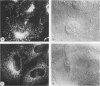
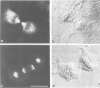
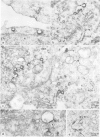
Abstract
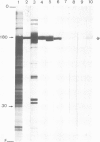
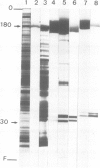
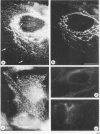
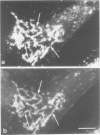
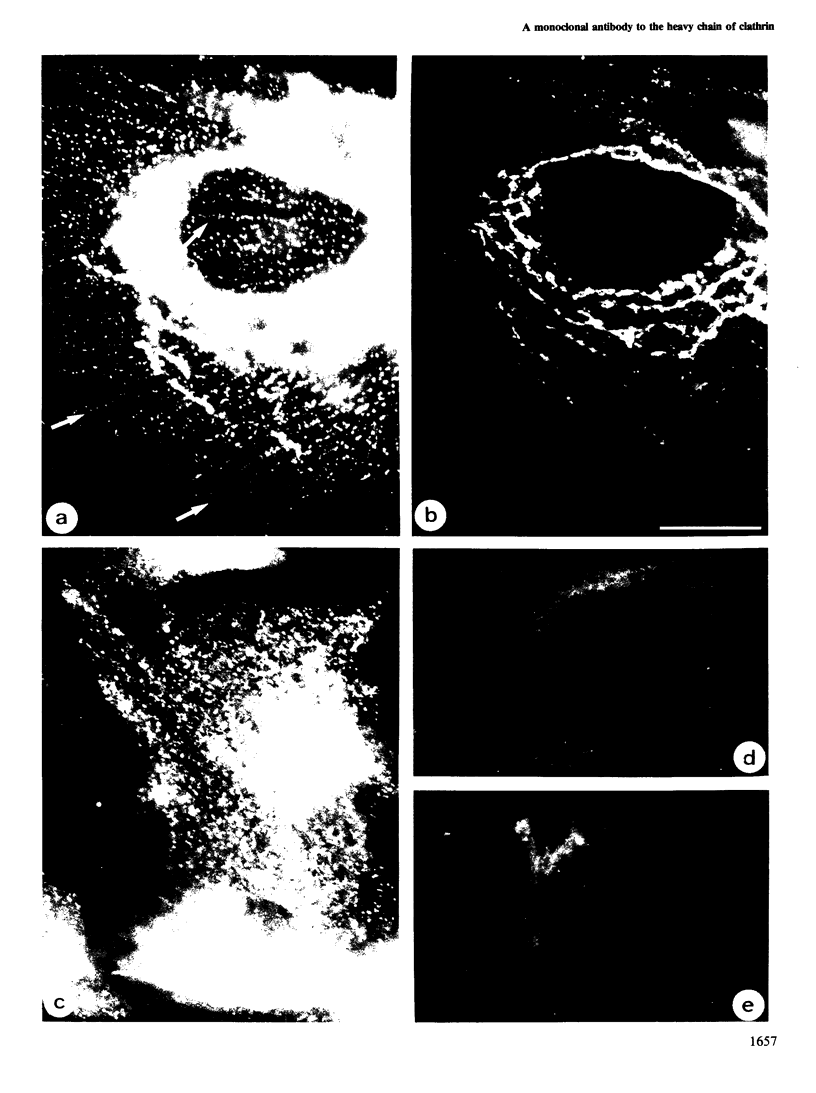
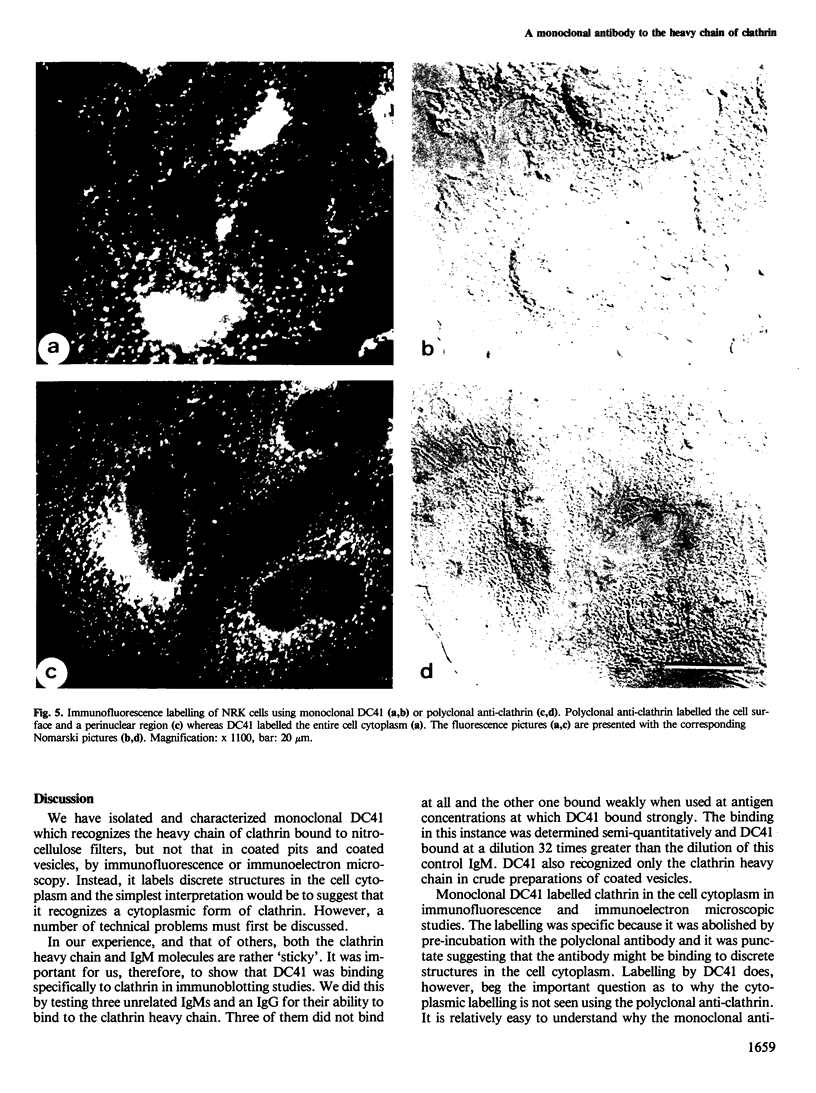
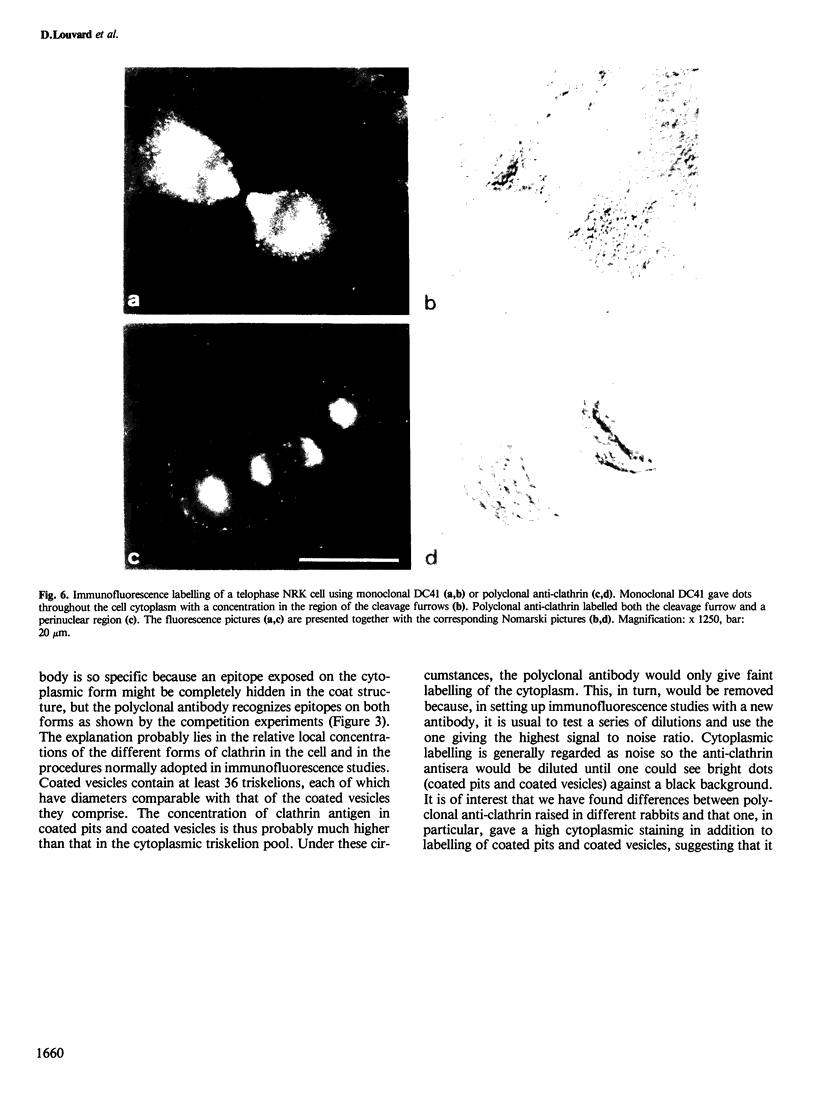
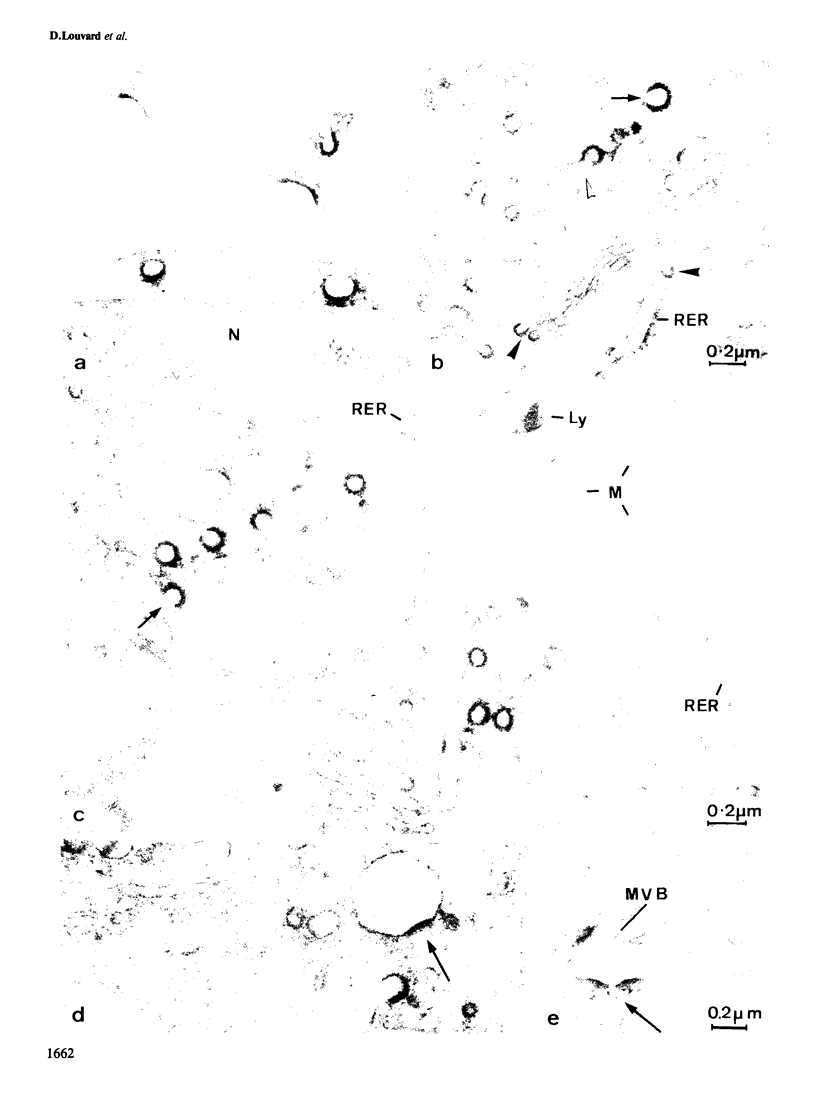
Monoclonal antibodies have been raised to pig brain triskelions and one clone, DC41, was found to recognize the clathrin heavy chain by immunoblotting. However, both by immunofluorescence and immunoelectron microscopy, and in complete contrast to polyclonal anti-clathrin antibodies, monoclonal DC41 did not label either coated pits or coated vesicles anywhere in the cell. Instead it appeared to label the cell cytoplasm. These data suggest that DC41 recognizes a cytoplasmic form of clathrin, perhaps that form produced by uncoating of coated vesicles which is then ready to re-build another coated pit.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blitz A. L., Fine R. E., Toselli P. A. Evidence that coated vesicles isolated from brain are calcium-sequestering organelles resembling sarcoplasmic reticulum. J Cell Biol. 1977 Oct;75(1):135–147. doi: 10.1083/jcb.75.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Griffiths G., Reggio H., Louvard D., Warren G. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1982;1(12):1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Tooze J., Warren G. A monoclonal antibody which recognises each of the nuclear lamin polypeptides in mammalian cells. EMBO J. 1983;2(3):361–367. doi: 10.1002/j.1460-2075.1983.tb01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Immunolocalization of the 110,000 molecular weight cytoskeletal protein of intestinal microvilli. J Mol Biol. 1981 Oct 15;152(1):49–66. doi: 10.1016/0022-2836(81)90095-4. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol. 1980 Mar;84(3):560–583. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J., Schmid E., Müller H., Franke W. W. Immunological identification and localization of clathrin and coated vesicles in cultured cells and in tissues. Exp Cell Res. 1981 May;133(1):191–211. doi: 10.1016/0014-4827(81)90369-4. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C., Parham P., Brodsky F. M. Location and distribution of the light chains in clathrin trimers. Proc Natl Acad Sci U S A. 1983 May;80(9):2481–2485. doi: 10.1073/pnas.80.9.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Gerhard W., Croce C. M. Production of antibodies against influenza virus by somatic cell hybrids between mouse myeloma and primed spleen cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2985–2988. doi: 10.1073/pnas.74.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer E. J., Schlossman D. M., Rothman J. E. Release of clathrin from coated vesicles dependent upon a nucleoside triphosphate and a cytosol fraction. J Cell Biol. 1982 Apr;93(1):230–236. doi: 10.1083/jcb.93.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tougard C., Picart R., Tixier-Vidal A. Electron-microscopic cytochemical studies on the secretory process in rat prolactin cells in primary culture. Am J Anat. 1980 Aug;158(4):471–490. doi: 10.1002/aja.1001580409. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Branton D. Assembly units of clathrin coats. Nature. 1981 Jan 29;289(5796):420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Ekblom P., Laurila P. Subcellular compartmentalization of saccharide moieties in cultured normal and malignant cells. J Cell Biol. 1980 May;85(2):429–434. doi: 10.1083/jcb.85.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Feramisco J. R., Ash J. F. Fluorescent localization of contractile proteins in tissue culture cells. Methods Enzymol. 1982;85(Pt B):514–562. doi: 10.1016/0076-6879(82)85050-7. [DOI] [PubMed] [Google Scholar]
- Winkler F. K., Stanley K. K. Clathrin heavy chain, light chain interactions. EMBO J. 1983;2(8):1393–1400. doi: 10.1002/j.1460-2075.1983.tb01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]