307–1314. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yakubenko VP, Solovjov DA, Zhang L, Yee VC, Plow EF, Ugarova TP. Identification of the binding site for fibrinogen recognition peptide γ383–395 within the αM I-domain of integrin αMβ2. J Biol Chem. 2001;275:13995–14003. doi: 10.1074/jbc.M010174200. [DOI] [PubMed] [Google Scholar]
- 39.Vogel DY, Heijnen PD, Breur M, de Vries HE, Tool AT, Amor S, Dijkstra CD. Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation. J Neuroinflammation. 2014;11:23. doi: 10.1186/1742-2094-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 41.Bellingan GJ, Xu P, Cooksley H, Cauldwell H, Shock A, Bottoms S, Haslett C, Mutsaers SE, Laurent GJ. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J Exp Med. 2002;196:1515–1521. doi: 10.1084/jem.20011794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphaD: a potential new anti- inflammatory treatment. Exp Neurol. 2000;166:52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- 43.Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedrea FC, Peters AA, O’Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 2004;156:42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Merched A, Tollefson K, Chan L. Beta2 integrins modulate the initiation and progression of atherosclerosis in low-density lipoprotein receptor knockout mice. Cardiovasc Res. 2010;85:853–863. doi: 10.1093/cvr/cvp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nie Q, Fan J, Haraoka S, Shimokama T, Watanabe T. Inhibition of mononuclear cell recruitment in aortic intima by treatment with anti-ICAM-1 and anti-LFA-1 monoclonal antibodies in hypercholesterolemic rats: implications of the ICAM-1 and LFA-1 pathway in atherogenesis. Lab Invest. 1997;77:469–482. [PubMed] [Google Scholar]
- 46.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubo N, Boisvert WA, Ballantyne CM, Curtiss LK. Leukocyte CD11b expression is not essential for the development of atherosclerosis in mice. J Lipid Res. 2000;41:1060–1066. [PubMed] [Google Scholar]
- 48.Patel SS, Thiagarajan R, Willerson JT, Yeh ET. Inhibition of alpha4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation. 1998;97:75–81. doi: 10.1161/01.cir.97.1.75. [DOI] [PubMed] [Google Scholar]
- 49.Antonov AS, Kolodgie FD, Munn DH, Gerrity RG. Regulation of macrophage foam cell formation by alphaVbeta3 integrin: potential role in human atherosclerosis. Am J Pathol. 2004;165:247–258. doi: 10.1016/s0002-9440(10)63293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van den Steen PE, Deroost K, Deckers J, Van HE, Struyf S, Opdenakker G. Pathogenesis of malaria-associated acute respiratory distress syndrome. Trends Parasitol. 2013;29:346–358. doi: 10.1016/j.pt.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 2005;106:3234–3241. doi: 10.1182/blood-2005-03-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyazaki Y, Vieira-de-Abreu A, Harris ES, Shah AM, Weyrich AS, Castro-Faria-Neto HC, Zimmerman GA. Integrin alphaDbeta2 (CD11d/CD18) is expressed by human circulating and tissue myeloid leukocytes and mediates inflammatory signaling. PLoS ONE. 2014;9:e112770. doi: 10.1371/journal.pone.0112770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- 55.Issekutz TB. Dual inhibition of VLA-4 and LFA-1 maximally inhibits cutaneous delayed-type hypersensitivity-induced inflammation. Am J Pathol. 1993;143:1286–1292. [PMC free article] [PubMed] [Google Scholar]
- 56.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS ONE. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chinetti-Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Curr Opin Lipidol. 2011;22:365–372. doi: 10.1097/MOL.0b013e32834a77b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int J Inflam. 2013;2013:503725. doi: 10.1155/2013/503725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.