ABSTRACT
In contrast to conventional, molecular medicine that focuses on targeting specific pathways, stem cell therapy aims to perturb many related mechanisms in order to derive therapeutic benefit. This emerging modality is inherently complex due to the variety of cell types that can be used, delivery approaches that need to be optimized in order to target the cellular therapeutic to specific sites in vivo, and non-invasive imaging methods that are needed to monitor cell fate. This review highlights advancements in the field, with focus on recent publications that use preclinical animal models for cardiovascular stem cell therapy. It highlights studies where cell adhesion engineering (CAE) has been used to functionalize stem cells to home them to sites of therapy, much like peripheral blood neutrophils. It also describes the current state of molecular imaging approaches that aim to non-invasively track the spatio-temporal pattern of stem cell delivery in living subjects.
KEYWORDS: cell adhesion, glycoengineering, mesenchymal stem cells, molecular imaging, multimodality imaging, reporter gene imaging, selectin, targeted delivery
Introduction
Stem cell therapy is fundamentally different from the conventional cornerstones of modern medicine that rely upon surgery or pharmaceutical therapy. Surgery treats physical ailments (e.g. inflamed appendix) and accompanying symptoms (abdominal pain) through “physical” tissue level resection (appendectomy) and/or reconstruction. Both the responses (wound-healing, regeneration) and known complications (coagulopathy, infections) can be anticipated based on a priori knowledge. Similarly, pharmaceuticals target specific receptors or pathways, with the goal of improving disease outcome. Here, predictive, pharmacokinetic models enable both the design of the therapeutic and its application regimen. In contrast to these conventional approaches that target specific processes using exogenous means, the field of cellular therapeutics aims to introduce stem cells to function as ‘catalysts’ that may either replace injured tissue or accentuate the endogenous repair mechanisms already at work in living organisms.1,2 Here, the molecular target is not one receptor or a localized feature. Instead, it is a series of related molecular processes and associated, heterogenous cell types that aim to increase tissue mass, augment differentiation, stimulate endogenous repair, establish supportive tissue regeneration (e.g., angiogenesis), and/or reduce inflammation. Validating this concept, numerous studies conducted in animals support the promise of this approach, with evidence that stem cells may either undergo differentiation3 or secrete paracrine factors that enhance tissue repair and functional outcome.4
The clinical application of stem cells has reached center stage. In the last 15 years, both adult and pluripotent-derived stem cells have proceeded through preclinical models, paving the way for commercialization, and motivating numerous clinical trials5. However, the successes of clinical studies have been scant, with successful clinical transplantation only being reported recently.6 While much effort has been focused on techniques for stem cells derivation, characterization, and cultivation, future success may hinge upon two less-appreciated areas of investigation: targeted delivery of therapeutic stem cell and noninvasive molecular imaging. This commentary reviews these two emerging areas, with focus on recent advances in cell delivery strategies7 and imaging.8-10
Targeted delivery of stem cells
Methods for the delivery of stem cells are broadly classified into: i. local methods where cells are directly injected into the damaged tissue; and ii. systemic methods that use intra-venous (i.v.), intra-arterial (i.a.) or intra-coronary (i.c.) infusion to introduce the stem cells. Both methods result in relatively low levels of engraftment with only 1–2% of the cells being retained.11 Although direct cell delivery to a focal area of tissue damage could be beneficial, poor vascularization leading to oxygen depletion, high cellular nutrient demand at sites of injury, along with the risk of tissue perforation likely limit the utility of this approach. Thus, there is growing interest in developing targeted, systemic cell injection techniques (Table 1). In this regard, i.v. injection introduces all therapeutic cells to the right-side of the heart, and this can lead to cell trapping, and retention within non-targeted lung alveolar capillaries.12 Arterial injections may offer more utility, but non-targeted delivery may simply result in passive entrapment within arterial microvasculature without extravasation.
Table 1.
Effect of stem cell modifications on in vivo targeted delivery.
| Experiment | Modification | Key finding | Citation |
|---|---|---|---|
| Rat BM-MSC transfused into the left ventricular cavity of MI rats | No modification | 1% of cells migrate to the infarcted myocardium at 4 h with significant retention in lung | 12 |
| Murine MSC-like cells were injected into the tail vein of 4-mo-old mice | Overexpression of CXCR4 on MSCs through adenovirus infection | ∼8 fold increase in retention to bone marrow | 35 |
| Murine MSCs were intramyocardially injected in mice with myocardial infarction | Overexpression of CCR-1 chemokine receptor on MSCs | Increase in MSC survival, migration, and engraftment in ischemic myocardium | 36 |
| Rat MSCs were intravenously infused into tail vain of myocardial infarcted rat | Overexpression of CXCR4 on MSCs | 2.5-fold increase engraftment to the infarcted myocardium, leading to reduced LV remodeling and enhanced recovery of function | 37 |
| Human and rat GRPs and MSCs were transplanted into the internal carotid artery of rats | Altering cell size, cell dose, and cell infusion velocity | Stroke at infusion velocity over 1 ml/min, profound decrease in cerebral blood flow for large cells infusion, stroke lesions for dosage injection more than 1 × 106 | 38 |
| Primary human MSCs were injected into the tail vein of an inflamed model of mice. | Immobilization of SLex on MSC surface using prior surface immobilization of biotin and streptavidin | 56% efficiency increase in cell localization to the inflamed ear | 20 |
| Human umbilical cord blood cells were injected intravenously into sublethally irradiated immunodeficient (NOD/SCID) mice | Enforced α(1,3)fucosylation and SLex expression on CB cells surface | Enhanced selectin binding and bone marrow engraftment of CB cells in irradiated NOD/SCID mice | 18 |
| Human MSCs were intravenously infused into the tail veins of immunodeficient (NOD/SCID) mice | Enforced α(1,3)fucosylation and SLex expression on MSCs surface | Robust tethering and rolling interactions and firm adherence of cells on sinusoidal vessels and rapid infiltration to the marrow parenchyma | 14 |
| Murine MSCs were injected into the mice with inflammatory bowel disease | Coating MSCs with VCAM-1 antibody using protein G | Highest delivery efficiency to inflamed mesenteric lymph node | 39 |
| Lin- Sca+ murine stem cells were intravenously injected into mice with infarcts created by ligation of LAD | Cells modified with bispecific antibodies against murine stem cell c-kit and VCAM-1 up-regulated on injured myocardial cells | Increased retention to injured myocardium | 40 |
| Human HSC intravenously injected into the xenogeneic rat model with ischemic injury induced by transient ligation LAD | Decorating HSCs with Bispecific antibodies that binds human CD45 and myosin light chain, an organ-specific injury antigen expressed by infarcted myocardium | Enhanced cell homing to myocardial infarcted tissue | 41 |
| Human MSCs intra-ventricularly injected through the left ventricle of mice with myocardial infarction | Coating MSCs with palmitated derivatives of phage-peptides (CRPPR, CRKDKC, KSTRKS, and CARSKNKDC) | Increased binding to infarcted regions | 42 |
| Swine CDC and MSC intracoronary infused into the brief cardiac IR injury swine model | Coupling CDCs and MSCs with 19Fc[FUT7+] plus FUT7 over-expression in the cells | 28% of cells localized in LAD proximal to IR site | 7 |
Abbreviations: BM-MSC: Bone Marrow-derived Mesenchymal Stem cells; MI: Myocardial Infarction; MSC: Mesenchymal stem cells; LV: Left ventricle; GRP: Glial restricted precursors; NOD/SCID: Nonobese diabetic/ sever combined immunodeficient; VCAM: vascular cell adhesion molecule; AD: Left anterior descending; HSC: haematopoietic stem cells; CDC: Cardiosphere derived cells; IR: Ischemia reperfusion.
Engineering of the cell delivery pathway should ideally target the well-vascularized, viable, but compromised, tissue that immediately surrounds the area of damage in specific organs. Since these vulnerable regions are often inflamed and susceptible to neutrophil recruitment, a number of laboratories have hypothesized that modifying stem cell surface adhesion molecules to more closely resemble peripheral-blood neutrophils may enable the efficient, systemic delivery of therapeutic cells. Broadly, this approach is termed ‘Cell Adhesion Engineering’ (CAE). With respect to this, while early studies suggested that mesenchymal stem cells (MSCs) may constitutively express the P-selectin ligands and VLA-4 that are necessary to target these regions,13 such expression may not be robust since the MSCs are a heterogeneous cell type and their surface markers may change during in-vitro propagation.14 Thus, there is a need to engineer simple but robust cell surface modifications to enable leukocyte-like stem cell capture. Key challenges in the field include:
Glycoengineering selectin-ligands on stem cells: The blood neutrophils are captured from flow when sialofucosylated carbohydrates expressed on their cell-surface bind E- and P-selectin expressed on the inflamed endothelium. These glycans are commonly decorated by α(2,3)sialic acid and α(1,3)fucose on Type-II lactosamine chains, with sialyl Lewis-X (sLeX) representing a prototypic selectin-ligand. In mammals, such structures can be synthesized by various glycosyltransferases including the α(1,3)fucosyltransferases FUT3-FUT7 and FUT9, and the α(2,3)sialyltransferase ST3Gal-4 and ST3Gal-6.15 Among these, the enzymes responsible for selectin-ligand biosynthesis in human neutrophils are FUT4, 7 and 9,16 and ST3Gal-4.17
Stem cells often lack the robust expression of α(1,3)fucosylated glycans. Thus, Xia et al.18 enforced fucosylation and sLeX expression on these cells, more specifically the human umbilical cord blood CD34+ cells, using the exogenous FUT6 along with the donor GDP-fucose (guanosine diphosphate-fucose). This modification enabled CD34+ cell rolling on P- and E-selectin at a shear stress of 0.5 dyn/cm2 in-vitro. Transplantation of these modified cells into immunodeficient mice also enhanced cell engraftment. Sackstein et al.14 extended this approach to MSCs and demonstrated that such α(1,3)fucosylation can enhance targeting of MSCs to the bone, since marrow vessels constitutively express E-selectin.
The high on- and off-rates of the physiological selectin-ligand bond are a key for the capture of cells from free flowing blood.19 Here, the expression of the sLeX glycan alone in the absence of the physological glycoprotein scaffold(s) results in low affinity cellular interactions and only rapid rolling.20 Based on this, Lo et al.21 engineered HEK (human embryonic kidney) cells for the high-level expression of a fusion protein 19Fc[FUT7+] (originally described in22), where the functional end of the P-selectin glycoprotein ligand-1 (PSGL-1, CD162) was fused to a human IgG1 tail. The protein was non-covalently and transiently coupled to MSC surface using palmitated-protein G chemistry.23 Such cell-surface PSGL-1 modification resulted in robust cell recruitment on substrates bearing P-selectin. Extending this approach, more recently, these authors showed that α(1,3)fucosylation of MSCs more prominently enhanced E-selectin recognition rather than P-selectin binding.7 Combining the PSGL-1 and fucosylation strategies thus resulted in robust binding on both selectin-types in in vitro flow chamber studies (Fig. 1). Glycoengineering of stem cells using both strategies also enabled short-term retention of 28% of the cells in the left anterior descending artery of the pig heart in a brief ischemia-reperfusion model.7 In addition to the demonstration of stem cell delivery, the study provides promising data suggesting that engineered stem cells may be safe for cardiovascular applications.
Figure 1.
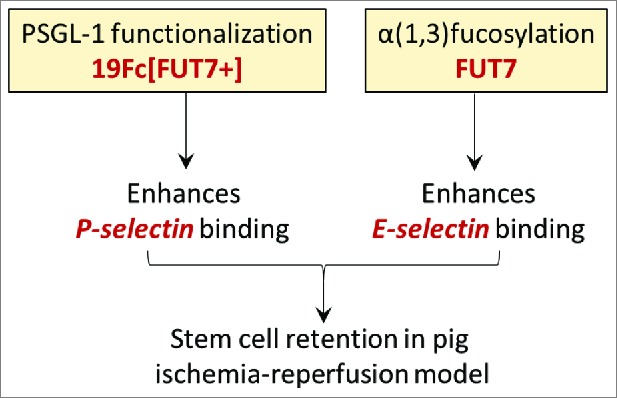
Complementary glycoengineering methods to enhance stem cell delivery. Coupling the recombinant PSGL-1 protein (19Fc[FUT7+]) to stem cell surface enhances cell binding to P-selectin. Overexpression of the α(1,3)fucosyltransferase FUT7, on the other hand, enhances cell binding to E-selectin. CDCs functionalized with both modifications were retained in the pig heart in a brief ischemia-reperfusion model (ref.7).
Optimizing conditions for the capture of stem cells from free flowing blood: Mesenchymal stem cells are often larger (20–25μm) than peripheral blood neutrophils (10–15μm). The fluid drag force that must be overcome in order to capture these cells from flow varies approximately as a square of the cell radius. Thus, the drag force applied on stem cells is ∼3.2 (=[22.5/12.5]2) fold higher than that applied on neutrophils. Additionally, while neutrophils are captured in the post-capillary venules where the wall shear stresses are low at 0–3 dyn/cm3, the engineered adhesion molecules on stem cells may have to be designed to overcome higher stresses at sites of inflammation in arteries. For these reasons, in addition to examining the simple rolling of stem cells on the endothelium, studies that aim to deliver stem cells following systemic injection must also emphasize cell adhesion engineering (CAE) methods that enhance cell recruitment or capture onto the endothelium. Beyond mimicking neutrophils, in the long run, it may also be necessary to identify additional stem-cell specific sialofucosylated glycoconjugates that can enhance cell capture. While CD44 has been implicated to be one of the stem cell glycoproteins that is prominently sialofucosylated to display the HCELL epitope,14 it is necessary to also identify other players that may be similarly modified.
Optimizing conditions for stem cell transmigration across the endothelium: Besides selectins, stem cells express a variety of endogenous chemokine and growth factor receptors that aid the activation of cell surface integrins and enhance cell homing to sites of inflammation and injury.11 Depending on the source of the cells, this includes chemokine receptors that bind SDF-1 (stromal cell-derived factor-1, CXCL12), MIP (Macrophage inflammatory protein, CCL3/4) and RANTES (Regulated on activation, normal T cell expressed and secreted, CCL5), and growth factor receptors that bind FGF (fibroblast growth factor), PDGF (platelet-derived growth factor) and VEGF (vascular endothelial growth factor). Expansion of stem cells can down-regulate many of these homing molecules in culture and thus methods to over-express these using non-viral techniques is of current interest. Among these, a large emphasis is on the receptor CXCR4 as it binds SDF-1 since numerous publications illustrate the importance of this binding on stem cell homing.24 Besides these, metalloproteinases (MMP-2 and MT1-MMP) may also play a role in degrading extracellular matrix components to enhance cell migration to sites of injury.
Molecular imaging of stem cells
Stem cell imaging is an emerging sub-topic within molecular imaging, a field where biological processes are visualized and quantified in living subjects.25 Current approaches for monitoring stem cells in vivo are commonly destructive and not quantitative, and they involve the use of methods like RT-PCR, immunohistochemistry, and fluorescence in situ hybridization.26 The inability to perform longitudinal studies that track cell number, location, and differentiation state in vivo, repeatedly, reduces the ability to relate cell in vivo fate to tissue regeneration, and our understanding of inter-individual variability. To address this major limitation, more recent investigations have attempted serial noninvasive cell imaging in living subjects. A comparison of these approaches follows.
Cell prelabeling: Cell prelabeling, consists of intracellular loading of imaging agent (molecules or nanoparticles) which enhance imaging sensitivity. This technique, which can be used in conjunction with SPECT (single photon emission computed tomography), PET (positron emission tomography) and MRI (magnetic resonance imaging), boosts imaging sensitivity because large amounts (µg to mg) of imaging agents can be specifically loaded into cells ex vivo prior to introduction into animals. The main issue here is cellular toxicity and dilution of imaging-probe mass over time due to cell proliferation in vivo. Cell prelabeling during SPECT can be accomplished with 111In oxine or [111In] Indium oxinate3, in which the 111In isotope of Indium complexes with the chelator 8-hydroxy quinolone.27 This chelator is subsequently released after intracellular entry. This technique is used in patients for imaging whole body leukocyte migration for fever of unknown origin. A minimum of 2 × 108 cells are labeled for adequate visualization, probe half-life is 2.8 d, and spatial resolution is ∼10 mm (Table 2, 27).
Table 2.
Comparison of cell prelabeling versus reporter gene for all major imaging modalities.
| Label | Modality | Cell sensitivity (Small animal) | Cell sensitivity (Large animal) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Prelabeling | |||||
| Near Infrared dye | IVM, FMT | 1 × 10031 | n/a | high sensitivity(10-17M) high spatial resolution (<1 µm) (IVM) inexpensive multiplex capability improved depth penetration (FMT) | low depth penetration (IVM) limited clinical use invasive procedure (IVM) loss of signal with depth |
| Indium oxine | SPECT | 6 × 10543 | 1 × 107 44 | high sensitivity (10-11M) ease of use multiplex capability | label dilution radioactive dose signal decay planar (not tomographic) low spatial resolution (2–10mm) |
| FDG | PET | 5 × 104 28 | 3 × 107 45 | high sensitivity (10-12M) tomographic natural molecule labeling quantitative | label dilution radioactive dose signal decay cyclotron required expensive low spatial resolution (2–10mm) |
| SPIO NP | MRI | 2 × 10246 | 1.5 × 107 10 | high spatial resolution improved sensitivity no radioactivity | label dilution toxicity low sensitivity (10-12M, MR) highly sequence dependent many imaging artifacts negative contrast method semiquantitative |
| Reporter Genes | |||||
| GFP | IVM | 1 × 10031 | n/a | high sensitivity (10-17M) high spatial resolution (<1 µm) inexpensive multiplex capability serial imaging | low depth penetration limited clinical use invasive procedure loss of signal with depth |
| Firefly Luciferase | BLI | 1 × 103 9 | n/a | high sensitivity (10-17M) inexpensive multiplex capability (Rluc) serial imaging | low spatial resolution loss of signal with depth low light cooled CCD required |
| HSV1TK/SR39TK 18F-FHBG | PET | 1 × 107 47 | 2.5 × 108 10 | high sensitivity (10-12M) tomographic serial imaging clinically approved | radioactive dose signal decay cyclotron required expensive |
Abbreviations: IVM: intravital microsopy; FMT: fluorescence molecular tomography; SPECT Single photon CT; PET: Positron emission tomography; MRI: Magnetic resonance imaging; BLI: Bioluminescence; SPIO: Superparamagnetic Iron Oxide Particles; NP: nanoparticles; HSV1-TK: Herpes Simplex Virus Type I truncated thymidine kinase; SR39TK: Mutant Herpes Simplex Virus Type I truncated mutated thymidine kinase; 18F-FHBG: 18F-radiolabelled 9-[4-fluoro-3-(hydroxyl methyl) butyl] guanine
During PET, positron-emitting 18F-fluorodeoxyglucose (18F-FDG) is commonly used to prelabel cells. PET has been used to image stem cells in mice.28,18F has a half live of 110 minutes, shorter than [111In], but PET is an order of magnitude more sensitive than SPECT (10−12 M Molar (M) vs. 10−11 M), and thus can detect fewer numbers of cells with the same mass of prelabel (Table 2).
Nanoparticle (30–100nm) (NP) based cell prelabeling use quantum dots, silica, polymer-based, gold or superparamagnetic (SPIO) particles for imaging with fluorescence, PET, SPECT, photoacoustics and magnetic resonance imaging (MRI). Among these, prelabeling with SPIO NP (MRI) is an established, clinically approved technique that has been used to monitor stem cell delivery.29 Here, SPIO NP mass, strength of magnetic field, signal to noise ratio, pulse sequence, and acquisition parameters all affect imaging quality and sensitivity.30 Using SPIO NP and a 3T MRI for cardiac imaging, Parashurama et al.9 demonstrate that a minimum of 1.5×107 MSCs are needed for large animal imaging. Here, only a fraction (∼20–30%) of the infused MSCs, rather than all MSCs, were SPIO NP loaded and this reduced overall toxicity. With this technique, the authors analyzed MRI data across the cardiac cycle, rather than in a single frame, leading to a linear relationship between signal and cell number. Because of the dilution of the prelabel with cell division, the above techniques are valuable for obtaining sensitive images for initial stem cell localization studies, for a duration of hours to days.
Optical Reporter gene (RG) imaging: Here, a reporter gene (RG) is stably expressed in cells. An imaging probe is then introduced which interacts with the RG to produce signal. Due to this, RG imaging signal sensitivity depends on reporter levels in cells, the number of cells expressing the RG, probe transport to the reporter, the strength of the signal generated, probe toxicity and safety, and the physics of the imaging device. In this case, as the RG is stably expressed, the imaging signal is constant rather than being diluted with cell division and can be repeated indefinitely. Promoter silencing is one process that can prevent long term, serial imaging. A short-half RG half-life (e.g. hours) is requisite so that the measured imaging signal reflects changes in reporter gene promoter levels. Here, in a prototypic example, green fluorescent protein (GFP) has been used for serial, intravital, stem cell imaging.31,32 However, the tissue depth is limited (∼150 microns) and this is not useful for whole body imaging due to visible-light absorption, scattering, and high background. Bioluminescent RG imaging using firefly luciferase (Fluc), on the other hand, enables highly sensitive, whole body imaging although the resulting optical signal also varies linearly with depth. Here, as little as 1000 MSCs can be detected in small animals (mouse) following localized subcutaneous injection, with the measured signal varying linearly with cell number.9 Interestingly, the measured bioluminescence signal persisted longer in the injured compared to the normal myocardium, suggesting fundamentally different MSC cell fates in these two environments.9 In addition to monitoring a single parameter, the use of two luciferase RGs (Fluc and Renilla(Rluc)) can enable multiplexing in vivo by usage of substrates specific to each of the luciferases. Using this approach, Ahn et al. engineered pluripotent stem cells co-expressing constitutively-active Fluc along with Rluc driven by Oct4/differentiation promoter.8 Stem cell proliferation and differentiation fates could then be independently monitored following local subcutaneous injection. In contrast to in vitro imaging data, the initial decrease in Rluc/Fluc signal was followed by an increase indicating complex stem cell regulatory mechanisms in vivo. Unfortunately, neither GFP nor bioluminescence studies are feasible in large animals due to lack of signal strength at greater tissue depths.
PET RG Imaging: PET RG imaging involves expressing a genetically encoded PET reporter gene (HSV1TK or its mutant SR39TK) in the transplanted stem cells. These RG expressing stem cells are then detected using the PET reporter probe (18F-FHBG (18F-radiolabelled 9-[4-fluoro-3-(hydroxyl methyl) butyl] guanine).33 Here, the PET RG in the stem cells selectively phosphorylates the PET reporter probe and traps it intracellularly, leading to a detectable signal. Only trace doses of the PET reporter probe are infused in order to limit patient exposure to radioactivity. Because the probe distributes throughout the body, less than 1% of the injected probe actually accumulates in the cells of interest. Due to this, in the first limit of detection study in larger animals, when different concentrations of MSCs expressing the PET-RG SR39TK were locally injected into the porcine left ventricle, a minimum of ∼2.5 ×108 cells were required for 18F-FHBG PET signal detection.10 As in previous studies, a number of parameters affect the imaging signal including PET RG expression levels, vascularity of target organ, cardiac motion, and animal fluid status. Thus, while PET is a powerful clinical imaging modality, additional improvements are necessary before the routine use of PET RG in stem cell based studies.
Multimodality molecular imaging: Since each imaging modality has its pros and cons (Table 2), it would be beneficial to combine complementary approaches by developing multimodal methods. For example, combining PET RG imaging (high sensitivity) with MRI (high spatial resolution) is a simple approach to improving visualization of stem cell therapies. In this case, the stem cell signal, derived from PET RG imaging, may be visualized in relationship to relevant, <10 µm, anatomical structures highlighted by MRI. Thus, imaging of the vascular endothelium with MRI can improve the ability to engineer and visualize cell delivery, and MR imaging of border tissue near myocardial infarction can help visualize stem cell migration and subsequent tissue repair. If the PET RG-expressing stem cells are also prelabeled with SPIO NP, then further benefit is gained from a multimodality perspective.10 Here, SPIO prelabeling can enable initial localization and validation of PET signals, and the PET signal can also validate the MR images of prelabeled cells. Further, because injections can be validated with two modalities, this approach strengthens the ability to track two injections in two locations in the same animal, potentially with two different cell numbers or two different RGs, independently. Other modalities can also be combined within a single study for benefit.10
Conclusion
Stem cell therapies represent a new and exciting approach for treating major health problems like ischemic heart disease. However, the results of clinical studies have been mixed. This highlights the need to better understand tissue specific stem cell differentiation mechanisms, delivery methods and imaging techniques.
In the case of cardiac cell therapy, preclinical data demonstrates that several cell types, including unfractionated bone marrow cells, bone marrow stromal cells (MSC), cardiac stem/progenitor cells (C-kit positive or Sca-1 positive), pluripotent stem cell-derived progenitors are all potential therapeutic candidates.34 The question naturally arises, which one cell type should be used therapeutically and/or how can they be used synergistically?
A vast literature has appeared on the mechanisms by which stem cells naturally home to sites of injury. These studies highlight caveats in the culture procedure of these cells that may alter the natural repertoire of homing receptors. To address this shortcoming, recent studies have begun to use ‘cell adhesion engineering’ approaches to accentuate the natural homing properties of the stem cells by glycoengineering selectin-ligands on their surface and also decorating chemokine receptors that aim to augment the natural tropism of these cells. Further studies may enable the improvement of such delivery strategies, and related simplifications that are necessary for clinical applications.
Both prelabeling and RG imaging enable noninvasive monitoring of stem cell fate. While prelabeling is sensitive, it is not ideal for long-term studies that last weeks to months. Reporter genes are more suitable for long term studies though improvements in sensitivity and spatial resolution are necessary. Further advancements in nanoparticle based prelabeling methods, RG design for PET and multimodal imaging may facilitate longitudinal stem cells studies, particularly in large animals. In the future, a combination of these advanced imaging modalities with the targeted cell adhesion engineering approach may pave the way for more robust validation of basic science concepts in large animals and their successful translation to humans.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Supported by grants from the American Heart Association (16IRG27770071) and National Institutes of Health (HL103411).
References
- [1].Sayed N, Liu C, Wu JC. Translation of Human-Induced Pluripotent Stem Cells: From Clinical Trial in a Dish to Precision Medicine. J Am Coll Cardiol 2016; 67(18):2161-76; PMID:27151349; https://doi.org/ 10.1016/j.jacc.2016.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics 2016; 3:16006; PMID:27162934; https://doi.org/ 10.1038/mto.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, et al.. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A 2009; 106(33):14022-7; https://doi.org/ 10.1073/pnas.0903201106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, et al.. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol 2006; 48(10):2116-24; PMID:17113001; https://doi.org/ 10.1016/j.jacc.2006.06.073 [DOI] [PubMed] [Google Scholar]
- [5].Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015; 17(1):11-22; PMID:26140604; https://doi.org/ 10.1016/j.stem.2015.06.007 [DOI] [PubMed] [Google Scholar]
- [6].Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature 2014; 513(7518):287-8; PMID:25230622; https://doi.org/ 10.1038/513287a [DOI] [PubMed] [Google Scholar]
- [7].Lo CY, Weil BR, Palka BA, Momeni A, Canty JM Jr, Neelamegham S. Cell surface glycoengineering improves selectin-mediated adhesion of mesenchymal stem cells (MSCs) and cardiosphere-derived cells (CDCs): Pilot validation in porcine ischemia-reperfusion model. Biomaterials 2016; 74:19-30; PMID:26433489; https://doi.org/ 10.1016/j.biomaterials.2015.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahn BC, Parashurama N, Patel M, Ziv K, Bhaumik S, Yaghoubi SS, Paulmurugan R, Gambhir SS. Noninvasive reporter gene imaging of human Oct4 (pluripotency) dynamics during the differentiation of embryonic stem cells in living subjects. Mol Imaging Biol 2014; 16(6):865-76; https://doi.org/ 10.1007/s11307-014-0744-1 [DOI] [PubMed] [Google Scholar]
- [9].Parashurama N, Ahn BC, Ziv K, Ito K, Paulmurugan R, Willmann JK, Chung J, Ikeno F, Swanson JC, Merk DR, et al.. Multimodality Molecular Imaging of Cardiac Cell Transplantation: Part I. Reporter Gene Design, Characterization, and Optical in Vivo Imaging of Bone Marrow Stromal Cells after Myocardial Infarction. Radiology 2016; 140049.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Parashurama N, Ahn BC, Ziv K, Ito K, Paulmurugan R, Willmann JK, Chung J, Ikeno F, Swanson JC, Merk DR, et al.. Multimodality Molecular Imaging of Cardiac Cell Transplantation: Part II. In Vivo Imaging of Bone Marrow Stromal Cells in Swine with PET/CT and MR Imaging. Radiology 2016; 280(3):826-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells 2016; 8(3):73-87; PMID:27022438; https://doi.org/ 10.4252/wjsc.v8.i3.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH. Systemic delivery of bone marrow–derived mesenchymal stem cells to the infarcted myocardium feasibility, cell migration, and body distribution. Circulation 2003; 108(7):863-868; PMID:12900340; https://doi.org/ 10.1161/01.CIR.0000084828.50310.6A [DOI] [PubMed] [Google Scholar]
- [13].Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006; 108(12):3938-44; PMID:16896152; https://doi.org/ 10.1182/blood-2006-05-025098 [DOI] [PubMed] [Google Scholar]
- [14].Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 2008; 14(2):181-7; PMID:18193058; https://doi.org/ 10.1038/nm1703 [DOI] [PubMed] [Google Scholar]
- [15].Taniguchi N, Honke K, Fukuda M, Narimatsu H, Yamaguchi Y, Angata TE. Handbook of glycosyltransferases and related genes. 2nd ed. 2014, Tokyo, Japan: Springer; 1707. [Google Scholar]
- [16].Buffone A Jr, Mondal N, Gupta R, McHugh KP, Lau JT, Neelamegham S. Silencing alpha1,3-fucosyltransferases in human leukocytes reveals a role for FUT9 enzyme during E-selectin-mediated cell adhesion. J Biol Chem 2013; 288(3):1620-33; PMID:23192350; https://doi.org/ 10.1074/jbc.M112.400929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mondal N, Buffone A Jr, Stolfa G, Antonopoulos A, Lau JT, Haslam SM, Dell A, Neelamegham S. ST3Gal-4 is the primary sialyltransferase regulating the synthesis of E-, P-, and L-selectin ligands on human myeloid leukocytes. Blood 2015; 125(4):687-96; PMID:25814486; https://doi.org/ 10.1182/blood-2014-07-588590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xia L, McDaniel JM, Yago T, Doeden A, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood 2004; 104(10):3091-6; https://doi.org/ 10.1182/blood-2004-02-0650 [DOI] [PubMed] [Google Scholar]
- [19].Beauharnois ME, Lindquist KC, Marathe D, Vanderslice P, Xia J, Matta KL, Neelamegham S. Affinity and kinetics of sialyl Lewis-X and core-2 based oligosaccharides binding to L- and P-selectin. Biochemistry 2005; 44(27):9507-19; PMID:15996105; https://doi.org/ 10.1021/bi0507130 [DOI] [PubMed] [Google Scholar]
- [20].Sarkar D, Spencer JA, Phillips JA, Zhao W, Schafer S, Spelke DP, Mortensen LJ, Ruiz JP, Vemula PK, Sridharan R, et al.. Engineered cell homing. Blood 2011; 118(25):e184-91; https://doi.org/ 10.1182/blood-2010-10-311464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lo CY, Antonopoulos A, Dell A, Haslam SM, Lee T, Neelamegham S. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials 2013; 34(33):8213-22; PMID:23891082; https://doi.org/ 10.1016/j.biomaterials.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al.. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell 1993; 75(6):1179-86; PMID:7505206; https://doi.org/ 10.1016/0092-8674(93)90327-M [DOI] [PubMed] [Google Scholar]
- [23].Kim SA, Peacock JS. The use of palmitate-conjugated protein A for coating cells with artificial receptors which facilitate intercellular interactions. J Immunol Methods 1993; 158(1):57-65; PMID:8429217; https://doi.org/ 10.1016/0022-1759(93)90258-9 [DOI] [PubMed] [Google Scholar]
- [24].Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1) homing factor for enigneered regenerative medicine. Expert Opin Biol Ther 2011; 11(2):189-97; PMID:21219236; https://doi.org/ 10.1517/14712598.2011.546338 [DOI] [PubMed] [Google Scholar]
- [25].James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 2012; 92(2):897-965. [DOI] [PubMed] [Google Scholar]
- [26].Kim YJ, Kim DW, Lee S, Kim HJ, Kim YL, Hwang JY, Oh IH, Park YH, Lee YK, Min CK, et al.. Comprehensive comparison of FISH, RT-PCR, and RQ-PCR for monitoring the BCR-ABL gene after hematopoietic stem cell transplantation in CML. Eur J Haematol 2002; 68(5):272-80; PMID:12144533; https://doi.org/ 10.1034/j.1600-0609.2002.00671.x [DOI] [PubMed] [Google Scholar]
- [27].Roca M, de Vries EF, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (111)In-oxine. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging 2010; 37(4):835-41; https://doi.org/ 10.1007/s00259-010-1393-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wolfs E, Struys T, Notelaers T, Roberts S.J, Sohni A, Bormans G, Van Laere K, Luyten FP, Gheysens O, Lambrichts I, et al.. 18F-FDG labeling of mesenchymal stem cells and multipotent adult progenitor cells for PET imaging: effects on ultrastructure and differentiation capacity. J Nucl Med 2013; 54(3):447-54; PMID:23353687; https://doi.org/ 10.2967/jnumed.112.108316 [DOI] [PubMed] [Google Scholar]
- [29].Barczewska M, Wojtkiewicz J, Habich A, Janowski M, Adamiak Z, Holak P, Matyjasik H, Bulte JW, Maksymowicz W, Walczak P. MR monitoring of minimally invasive delivery of mesenchymal stem cells into the porcine intervertebral disc. PLoS One 2013; 8(9):e74658; PMID:24058619; https://doi.org/ 10.1371/journal.pone.0074658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Heyn C, Bowen CV, Rutt BK, Foster PJ. Detection threshold of single SPIO-labeled cells with FIESTA. Magn Reson Med 2005; 53(2):312-20; PMID:15678551; https://doi.org/ 10.1002/mrm.20356 [DOI] [PubMed] [Google Scholar]
- [31].Parashurama N, Lobo NA, Ito K, Mosley AR, Habte FG, Zabala M, Smith BR, Lam J, Weissman IL, Clarke MF, et al.. Remodeling of endogenous mammary epithelium by breast cancer stem cells. Stem Cells 2012; 30(10):2114-27; https://doi.org/ 10.1002/stem.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kotsuma M, Parashurama, N, Smith BR, Wo BR, Ito K, Gambhir SS. Nondestructive, serial in vivo imaging of a tissue-flap using a tissue adhsion barrier. Intravital 2012; 1(1):69-76; https://doi.org/ 10.4161/intv.21769 [DOI] [Google Scholar]
- [33].Willmann JK, Paulmurugan R, Rodriguez-Porcel M, Stein W, Brinton TJ, Connolly AJ, Nielsen CH, Lutz AM, Lyons J, Ikeno F, et al.. Imaging gene expression in human mesenchymal stem cells: from small to large animals. Radiology 2009; 252(1):117-27; PMID:19366903; https://doi.org/ 10.1148/radiol.2513081616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schulman IH, Hare JM. Key developments in stem cell therapy in cardiology. Regen Med 2012; 7(6 Suppl):17-24; PMID:23346572; https://doi.org/ 10.2217/rme.12.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lien CY, Chih‐Yuan Ho K, Lee OK, Blunn GW, .Su Y. Restoration of Bone Mass and Strength in Glucocorticoid‐Treated Mice by Systemic Transplantation of CXCR4 and Cbfa‐1 Co‐Expressing Mesenchymal Stem Cells. J Bone Miner Res 2009; 24(5):837-48; PMID:19113920; https://doi.org/ 10.1359/jbmr.081257 [DOI] [PubMed] [Google Scholar]
- [36].Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res 2010; 106(11):1753-62; PMID:20378860; https://doi.org/ 10.1161/CIRCRESAHA.109.196030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 2008; 16(3):571-9; https://doi.org/ 10.1038/sj.mt.6300374 [DOI] [PubMed] [Google Scholar]
- [38].Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW, Walczak P. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab 2013; 33(6):921-7; PMID:23486296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis JE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther 2010; 18(7):1365-72; https://doi.org/ 10.1038/mt.2010.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lum LG, Fok H, Sievers R, Abedi M, Quesenberry PJ, Lee RJ. Targeting of Lin− Sca+ hematopoietic stem cells with bispecific antibodies to injured myocardium. Blood Cells Mol Dis 2004; 32(1):82-7; PMID:14757418 [DOI] [PubMed] [Google Scholar]
- [41].Lee RJ, Fang Q, Davol PA, Gu Y, Sievers RE, Grabert RC, Gall JM, Tsang E, Yee MS, Fok H. Antibody targeting of stem cells to infarcted myocardium. Stem Cells 2007; 25(3):712-7; PMID:17138964; https://doi.org/ 10.1634/stemcells.2005-0602 [DOI] [PubMed] [Google Scholar]
- [42].Kean TJ, Duesler L, Young RG, Dadabayev A, Olenyik A, Penn M, Wagner J, Fink DJ, Caplan AI, Dennis JE. Development of a peptide-targeted, myocardial ischemia-homing, mesenchymal stem cell. J Drug Target 2012; 20(1):23-32; PMID:22047107; https://doi.org/ 10.3109/1061186X.2011.622398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Elster JL, Rathbone CR, Liu Z, Liu X, Barrett HH, Rhoads RP, Allen RE. Skeletal muscle satellite cell migration to injured tissue measured with 111In-oxine and high-resolution SPECT imaging. J Muscle Res Cell Motil 2013; 34(5-6):417-27; https://doi.org/ 10.1007/s10974-013-9368-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chin BB, Nakamoto Y, Bulte JW, Pittenger MF, Wahl R, Kraitchman DL. 111In oxine labelled mesenchymal stem cell SPECT after intravenous administration in myocardial infarction. Nucl Med Commun 2003; 24(11):1149-54; PMID:14569169; https://doi.org/ 10.1097/00006231-200311000-00005 [DOI] [PubMed] [Google Scholar]
- [45].Doyle B, Kemp BJ, Chareonthaitawee P, Reed C, Schmeckpeper J, Sorajja P, Russell S, Araoz P, Riederer SJ, Caplice NM. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med 2007; 48(10):1708-14; https://doi.org/ 10.2967/jnumed.107.042838 [DOI] [PubMed] [Google Scholar]
- [46].Tai J.H., Foster P, Rosales A, Feng B, Hasilo C, Martinez V, Ramadan S, Snir J, Melling CW, Dhanvantari S, et al.. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes 2006; 55(11):2931-8; https://doi.org/ 10.2337/db06-0393 [DOI] [PubMed] [Google Scholar]
- [47].Cao F., Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla S.J, Connolly A.J, Chen X, et al.. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation 2006; 113(7):1005-14; PMID:16476845; https://doi.org/ 10.1161/CIRCULATIONAHA.105.588954 [DOI] [PMC free article] [PubMed] [Google Scholar]


