ABSTRACT
As the second most common gynecologic malignant tumors with a high mortality rate, cervical cancer jeopardizes women's life worldwide. The low cure rate in cervical cancer patients is mainly attributed to the lack of effective therapies. One feasible novel strategy is to develop immune-based approaches such as adoptive cell immunotherapy of DCCIKs which represents a promising nontoxic antineoplastic immunotherapy preferred in clinic practice. However, the therapeutic effect is not as efficient as anticipated. Possible explanations are tumors exploit immunoregulatory check-points such as programmed death 1(PD1)/PDL1 which provides tumor cells an escape strategy of circumventing immunologic rejection from immune surveillance by hampering activated tumor-specific T cell activities and rendering them functionally exhausted. With reduced transformation activity and enhanced antigenicity, a modified HPV16 E7 (HPV16mE7) was used to load DCs with silenced SOCS1 mediated by a recombinant adenovirus to improve the targetability and efficiency against cervical cancer. Combined with anti-PDL1 antibody MPDL3280A therapy, the co-cultured DCCIKs were transfused into murine models bearing tumor of HPV16 E6/E7 expressing CaSki cells for in vitro/in vivo antitumor activity assay. Although all of the animals succumbed to CaSki tumors even after adoptive DCCIKs transfer or MPDL3280A immunotherapy, the infusion of PDL1 blocking monoclonal antibody with activated T cells cured 40% of animals. These data support PDL1 blockade improves the efficacy of adoptive DCCIKs therapy, providing a new approach of immunotherapy against cervical cancer.
KEYSWORDS: cervical cancer, DCCIKs, MPDL3280A, PDL1, SOCS1s
Introduction
ervical cancer is the second most common gynecologic malignant tumors. Human papillomavirus (HPV) infection has been identified responsible for nearly all cervical cancer cases by molecular, clinical and epidemiological studies.1,2 Associated with oncogenesis cause of 70% cervical cancer cases, HPV16 and HPV18 are considered as “high risk” (HR) viruses.3,4 As the 2 E6 and E7 oncoproteins expressed by HPV16 and HPV18 are essential in the transformation and maintenance of the malignant phenotype, these 2 oncoproteins turn into targets of various immunotherapeutic approaches such as adoptive cellular immunotherapy of dendritic cells (DCs), cytokine-induced killer cells (CIKs), tumor-infiltrating lymphocytes (TILs), and cytotoxic T lymphocytes (CTLs).5,6
Characterized with a MHC-unrestricted tumoricidal activity and a mixed T-NK phenotype, cytokine-induced killer cells (CIKs) are a heterogeneous subset of ex vivo expanded T lymphocytes. When co-cultured with DCs which can generate antigen-specific immune responses, the activated CIKs (DCCIKs) acquire the advantages of high cytotoxicity and a broad tumor-killing spectrum.7,8 However, in clinical practice, the therapeutic activity of DCCIKs is not as efficient as anticipated, mainly attributed to the tumor-induced immuno-suppressive factors which limit the therapeutic potential in tumor microenvironment. The functional integrity of tumor specific T cells was paralyzed by a variety of countermeasures including the expression of ligands such as PDL1 and CTLA4 for the inhibitory receptors on T cells.9,10
Identified previously to inhibit T cell receptor (TCR) signaling by recruiting the SHP-2 phosphatases which decrease CD3ξ and Zap-70 phosphorylation and introduce a TCR stop signal that limits T cell interactions with DCs, the PD1/PDL1 interaction induces T cell anergy and exhaustion manifested by the loss of cytolytic activity, proliferative capacity, cytokine secretion such as INF-γ, IL-2 and TNF-α, and finally T cell apoptosis within the tumor microenvironment.11-14
In most human cancers such as renal, pancreatic, gastric, ovarian, breast, myeloma and esophageal carcinomas, PDL1 is found expressed on cell surface, which was identified as poor clinical prognosis.15-24 Moreover, PD1 or PDL1 blockade by monoclonal antibodies such as MPDL3280A which was modified in the Fc domain for eliminating antibody-dependent cellular cytotoxicity to prevent the depletion of T cells can restore the function of T cells and improve the tumorcidal efficacy of adoptively transferred T cells in vivo.25-29
Overall, the immune checkpoint inhibitors targeting PD1/PDL1 have shown unprecedented rates of durable clinical responses, with an activity range from 10% to 45% in the context of unselected populations affected by advanced solid tumors.27-31
As an inducible negative inhibitor of the JAk/STAT signal pathway,32 suppressor of cytokine signaling 1 (SOCS1) exerts potent immunosuppressive effect on blocking the activation of constitutive immune response.33 Silencing SOCS1 in DCs irritates the maturation of DCs and triggers an allogeneic T-cell expansion.34-36
As activated (mature) and antigen-loaded DCs ex vivo cultured can induce the differentiation of antigen-specific T cells into effector T cells. Therefore, in this research, a modified HPV16 E7 (HPV16mE7) with reduced transformation activity and enhanced antigenicity was employed to load DCs with silenced SOCS1 mediated by Ad-shSOCS1. The HPV16mE7-pulesed and SOCS1 silenced DCs were co-cultured with CIKs generated from PBMCs. The generated DCCIKs were transfused into mouse models bearing tumor of viral HPV16E6E7 oncoproteins expressing CaSki cells. Then, anti-PDL1 mono-antibody MPDL-3280A was administered i.v. simultaneously. The tumor volume and the survival days from different treatment groups were investigated to evaluate the therapeutic efficacy of the DCCIKs in combination with MPDL-3280A.
Materials and methods
Cells and reagents
CaSki and HEK293 cell lines were purchased from American Type Culture Collection (ATCC). Cells were maintained in the MEM or RPMI-1640 culture media (Gibco, Life Technologies, US) supplemented with 10% (v/v) fetal bovine serum (FBS) (HyClone Laboratories, US). PBMCs were prepared from healthy donors whose informed consent was obtained in accordance with the study protocols approved by the Institutional Review Board of the Hospital Authority of Tongji Medical College. Anti-CD3 antibody, rhIL-2, rhIL-4, rhTNF-γ, TNF-α, and rhGM-CSF were acquired from Peprotech Inc. Normal human AB serum were purchased from Gibco, Life Technologies (USA). Normal human IgG and PE-conjugated PD-L1 monoclonal antibody were purchased from R&D Systems (USA). Mouse anti-human CD80-PE monoclonal antibody, mouse antihumanCD40-FITC monoclonal antibody, mouse anti-human CD83-PE monoclonal antibody, mouseanti-human CD86-PE monoclonal antibody, and mouse antihumanCD1a-FITC monoclonal antibody were productsof Santa Cruz Co, Ltd. Lymphocyte separation medium Ficoll was sourced from GE Healthcare (USA). Female BALB/c mice weighing 16 to 22 g and 6 to 8 weeks of age raised under SPF circumstance were purchased from the Guangzhou Traditional Chinese Medicine University. All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
Preparation of DCs and CIKs
Separated from 70mL peripheral blood sterilely collected from a healthy adult volunteer by using Ficoll-Paque, PBMCs were cultured in RPMI-1640 at 1 × 106 /mL supplemented with 10% (V/V) autologous serum. The obtained cells were subsequently transferred into culture flasks and cultured for 2 h. Collected as the progenitor of CIKs, Nonadherent cells were resuspended at 1 × 106 cells/mL in RPMI-1640 medium supplemented with 10% human AB serum and cultured with rhIFN-γ (1000 UmL) and rhIL-2 (500 U/mL) as well as anti-CD3 antibody (10 μg/mL) immobilized in culture flask. Half the amount of medium supplemented with rhIL-2 and rhIFN-γ was exchanged every 3 d. The remaining adherent cells were cultured in RPMI-1640 medium supplemented with 40 ng/mL rhGM-CSF and 40 ng/mL rhIL-4. The supernatant was replaced with fresh medium containing rhGM-CSF and rhIL-4 every 3 d. All cultures were incubated at 37 °C in 5% humidified CO2. After 7 d of culture, >80 % of the cells expressed characteristic DC-specific markers demonstrated by flow cytometric analysis. Then, the DCs were exposed to Ad-shSOCS1 at 100 MOI for 8 h. The transducted cells were washed with PBS and pulsed with the HPVm16E7 protein at 25 µg/mL in fresh culture medium for 6 hrs (The recombinant adenovirus Ad-shSOCS1 and HPVm16E7 were prepared as we previously reported in36), followed by stimulation with TNF-α (10 ng/mL) for 24 h to develop mature DCs. The finally matured DCs were co-cultured with CIKs at a ratio of 10:1 in CIK medium for 3 d to generate DCCIKs.
In vitro antitumor activity analysis
The in vitro cytotoxicity of the DCCIKs used as effector cells in the absence or presence of 5 µg/mL MPDL3280A against CaSki cells employed as target cells at a ratio of 10:1, 30:1 and 90:1 was determined using a CCK8 kit, (Dojindo Molecular Technologies, Inc., Kumamoto, Japan). The effector and target cells were added to 96-well plates and incubated for 24 h. The groups comprising a mixture of cell types were the experimental groups, whereas the control groups contained only one cell type of the CaSki cells, DCCIKs or 1640 RPMI cultivating solution. The CCK8 assay was performed in triplicate and optical density (OD) was read at 570 nm. The cytotoxic activity was calculated as follows:
In vivo antitumor assay
Xenografted tumor mouse models were established by subcutaneous inoculation of 1 × 106 CaSki cells into the right flank of female BALB/c mice. At day 7, all mice had palpable tumors approximately 100 mm3 were assigned to 4 groups: normal human IgG served as control; DCCIKs; MPDL3280A (0.1 mg in 0.1mL PBS administered intravenously); and DCCIKs in combination with 0.1mg MPDL3280A in 0.1mL PBS administered i.v., 10 mice in each group. And therapeutic treatments were initiated respectively. Adoptive cell immunotherapy consisted of 2 intravenous transfusion of DCCIKs at d 7 and d 14 (1 × 107 cells per dose in a total volume of 0.1 mL in the presence of o.1 mg control normal human IgG or MPDL3280A, respectively). For the treatment with Ab, 0.1mg MPDL3280A or normal human IgG as a control was i.v. injected at the same time as adoptive cell immunotherapy was administered. The tumor volumes were recorded daily. And the tumor volume was calculated by the following formula: (major axis × minor axis2) × 0.52. When the tumor volume reached ∼4000 mm3, the tumor-bearing mice were euthanized to avoid un-necessary pain and recorded as dead.
Statistical analysis
The Kaplan-Meier (log-rank test) estimator was used to generate survival curves for the treated animals in different groups. Data of the tumor volume after inoculation of CaSki cells were analyzed using Student's t-test.
Results
Morphology observation of DCs and CIKs
Under microscop, DCs in irregular shape presented mature characteristics of larger soma with plenty of dendritic long and small bulges on their surfaces after stimulated with TNF-α (Fig. 1). The expression levels of all antigens were significantly increased by 2–4 folds during maturation in Ad-shSOCS1 group with respect to control (Table 1). The expression levels of CD1a, CD80 and CD83 increased about 10% in Ad-shSOCS1 group than TNF-α group. CIK cells demonstrated clustered growth. Masses of CIKs gradually multiplied and became larger after 3 d incubation.
Figure 1.
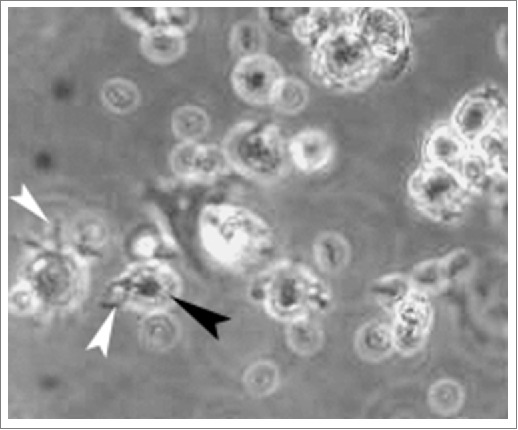
The morphology of mature DCs After stimulated with TNF-α, HPVm16E7-pulsed DCs in irregular shape showed mature characteristics of larger soma with plenty of dendritic long and small bulges on their surfaces (400 × magnification). White arrow indicates long bulge and black arrow indicates small bulge.
Table 1.
Percentage of DCs expressing CD1 a, CD80, CD83, CD86, HLA-DR.
| CD1a | CD80 | CD83 | CD86 | HLA-DR | |
|---|---|---|---|---|---|
| Control | 24.2 ± 3.6 | 33.2 ± 6.7 | 36.9 ± 4.3 | 45.5 ± 2.3 | 59.6 ± 4.7 |
| TNF-α | 83.5 ± 4.6a | 73.4 ± 7.4a | 78.8 ± 7.2a | 86.3 ± 5.4a | 91.2 ± 4.2a |
| Ad-shSOCS1-DCs | 92.8 ± 4.2a,c | 88.6 ± 6.2a,c | 93.3 ± 3.8a,c | 93.6 ± 3.7a,b | 92.6 ± 3.4a,b |
Immature DCs were stimulated by Ad-shSOCS1. Percent of DCs expressing CD1a, CD80, CD83, CD86 and HLA-DR were detected using flow cytometry before and after 24 h TNF-α stimulation and analyzed by SPSS one-way ANOVA analysis.
Significant differences compared with that of the DCs control, p < 0.001;
no significant differences compared with that of the TNF-α control, p > 0.05;
significant differences compared with that of the TNF-α control or, p < 0.05.
In vitro antitumor activity analysis
The cytotoxicity of effector cells at 10:1, 30:1 and 90:1 E/T ratios against CaSki cells in which about 23.6% express PDL1 was analyzed using CCK 8 assay. It revealed that DCCIKs lymphocytes incubated with MPDL3280A increased the cytotoxic activity with 37.9% at the E:T of 30 in relative to DCCIKs in the absence of MPDL3280A (p < 0 .01, Fig. 2A, B).
Figure 2.
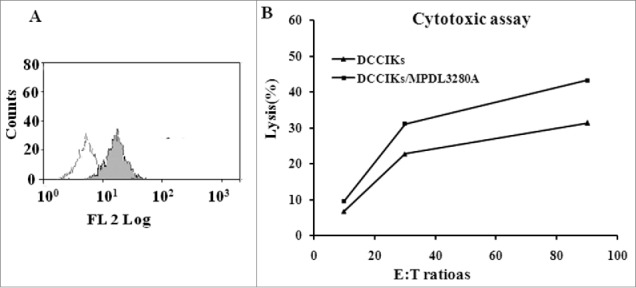
Cytotoxicity assay (A) Detected by flow cytometry, 23.6% CaSki cells expressed PDL1. (B)The cytotoxicity of effector cells at 10:1, 30:1 and 90:1 E/T ratios against CaSki cells was analyzed using CCK 8 assay. When lymphocytes incubated with MPDL3280A, DCCIKs increased the cytotoxic activity against CaSki cells with 37.9% at the E:T of 30 in relative to DCCIKs in the absence of MPDL3280A (p < 0 .01).
In vivo antitumor assay
To determine whether the combination of MPDL3280A with DCCIKs has any improved antitumor efficacy against cervical cancer. Xenografted tumor mouse models were established by subcutaneous inoculation of 1 × 106 constitutively HPV16 E6E7 expressing CaSki cells into the right flank of female BALB/c mice. Transient therapeutic effect of the anti-PDL1 MPDL3280A on the CaSki cells was observed in mice treated with single MPDL3280A. Injection with MPDL3280A after tumor inoculation presented suppressed carcinoma cell growth within 12 d or so, although the tumors progressed rapidly in most of the mice 12 d post treatment of single MPDL3280A (Fig. 3A). DCCIKs demonstrated moderate therapeutic effect on cervical cancer in relative to IgG control in which progressive tumor growth was observed (p < 0 .01). The survival time was prolonged more than 60 d in 30% of the mice treated with DCCIKs in respect to the mice of IgG control group which were all dead in 40 d (Fig. 3B, p < 0.05). On the contrary, combination of MPDL3280A with adoptive DCCIKs transfer significantly inhibits the growth of cervical carcinoma in vivo compared with monotherapy of DCCIKs transfer, MPDL3280A or IgG control (Fig. 3A). The survival time was extended more than 80 d in 40% of the mice treated with the combination of DCCIKs and MPDL3280A (Fig. 3B).The experiments were repeated, producing a significant statistical difference (p < 0 .01).
Figure 3.
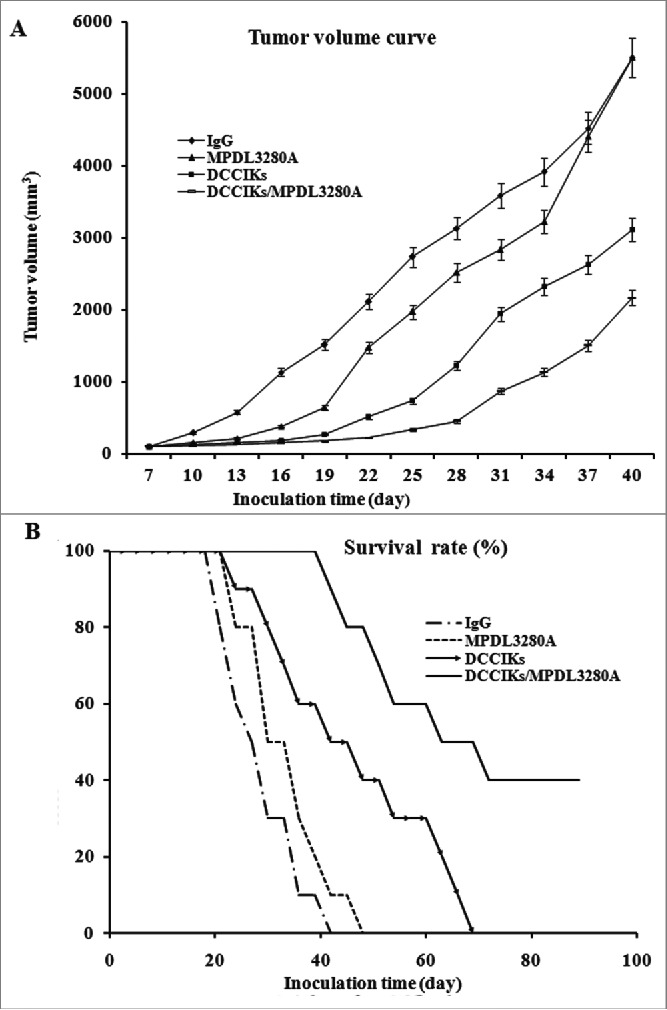
In vivo antitumor activity analysis. (A) The therapeutic effect of DCCIKs in combination with MPDL3280A against mice bearing tumors was investigated in the groups of 10 BALB/C mice received 1×106 CaSki cells. The therapy was initiated with normal human IgG, DCCIKs, MPDL3280A and DCCIKs in combination with MPDL3280A on day 7. Tumor volume and time experiment was analyzed using student's t-test. *p < 0.01(DCCIKs/MPDL3280A vs DCCIKs or MPDL3280A or IgG control); <0.05 (DCCIKs vs MPDL3280A or IgG control) (B) Survival rates were analyzed using the Kaplan-Meier method (log-rank test). The experiments were repeated, producing a significant statistical difference. *p < 0.01(DCCIKs/MPDL3280A vs DCCIKs or MPDL3280A or IgG control); <0.05 (DCCIKs vs MPDL3280A or IgG control).
Discussion
CD3/CD56 surface markers expressing CIKs is a cell population obtained from PBMCs by stimulating with IFN-γ, IL-2, and OKT3.16 As CIKs can both directly suppress carcinoma cells and regulate the body immune system against tumor cells indirectly, it can restrain tumor growth and recurrence by immediate cytotoxicity and improving the patients' immunity which results in a long-term effect. At the same time, as the antigen-presenting DCs are able to induce robust cell-mediated immunity capable of attacking and eliminating abnormal antigen-bearing cells, activated CIKs by tumor antigen-pulsed DCs can both exert non-MHC restrictive cytotoxicity and MHC restrictive cytotoxicity reinforced by antigen-pulsed DCs on targeted tumor cells. Furthermore, when SOCS1 was silenced, HPV16mE7-pulsed DCs showed enhanced efficacy against cervical cancer.36 In this study, although the co-cultured DCCIKs hardly ever eradicate the tumor completely, it demonstrated moderate activity in inhibiting tumor growth and improved survival rate (30%) longer than 60 d in xenografted mice.
The generated effector T cells are anticipated to recognize and execute the tumor targets. However, in clinic practice, adoptive cell immunotherapy presents less therapeutic efficiency than anticipated, mainly attributed to that tumors employ multiple strategies to attenuate the efficacy of T cell-mediated attack through interfering with nearly every step requisite for effective immunity. The progression of HPV infection to cervical cancer is a slow process. Under continuous immunological pressure in development and progression, the tumor evolves several mechanisms to escape immune surveillance. One of the strategies is to express inhibitory coreceptors such as CTLA4 and PDL1, under normal circumstances, which protect cells from immune-mediated harm, whereas induced by tumors to predominately paralyze the activated effector T lymphocytes via phosphatase activity and other signaling pathways within the tumor microenvironment.11-14
Expressed in a variety of malignancies, PDL1 seems to be associated with worse prognosis in cancer of colorectal, breast ovarian and esophageal,15-24 while with good prognosis in cervical cancer with improved survival via incapacitation of the infiltrating PD1+ regulatory T cells (Tregs) through PD1/PDL1 interactions.23 The reason for the low expression in cervical cancer may be attributed to patients severe immune defects. Reported by Herbst RS, most progressing patients with MPDL3280A on-treatment biopsies showed a lack of PDL1 upregulation by either tumor cells or tumor infiltrating immune cells. These growing tumors displayed one of 3 patterns: (1) immunological ignorance: little or no tumor-infiltrating immune cell infiltration; (2) non-functional immune response: presence of an intra-tumoral immune infiltrate with minimal to no expression of PDL1; (3) excluded infiltration of tumor-infiltrating immune cells. And chip analysis of samples from these non-responders failed to provide evidence of activated T cells.25
Given the severe cellular immune function defects of cancer patients, improving the cellular immunity functions is essential to improve their survival rate as well as the inhibition of tumor recurrence and metastasis. And the therapeutic effect of adoptive cell immunotherapy has been proven in recent years in clinic. In order to further enhance the efficacy of the transferred adoptive cells, circumventing the inhibition induced by tumors is a promising strategy, given that PLD1/PD1 blockade has been confirmed in the treatment of advanced melanoma, non-small cell lung cancer (NSCLC) and genitourinary cancer.
As no clinic trials of PDL1/PD1 blockade for cervical cancer have been reported, therefore, in this study we intend to evaluate whether MPDL3280A as a single agent or MPDL3280A in combination with adoptive cell immunotherapy (DCCIKs) could exert better therapeutic effect on cervical cancer. Although MPDL3280A as a single agent restrained the tumor volume significantly within the initial 12 days, the tumor expanded rapidly after the suppression in the xenografted tumor mouse models. The transient therapeutic effect may be attributed to less T cell immunogenicity because of the immunodeficiency condition of BALB/c mice, which is similar to the immune status in cervical cancer patients. CIKs activated by HPV16mE7 pulsed and SOCS1 silenced DCs demonstrated moderate antitumor effect. On the contrary, MPDL3280A in combination with DCCIKs presented potent antitumor efficacy. The survival time of 40 % mice were prolonged than 80 d. In conclusion, combining PD1/PDL1 signal pathway blockade with adoptive cell immunotherapy presents a promising prospect against cervical cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The authors are grateful for the National Natural Science Fund (No.81572431), the Brainstorm Project on Science and Technology by Department of Science and Technology of Anhui Province(No.1604a0802094), the National Natural Science Foundation of China (No.81170564), the Knowledge Innovation Program of Shenzhen Innovation Committee (No. JCYJ20150402095058890), and the shenzhen Venture Funding (No.CYZZ20140509142642226).
Reference
- [1].Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121:621-32; PMID:17405118; https://doi.org/ 10.1002/ijc.22527 [DOI] [PubMed] [Google Scholar]
- [2].Walboomers JM. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12-9; PMID:10451482; https://doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- [3].Kanodia S, Da Silva DM, Kast WM. Recent advances in strategies for immunotherapy of human papillomavirus-induced lesions. Int J Cancer 2008; 122:247-259; PMID:17973257; https://doi.org/ 10.1002/ijc.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Psyrri A, Daniel D. Human papillomavirus in cervical and head-and-neck cancer. Nat Clin Pract Oncol 2008; 5:24-31; PMID:18097454; https://doi.org/ 10.1038/ncponc0984 [DOI] [PubMed] [Google Scholar]
- [5].Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015; 348:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; https://doi.org/ 10.1038/nrc3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch dendritic cell-based interventions for cancer therapy. OncoImmunology 2012; 1:1111-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Adoptive cell transfer immunotherapy. OncoImmunology 2012; 1:306-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Momtaz P, Postow MA. Immunologic checkpoints in cancer therapy: focus on the programmed death-1 (PD-1) receptor pathway. Pharmacogenomics and Personalized Medicine 2014; 7:357-65; PMID:25484597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, et al.. The PDL1-PD1 axis converts human Th1 cells into regulatory T Cells. Sci Transl Med 2011; 3:111-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004; 173:945-54; PMID:15240681; https://doi.org/ 10.4049/jimmunol.173.2.945 [DOI] [PubMed] [Google Scholar]
- [12].Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009; 10:1185-92; PMID:19783989; https://doi.org/ 10.1038/ni.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol 2009; 21:1065-77; PMID:19651643; https://doi.org/ 10.1093/intimm/dxp072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 2009; 15:517-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Badoual C, Combe P, Gey A, Granier C, Roussel H, De Guillebon E, Oudard S, Tartour E. PD-1 and PDL-1 expression in cancer: significance and prognostic value. Med Sci (Paris) 2013; 29:570-2. [DOI] [PubMed] [Google Scholar]
- [16].Droeser RA, Hirt C, Viehl CT. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013; 49:2233-42; PMID:23478000; https://doi.org/ 10.1016/j.ejca.2013.02.015 [DOI] [PubMed] [Google Scholar]
- [17].Fullár A, Kovalszky I, Bitsche M, Romani A. Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma. Exp Cell Res 2012; 318:1517-27; https://doi.org/ 10.1016/j.yexcr.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ghebeh H, Mohammed S, Al-Omair A. The B7-1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006; 8:190-8; PMID:16611412; https://doi.org/ 10.1593/neo.05733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hamanishi J, Mandai M, Iwasaki M. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 2007; 104:3360-5; PMID:17360651; https://doi.org/ 10.1073/pnas.0611533104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ohigashi Y, Sho M, Yamada Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005; 11:2947-53; PMID:15837746; https://doi.org/ 10.1158/1078-0432.CCR-04-1469 [DOI] [PubMed] [Google Scholar]
- [21].Thompson RH, Kuntz SM, Leibovich BC. Tumor B7-1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow- up. Cancer Res 2006; 66:3381-5; PMID:16585157; https://doi.org/ 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- [22].Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006; 108:19-24; PMID:16530813; https://doi.org/ 10.1016/j.acthis.2006.01.003 [DOI] [PubMed] [Google Scholar]
- [23].Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009; 15:6341-7; PMID:19825956; https://doi.org/ 10.1158/1078-0432.CCR-09-1652 [DOI] [PubMed] [Google Scholar]
- [24].Geng WP, Xu M, Wang YQ. An investigation of PDL1, PDL2 and B7-H4 expressions in human cervical cancer and their significance. J of Chongqing medical university 2011; 36:1186-9. [Google Scholar]
- [25].Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al.. Predictive correlates of response to the anti-PDL1 antibody MPDL3280A in cancer patients. Nature 2014; 27:563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Strome SE, Dong H, Tamura H. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res 2003; 63:6501-5; PMID:14559843 [PubMed] [Google Scholar]
- [27].Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al.. Fine. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 27:558-62; https://doi.org/ 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- [28].Yoon SH. Immunotherapy for non-small cell lung cancer. Tuberc Respir Dis 2014; 77:111-5; https://doi.org/ 10.4046/trd.2014.77.3.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy to prevent immune decline in ovarian cancer. Cancer Res 2013; 73:6900-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32:1020-30; PMID:24590637; https://doi.org/ 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wolchok JD, Kluger H, Callahan MK, Postow MA. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; https://doi.org/ 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Albert ML, Bhardwaj N. Resurrecting the dead: DCs cross-present antigen derived from apoptotic cells on MHCI. Immunologist 1998; 6:194-8. [Google Scholar]
- [33].Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol 2003; 4:1169-76; PMID:14639467; https://doi.org/ 10.1038/ni1012 [DOI] [PubMed] [Google Scholar]
- [34].Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity 2003; 19:437-50; PMID:14499118; https://doi.org/ 10.1016/S1074-7613(03)00240-1 [DOI] [PubMed] [Google Scholar]
- [35].Guenterberg KD, Lesinski GB, Mundy-Bosse BL, Karpa VI, Jaime-Ramirez AC, Wei L, Carson WE 3rd. Enhanced anti-tumor activity of interferon-α in SOCS1-defificient mice is mediated by CD4+ and CD8+ T cells. Cancer Immunol Immun 2011; 60:1281-8; https://doi.org/ 10.1007/s00262-011-1034-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhu Y, Zheng Y, Mei L, Liu M, Li S, Xiao H, Zhu H, Wu S, Chen H, Huang L. Enhanced immunotherapeutic effect of modified HPV16 E7-pulsed dendritic cell vaccine by an adeno-shRNA-SOCS1 virus. Int J Oncol 2013; 43:1151-9; PMID:23877655 [DOI] [PubMed] [Google Scholar]


