Key Points
Intravascular ERp72 supports platelet accumulation and fibrin generation through the a and a′ active sites.
ERp72 functions separately from protein disulfide isomerase and ERp57 in supporting platelet aggregation.
Abstract
Several CGHC motif–containing disulfide isomerases support thrombosis. We here report that endoplasmic reticulum protein 72 (ERp72), with 3 CGHC redox-active sites (ao, a, and a′), supports thrombosis. We generated a new conditional knockout mouse model and found that Tie2-Cre/ERp72fl/fl mice with blood and endothelial cells lacking ERp72 had prolonged tail bleeding times and decreased platelet accumulation in laser-induced cremaster arteriole injury and FeCl3-induced mesenteric arterial injury. Fibrin deposition was decreased in the laser injury model. Both platelet and fibrin accumulation defects were fully rescued by infusion of recombinant ERp72 containing functional a and a′ CGHC motifs (ERp72(oo-ss-ss)). Infusion of ERp72 containing inactivated a and a′ CGHC motifs (ERp72(ss-oo-oo)) inhibited platelet accumulation and fibrin deposition in wild-type mice. Infusion of ERp72(oo-ss-ss) into β3-null mice increased fibrin deposition in the absence of platelets. ERp72-null platelets had defective aggregation, JON/A binding, P-selectin expression, and adenosine triphosphate (ATP) secretion. The aggregation and ATP secretion defects were fully rescued by ERp72(oo-ss-ss) but partially rescued by ERp72(ss-oo-ss) and ERp72(ss-ss-oo). Aggregation and ATP secretion of human platelets was potentiated by ERp72(oo-ss-ss) but inhibited by ERp72(ss-oo-ss) and ERp72(ss-ss-oo). These data suggest that both the a and a′ active sites are required for platelet function. ERp72 bound poorly to β3-null mouse platelets, and the addition of ERp72(oo-ss-ss) to human platelets generated thiols in αIIbβ3, suggesting a direct interaction of ERp72 with αIIbβ3. Defective aggregation of ERp72-null platelets was recovered by ERp72, but not other thiol isomerases. In summary, ERp72 plays a critical role in platelet function and coagulation through the a and a′ CGHC motifs.
Introduction
Endoplasmic reticulum protein 72 (ERp72) is a member of a family of enzymes important in disulfide bond formation in the endoplasmic reticulum, of which protein disulfide isomerase (PDI) is the prototypical member.1 Thioredoxin-like domains of these enzymes contain CXYC motifs, which catalyze the formation, reduction, or rearrangement of disulfide bonds. A growing body of data indicates distinct roles of several PDI family members in promoting thrombus formation. Platelets contain most PDI family members, some of which are secreted during platelet activation.2 Using targeted knockout mouse models, we and others have shown that 2 secreted PDI family proteins, PDI and ERp57, play a critical role in platelet accumulation and fibrin generation in vivo.3-6 These observations have greatly expanded our understanding of the physiological roles of this PDI family beyond the traditional functions of these enzymes in the endoplasmic reticulum. Using an inhibitory antibody, ERp5 is a third PDI family member found to have a role in thrombosis.7 Whether additional PDI family members have a role in thrombosis remains a critical unanswered question in understanding the mechanism underlying thrombus formation.
Recent research has begun to clarify why there are so many members of the PDI family in the endoplasmic reticulum of mammalian cells. Some proteins appear to be substrates for only a single member of the PDI family, while others are substrates of multiple members of the PDI family.8 Laminin, a large heavily disulfide-linked protein, forms mixed disulfides with ERp57, ERp46, and ERp18.8 Similarly, newly synthesized thyroglobulin (∼2750 residues containing 122 cysteine residues) is a substrate of ERp72, ERp57, ERp5, and PDI, with each oxidoreductase contributing to oxidative folding in different regions of thyroglobulin.9 PDI, ERp57, and ERp72 act distinctly and coordinately to facilitate polyomavirus infection,10 while ERdj5 (another member of the PDI family) cooperates with PDI for endoplasmic reticulum translocation of the simian virus.11 ERp57 and ERp72 catalyze formation of certain disulfide bonds in bovine pancreatic trypsin inhibitor better than PDI, while PDI is much more efficient at catalyzing a final rate-limiting step.12 ERp72 and PDI cooperated together for maximum folding efficiency. Thus, optimal protein folding involves the complimentary enzymatic activities of multiple members of the PDI family.
ERp72 is one of the largest PDIs and the only PDI family member with 5 thioredoxin-like domains due to an additional N-terminal a° domain to give an a°-a-b-b′-a′ architecture.13 The a°, a, and a′ domains each contain a redox-active CGHC active site. ERp72 contains 2 noncatalytic domains, b and b′, and has an endoplasmic reticulum retention signal (KEEL) at the COOH terminus. While the bb′ domains of ERp72 are similar to ERp57, surface charge differences allow the b′ domain of ERp57, but not ERp72, to bind calnexin.14 While the principal substrate-binding domain of PDI is the hydrophobic b′ domain that facilitates binding of PDI to small peptides and scrambled RNase,1 the b′ domain of ERp72 is not hydrophobic, and ERp72 does not bind to small peptides and scrambled RNase.1,15 Instead, hydrophobic patches in the a°, a, and a′ active site domains of ERp72 appear to mediate substrate binding.14 Differences in substrate binding ability of each enzyme likely contribute to differences in selectivity for specific substrates or specific cysteine residues in a substrate.8,9
While substantial amounts of ERp72 are secreted from platelets upon activation and bind to the platelet surface,2 there are no reports on the role of ERp72 in thrombosis. There is 1 report showing distinct catalytic activities of the 3 active sites in the insulin reductase assay,12 but nothing is known about the relative importance of the active sites of ERp72 in physiologic reactions. Using targeted knockout mice, we document a role for this member of the PDI family in platelet aggregation and platelet accumulation and fibrin deposition in vivo. We found the a and a′, but not the a°, active sites are critical for these functions.
Materials and methods
Materials and the methods for isolation of endothelial cells from mice, RNA extraction, reverse transcription polymerase chain reaction (RT-PCR) and PCR, western blotting, bleeding time analysis, coagulation assays, flow cytometry and aggregation and secretion studies of human and mouse platelets, FeCl3-induced platelet accumulation and thrombosis of the mesenteric artery, and intravital microscopy of laser-induced thrombosis of the cremaster muscle arterioles are all previously described.6
The detailed methods for these techniques and assays are included with minor revisions in supplemental Methods (available on the Blood Web site). All experiments with mice were performed in accordance with Temple University and Soochow University institutional guidelines and approval of the animal care committees.
Methods for the generation and characterization of tissue-specific ERp72-deficient mice, labeling of thiols in αIIbβ3 with biotin-HPDP and detection by mass spectrometry, and statistics are detailed in supplemental Methods.
Generation of recombinant human ERp72 protein and catalytically inactive a°, a, and a′ active sites
Human ERp72 (UniProtKB: P13667) was expressed in Escherichia coli strain BL21 (DE3) pLysS (Promega), respectively, and purified on an Ni Sepharose High Performance column (GE Healthcare). The different mutant recombinant ERp72 enzymes were generated by GenScript, with cysteine residues of 1 active site mutated to serine residues representing the Cys/SH (s) change to Ser/OH (o). The mutant proteins were C91S and C94S at the N-terminal a° domain active site designated ERp72(oo-ss-ss); C206S and C209S at the a domain active site, designated ERp72(ss-oo-ss); and C555S and C558S at the a′ domain active site, designated ERp72(ss-ss-oo). Recombinant wild-type ERp72, designated ERp72(ss-ss-ss); inactivated a plus a′ domain active sites (C206S, C209S; C555S and C558S), designated ERp72(ss-oo-oo); and inactivated a° plus a domain active sites, designated ERp72(oo-oo-ss) were also generated. DNA sequencing confirmed the correct base substitutions. The purified proteins were run on 4% to 20% SDS-PAGE gels and the purity was over 85% to 90% by Coomassie staining (see supplemental Methods and supplemental Figure 14).
Labeling of αIIbβ3 on platelets with 3-(N-maleimidylpropionyl)biocytin
We have previously characterized labeling of thiols in αIIbβ3 with 3-(N-maleimidylpropionyl) biocytin (MPB),16-19 documenting optimal labeling conditions.17,18 We confirmed specificity of labeling for thiols18 and confirmed labeling in both αIIb and β3 using immunoprecipitation and mass spectrometry17,18 (the major proteins labeled on platelets were GPIbα, αIIb, β3, and actin).18 In the current studies, MPB (100 μM) was added to washed nonactivated platelets or platelets incubated with varying amounts of ERp72(oo-ss-ss), ERp72(ss-oo-oo), or dithiothreitol (2.5 μM) and labeling detected by blotting with avidin.18
Results
Generation of conditional ERp72-knockout mice
To determine the role of intravascular ERp72 in platelet function and thrombus formation, we generated conditional ERp72-knockout mice (supplemental Figure 1). The conditional knockout alleles were produced by introducing loxP sites flanking the ERp72 gene encoding exon 2 using the cloned stem cells (supplemental Figure 1A). The genotyping PCR using primers 1F and 1R yielded the expected 289-bp product for the first FRT site, while the genotyping PCR using primers 2F and 2R produced the predicted 356-bp product for second loxP site and the corresponding 300-bp product from the wild-type allele (supplemental Figure 1B). The mice carrying recombined locus were mated with the mice expressing Flp enzyme to induce the combination of 2 FRT sites, the genotyping PCR of their offspring using primers 1F and 1R2 yielded the expected 584-bp product for Flp-excised allele and the corresponding 414-bp product from the wild-type allele (supplemental Figure 1C). The mice with Flp-excised allele (ERp72-floxed mice) were mated with mice expressing Tie2-Cre to induce the combination of 2 loxP sites, the genotyping PCR of the offspring using primers 3F and 3R produced the expected 221-bp product for the Cre-excised allele, these mice were also positive for Cre gene in genotyping (supplemental Figure 1D).
To test the knockout efficiency of homozygous ERp72fl/fl mice expressing the Cre gene (Tie2-Cre/ERp72fl/fl), we measured the messenger RNA (mRNA) and protein levels by RT-PCR and western blotting. Tie2-Cre/ERp72fl/fl mice did not express ERp72 mRNA or protein in platelets and endothelial cells (Figure 1A-B). Leukocytes also had decreased ERp72 (supplemental Figure 2A). The mRNA or protein levels of other PDI family members, such as PDI, ERp57, ERp5, and ERp46, were comparable with wild-type mice (Figure 1A-B; supplemental Figure 2B). These results indicate successful targeting of ERp72. Complete blood counts in Tie2-Cre/ERp72fl/fl mice revealed no abnormalities, and platelet counts were comparable to those of Cre-negative (supplemental Figure 2C). Platelet size was comparable to those of Cre-negative littermates (mean platelet volume: 6.82 ± 0.07 fL vs 6.79 ± 0.04 fL, P = not significant, n = 13). Platelets from Tie2-Cre/ERp72fl/fl mice had normal expression of the major platelet surface glycoproteins αIIbβ3, GpIbα, and GpVI (supplemental Figure 2D). The prothrombin time, partial thromboplastin time, and fibrinogen levels in Tie2-Cre/ERp72fl/fl mice were normal (supplemental Figure 3).
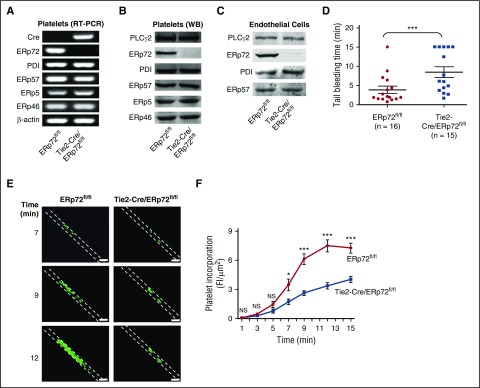
Figure 1.
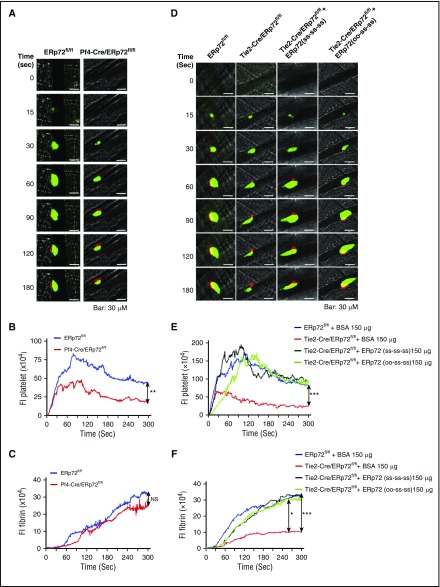
Intravascular ERp72 is required for hemostasis and platelet accumulation into a growing thrombus. (A-C) Characterization of Tie2-Cre/ERp72fl/fl mice. (A) Platelet mRNA expression was evaluated by RT-PCR to demonstrate the absence of ERp72 mRNA. The mRNA expressions of other PDIs serve as control. Western blots of platelet (B) and endothelial (C) lysates using a polyclonal rabbit anti–ERp72 antibody and antibodies against PDI, ERp57, ERp46, and ERp5. Shown are the PLCγ2 loading controls for ERp72. Separate loading controls were run for ERp57, ERp5, and ERp72 with similar amounts of protein found in each sample (not depicted). Blots are representative of 3 separate experiments. (D) Tail bleeding times; ***P < .001, Student t test. (E and F) Incorporation of platelets into growing thrombus in ERp72fl/fl mice and Tie2-Cre/ERp72fl/fl mice was detected by Alexa 488 anti-CD41 using FeCl3-induced mesenteric arterial injury. Mean artery diameters were 125.2 ± 2.6 μm in ERp72fl/fl mice and 121.3 ± 2.1 μm in Tie2-Cre/ERp72fl/fl mice (P = not significant). (E) Images at 7, 9, and 12 minutes. Dotted lines mark the vessel wall. Scale bar, 200 μm. Images are original magnification ×100. (F) Composite of fluorescence intensity (FI) per area analyzed (FI/μm2) in ERp72fl/fl (n = 20 from 8 mice) and Tie2-Cre/ERp72fl/fl (n = 17 from 8 mice) mice; mean ± standard error of the mean (SEM), *P < .05, ***P < .001, Student t test.
Tie2-Cre/ERp72fl/fl mice have prolonged tail bleeding times and decreased platelet incorporation into a growing thrombus
Tail bleeding times of Tie2-Cre/ERp72fl/fl mice were approximately doubled relative to their littermate controls (Figure 1D). Since there was no decrease in von Willebrand factor multimers or antigen levels in plasma from Tie2-Cre/ERp72fl/fl mice (supplemental Figure 4), the defect in tail bleeding does not result from the effect of ERp72 deficiency on biogenesis of von Willebrand factor in endothelial cells. Incorporation of platelets into the thrombus in Tie2-Cre/ERp72fl/fl mice was substantially decreased in a FeCl3-induced mesenteric artery injury model (Figure 1E), with the decrease being statistically significant over the 7- to 15-minute time interval (Figure 1F). Because fibrinogen is required for the stable thrombi in the FeCl3 model,20-23 we measured fibrinogen levels in Tie2-Cre/ERp72fl/fl mice, which were normal (supplemental Figure 3B). These results indicate a role for intravascular ERp72 in platelet accumulation.
ERp72 deficiency impairs platelet function
Since the prolonged tail bleeding times and impaired platelet accumulation suggests a role for ERp72 in platelet responses, we analyzed the function of ERp72-null platelets.
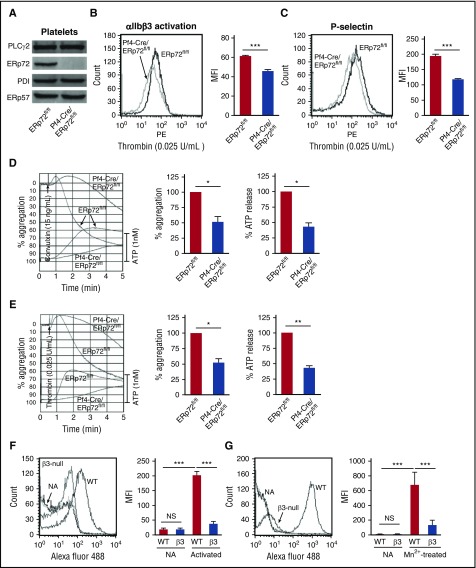
Pf4-Cre/ERp72fl/fl mice were selected by genotyping (supplemental Figure 5A). Platelets from these mice did not express ERp72 protein (Figure 2A). Complete blood counts in these mice revealed no abnormalities, and platelet counts and platelet size were normal. Platelets from Pf4-Cre/ERp72fl/fl mice had normal expression of the major platelet surface glycoproteins αIIbβ3, GpIbα, and GpVI (supplemental Figure 5B). Thrombin-induced activation of αIIbβ3 (Figure 2B), P-selectin expression (Figure 2C), and thrombin- and convulxin-induced aggregation and ATP release were decreased in platelets lacking ERp72 (Figure 2D-E). Higher doses of thrombin and convulxin overcame the aggregation defect (supplemental Figure 6). ADP-induced aggregation was also defective in ERp72-null platelets (supplemental Figure 7).
Figure 2.
ERp72 is critical for platelet aggregation and interacts with β3 integrins. (A) Western blots of platelet lysates using a polyclonal rabbit anti–ERp72 antibody and antibodies against ERp57 and PDI. Shown are the PLCγ2 loading controls for ERp72. Blots are representative of 3 separate experiments, with separate loading controls run for ERp57 and PDI (not depicted). (B) ERp72-deficient platelets have defective thrombin (0.025 U/mL)–induced activation of αIIbβ3 (detected by the JON/A activation-dependent antibody). (C) P-selectin expression is decreased in thrombin-stimulated ERp72-null platelets. (B and C) Representative histogram (left) and combined results (right); mean ± SEM, n = 6 for each group, ***P < .001, Student t test. (D and E) Representative aggregation and ATP release tracings (left) and combined results (right) showing the defect in ERp72-deficient platelets using convulxin (15 ng/mL) or thrombin (0.025 U/mL); mean ± SEM, n = 3, *P < .05, **P < .01, Student t test. Aggregation and ATP secretion were monitored in the lumiaggregometer. (F and G) ERp72 interacts with β3 integrins on mouse platelets. Binding of Alexa Fluor 488–conjugated ERp72 to nonactivated (NA) and thrombin-activated (F) or Mn2+-treated (G) wild-type and β3-null mouse platelets. Cumulative data for ERp72 binding to thrombin-activated platelets (right panel of F) and to Mn2+-treated platelets (right panel of G); mean ± SEM, n = 3 for each group, ***P < .001, analysis of variance. Washed mouse platelets (3 × 108/mL) were preincubated with Alexa Fluor 488 ERp72 (30 μg/mL) for 10 minutes at room temperature and then activated by thrombin (0.1 U/mL) (F) or treated with Mn2+ (12 mM) (G) for 5 minutes at room temperature. Surface binding of Alexa Fluor 488 ERp72 was detected by flow cytometry. ATP, adenosine triphosphate.
ERp72 interacts with αIIbβ3 on the platelet surface
Since platelet aggregation depends on activation of αIIbβ3, we examined binding of ERp72 to αIIbβ3 on platelets. ERp72 binding to thrombin-activated β3-null platelets was substantially decreased compared with wild-type platelets (Figure 2F). To study ERp72 binding to activated αIIbβ3 in the absence of platelet activation, we treated platelets with Mn2+, which directly induces conformational changes in αIIbβ3 without causing platelet activation.24 Binding of ERp72 to Mn2+-treated β3-null platelets was substantially decreased compared with binding to wild-type platelets (Figure 2G). ERp72 competed more effectively than ERp57 or PDI for binding of Alexa PDI to activated human platelets (supplemental Figure 8).
The a and a′ active sites of ERp72 support platelet aggregation
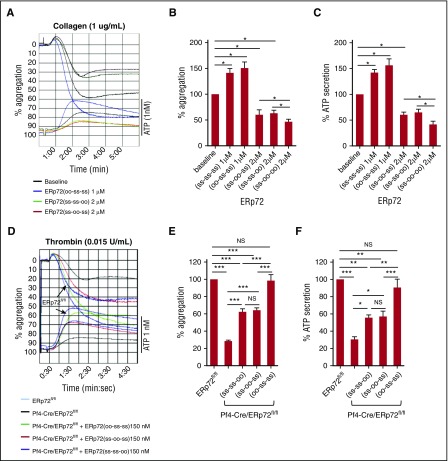
To determine the relative importance of the 3 active sites of ERp72 in platelet function, we generated 3 single active site mutants of ERp72. One mutant, ERp72(oo-ss-ss), had the a° CGHC motif inactivated, and the other mutants, ERp72(ss-oo-ss) and ERp72(ss-ss-oo), had the a or a′ CGHC motif inactivated, respectively. The ERp72(oo-ss-ss) mutant that retained the a and a′ CGHC motifs potentiated collagen-induced platelet aggregation and ATP release of human platelets to a similar extent as wild-type ERp72(ss-ss-ss) (Figure 3A-C). The single active site mutants with the a or a′ CGHC motif inactivated inhibited collagen-induced platelet aggregation and ATP release to similar extents (Figure 3A-C). The double active site mutant of both the a and a′ CGHC motifs caused additional inhibition of aggregation and ATP release compared with the single a or a′ active site mutants (Figure 3B-C). In contrast to the inhibitory effect of ERp72(ss-oo-ss) and ERp72(ss-ss-oo) in human platelets, both ERp72(ss-oo-ss) and ERp72(ss-ss-oo) partially recovered aggregation and ATP release of ERp72-null platelets, while addition of ERp72(oo-ss-ss) resulted in full recovery (Figure 3D-F). ERp72(ss-oo-ss) and ERp72(ss-ss-oo) likely compete with endogenous ERp72 in human platelets inhibiting aggregation, while a single functional a or a′ CGHC motif in these mutants partially recovers aggregation of ERp72-null mouse platelets. The mutants bound similarly to platelets, indicating the functional consequences of the different mutants are not due to alterations in binding (supplemental Figure 9).
Figure 3.
The a and a′ active sites of ERp72 are critical for platelet aggregation and ATP release. (A-C) Effect of preincubating human platelets (2 × 108 platelets/mL) with single active site mutants of ERp72, ERp72(oo-ss-ss), ERp72(ss-oo-ss), and ERp72(ss-ss-oo). Submaximal aggregation (baseline) was stimulated with collagen (1 µg/mL). The concentrations for each type of protein are indicated. Representative aggregation tracings with ATP release for the 3 single active site mutants (A) and corresponding cumulative data for aggregation (B) and secretion (C) with wild-type ERp72(ss-ss-ss) and the double active site mutant ERp72(ss-oo-oo) results added to the cumulative data; mean ± SEM, n = 3, *P < .05, analysis of variance. (D-F) Correction of the aggregation and secretion defects of ERp72-null platelets (2 × 108 platelets/mL) from Pf4-Cre/ERp72fl/fl mice compared with wild type (ERp72fl/fl) littermate mice by ERp72 mutants. The mutant proteins were added 5 minutes prior to the addition of thrombin (0.015 U/mL). Representative tracings (D) and cumulative data for aggregation (E) and secretion (F); mean ± SEM, n = 3, *P < .05, **P < .01, ***P < .001, analysis of variance. ERp72fl/fl, PDIfl/fl, and ERp57fl/fl represent the Cre-negative littermate controls. NS, not significant.
Additional studies using mutants with only a single functional active site (double active site mutants) confirmed these results. ERp72(ss-oo-oo) containing only the a° active site did not result in any recovery of the aggregation defect of ERp72-null platelets (supplemental Figure 10A-B). ERp72(oo-ss-oo) and ERp72(oo-oo-ss), with either a functional a or a′ active site, partially recovered aggregation. Aggregation was again fully recovered by ERp72(oo-ss-ss). Together, these findings demonstrate the importance of the a and a′ active sites in platelet aggregation. In contrast, the a° active site does not have a detectable role in platelet aggregation.
Distinct roles of ERp72, PDI, and ERp57 in platelet function
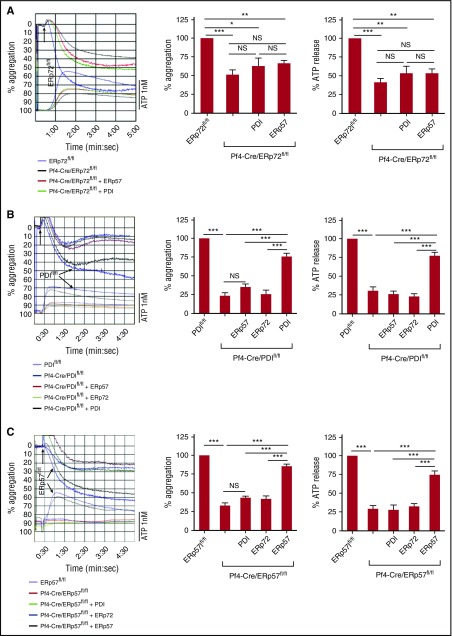
Absence of platelet ERp72 can decrease aggregation by ∼70% (Figure 3D; supplemental Figure 10), suggesting that ERp72 has a distinct role from the other PDIs in activation of αIIbβ3. However, it is possible that the combined redox function of the PDIs is limiting and their mode of action redundant so that deficiency of any PDI enzyme has a similar functional consequence. To further address this question, we tested whether PDI or ERp57 could recover aggregation of ERp72-null platelets. While ERp72 recovered aggregation of ERp72-null platelets (Figure 3D; supplemental Figure 10), adding PDI or ERp57 to ERp72-null platelets did not recover aggregation (Figure 4A). Moreover, PDI, but not ERp72 or ERp57, recovered aggregation of PDI-null platelets (Figure 4B). Similarly, ERp57, but not ERp72 or PDI, recovered aggregation of ERp57-null platelets (Figure 4C). These data imply each enzyme has a different role in the activation of αIIbβ3.
Figure 4.
Preferential recovery of aggregation of ERp72-, PDI-, and ERp57-null platelets by the specific PDI that is deleted. (A) ERp72-null platelets were incubated with 150 nM PDI or ERp57 (as in Figure 3D). (B) PDI-null platelets were incubated with 100 nM ERp57, ERp72, or PDI. (C) ERp57-null platelets were incubated with 100 nM ERp57, ERp72, or PDI. (A-C) Platelet aggregation was stimulated using thrombin (0.015 U/mL) (right); cumulative data, mean ± SEM, n = 3, *P < .05, **P < .01, ***P < .001, analysis of variance. ERp72fl/fl, PDIfl/fl, and ERp57fl/fl represent the Cre-negative littermate controls. NS, not significant.
Intravascular ERp72 is required for fibrin deposition
To confirm the role of ERp72 in platelet accumulation in a second injury model and to simultaneously analyze fibrin deposition, we used a laser-induced injury model. While Pf4-Cre/ERp72fl/fl mice that lack ERp72 in platelets (Figure 2A) have decreased platelet accumulation, they do not have a defect in fibrin deposition (Figure 5A-C).
Figure 5.
Intravascular ERp72 is required for platelet accumulation and fibrin formation in vivo. (A-C) Cremaster arteriole injury was induced in Pf4-Cre/ERp72fl/fl mice and their Cre-negative ERp72fl/fl littermate control mice. Platelets and fibrin accumulated at the site of injury were detected using anti-CD41 F(ab)2 fragments conjugated to Alexa Fluor 488 and anti–fibrin antibody conjugated to Alexa Fluor 647. (A) Representative fluorescence images in intravital microscopy for platelet accumulation (green) and fibrin deposition (red) at the indicated time points after injury . Original magnification ×64. Scale bar, 30 μm. The median integrated fluorescence intensities (FIs) of anti-CD41 (platelet, B) and anti–fibrin (fibrin, C) antibodies over 300 seconds. (D-F) Cremaster arteriole injury was induced in Tie2-Cre/ERp72fl/fl mice and Cre-negative ERp72fl/fl littermate control mice. Tie2-Cre/ERp72fl/fl mice were infused with ERp72(ss-ss-ss) or ERp72(oo-ss-ss) (150 μg/mouse). (D) Representative fluorescence images of platelet accumulation (green) and fibrin deposition (red). Original magnification ×64. Scale bar, 30 μm. The data of fluorescence intensities of anti–CD41 antibody (platelet, E) and anti–fibrin antibody (fibrin, F) were obtained from 30 thrombi in 3 mice. Fluorescence signal was not observed using fluorescently labeled control immunoglobulin G (not depicted). The area under the curve of fluorescence intensity over 300 seconds was analyzed using a Kruskal-Wallis test. Only significant differences are shown; *P < .05; ***P < .001. The data were obtained from 30 thrombi in 3 mice for each experimental condition.
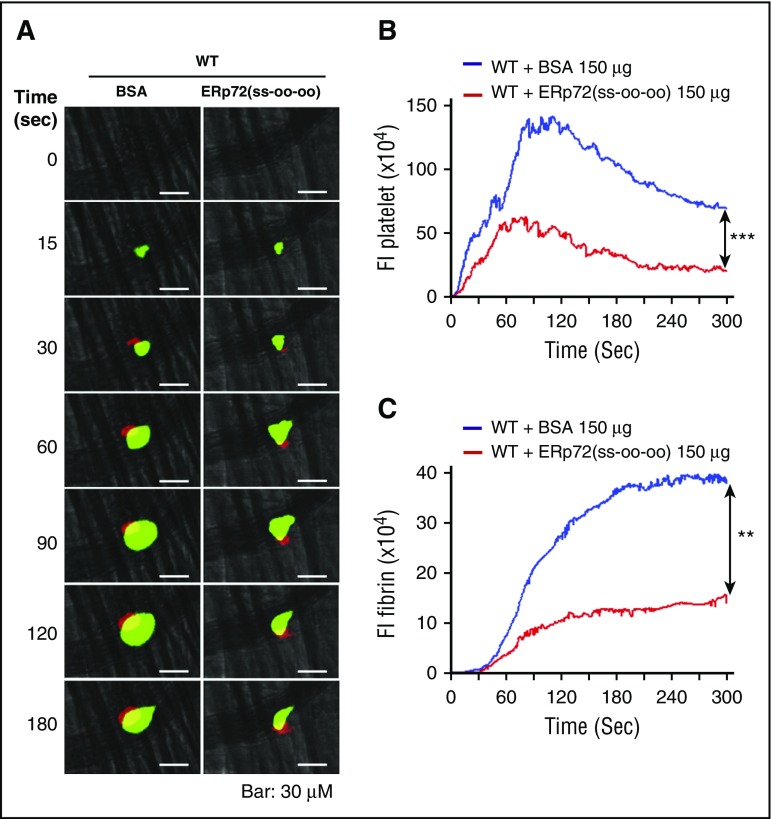
Previous studies implicated platelets and endothelial cells as the major in vivo sources of extracellular thiol isomerases.4,25,26 To eliminate ERp72 from endothelial cells in addition to platelets, we used Tie2-Cre/ERp72fl/fl mice,5 which have substantially decreased ERp72 in both endothelial cells and platelets (Figure 1B-C). Tie2-Cre/ERp72fl/fl mice displayed a significant reduction in both platelet accumulation and fibrin deposition at sites of injury (Figure 5D-F). Infusion of recombinant wild-type ERp72(ss-ss-ss) or ERp72(oo-ss-ss) recovered the defects in platelet accumulation and fibrin deposition to a similar degree (Figure 5D-F). These results support the role for the a and a′ CGHC active sites in these processes. ERp72(ss-oo-oo) infused into wild-type mice inhibited platelet accumulation and fibrin formation (Figure 6A-C). This suggests that ERp72(ss-oo-oo) competes with endogenous ERp72 to inhibit platelet accumulation and fibrin formation and is consistent with a role for the a and a′ CGHC sites of ERp72 in thrombosis.
Figure 6.
Recombinant ERp72(ss-oo-oo) protein inhibits platelet accumulation and fibrin deposition in wild-type mice. Wild-type (WT) C57BL/6 mice were infused with ERp72 (ss-oo-oo) or bovine serum albumin (BSA; control) (150 μg per mouse), followed by cremaster arteriole injury. Platelets and fibrin were detected using anti-CD41 F(ab)2 conjugated to Alexa Fluor 488 and anti–fibrin antibody conjugated to Alexa Fluor 647. (A) Representative fluorescence images of intravital microscopy for platelet accumulation (green) and fibrin deposition (red). Original magnification ×64. Scale bars, 30 μm. Median fluorescent intensities (FIs) of anti–CD41 antibodies (platelets) (B) and anti–fibrin antibody (C) with the area under curve were analyzed using a Mann-Whitney rank-sum test; **P < .01; ***P < .001. The data were obtained from 30 thrombi in 3 mice for each experimental condition.
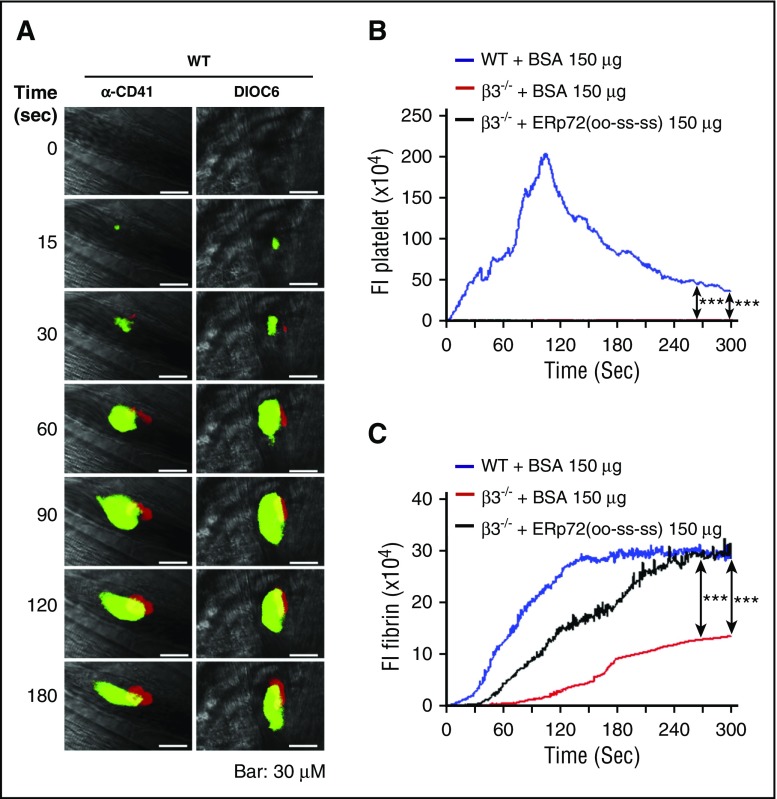
To determine whether ERp72 can directly mediate fibrin formation independently of platelet accumulation, we employed β3-null mice in which platelet accumulation and fibrin deposition is almost completely absent.27 Since platelets cannot be detected using anti–CD41 antibody in β3-null mice, we used the cationic lipophilic fluorochrome DiOC6 (used to study of platelet biology in vivo28 and in vitro).29 Platelet accumulation in wild-type mice visualized by DiOC6 was comparable to anti–CD41 antibody (Figure 7A). As expected, platelet accumulation was absent at the site of injury in β3-null mice, with a significant reduction of fibrin formation (Figure 7B-C; supplemental Figure 11). This suggests that platelet accumulation contributes to fibrin formation, as we previously documented.5,6 Infusion of ERp72(oo-ss-ss) into β3-null mice substantially recovered fibrin formation, without any recovery of platelet accumulation (Figure 7B-C). This suggests that ERp72 can directly contribute to activation of coagulation through the a and a′ active sites.
Figure 7.
Recombinant ERp72(oo-ss-ss) protein directly enhances fibrin deposition. (A) Cremaster arteriole injury was induced in wild-type mice after they received intravenous injection of anti–fibrin antibodies conjugated to Alexa Fluor 647, with anti-CD41 F(ab)2 conjugated to Alexa Fluor 488 or 3′-dihexyloxacarbocyanine iodide (DIOC6) (2.5 μL of a 100 μM solution per gram of body weight). Representative images for platelet accumulation visualized by these 2 methods (green) and fibrin formation (red). The total original magnification is ×64. Scale bar, 30 μm. (B) Wild-type mice and β3−/− mice received intravenous infusion of ERp72(oo-ss-ss) or BSA (control) (150 μg per mouse) as indicated, followed by cremaster arteriole injury. Platelets and fibrin formation were detected using DIOC6 and anti–fibrin antibody conjugated to Alexa Fluor 647. Median fluorescence intensities (FIs) of platelets (B) and fibrin (C) with the area under curve analyzed using a Kruskal-Wallis test. Only significant differences are shown; ***P < .001. The data were obtained from 40 thrombi in 5 mice for each experimental condition.
ERp72 generates thiols in αIIbβ3
Concentrations of ERp72 as low as 100 nM potentiated platelet aggregation (supplemental Figure 12A), and beginning at 100 nM, ERp72(oo-ss-ss) potentiated MPB labeling of sulfhydryls in αIIb and β3. ERp72(ss-oo-oo) and dithiothreitol had no effect (supplemental Figure 12B-D). Activation of platelets increased MPB labeling of αIIb and β3,17 and the addition of ERp72(oo-ss-ss) potentiated this increase (supplemental Figure 12E). These findings further suggest that ERp72 directly interacts with αIIbβ3 on intact platelets and show the a and a′ active sites of ERp72 can cleave disulfide bonds in both αIIb and β3.
Thiols in αIIbβ3
To characterize the reactions involving thiols and disulfides in αIIbβ3, we labeled αIIbβ3 purified from nonactivated and activated platelets with the pyridyldithiol-activated sulfhydryl-reactive biotin-HPDP. Results from 3 separate experiments reproducibly identified 7 and 9 labeled Cys residues in nonactivated and activated αIIb, respectively, with 5 Cys residues labeled in nonactivated and activated β3 (supplemental Figure 13; supplemental Table 1). Several additional Cys residues were labeled in only 1 experimental sample. The labeling results using MPB and biotin-HPDP cannot be compared, as the labeling reagents and conditions are different, and we did not quantitate the amount of each cysteine in the thiol versus disulfide form.
Discussion
In this study, using new genetically modified mouse models of conditional ERp72 deficiency with recombinant ERp72 inactive mutants, we provide the first evidence showing that ERp72 is important in hemostasis, thrombosis, and platelet function. Moreover, our results indicate that the primary active sites of ERp72 in thrombosis are localized in the a and a′ domains.
The study of redox-sensitive proteins and reactions requires the native physiological environment. Using high-speed digital intravital microscopy, we evaluated phenotypes of ERp72 deficiency in live mice. Both Pf4-Cre/ERp72fl/fl and Tie2-Cre/ERp72fl/fl mice exhibited a major decrease in platelet accumulation in laser arterial injury, and using Tie2-Cre/ERp72fl/fl mice, a major defect in platelet accumulation was also documented in the FeCl3-induced mesenteric arterial injury model. Since these 2 models are distinct in injury mechanisms, vessel size, and hemodynamics, the uniform phenotypes in these 2 injury conditions imply that ERp72 is necessary for platelet deposition at the sites of injury. In observing simultaneous fibrin formation in the laser model, we found that Tie2-Cre/ERp72fl/fl mice, but not Pf4-Cre/ERp72fl/fl mice, have decreased fibrin formation. These results imply that intravascular ERp72 has an essential role in platelet accumulation and fibrin generation and that ERp72 from endothelial cells is sufficient to support fibrin generation (while leukocyte ERp72 is decreased in Tie2-Cre/ERp72fl/fl mice, leukocytes are not present at the site of injury to contribute ERp72 over the first 180 to 240 s in our laser model).6
Holbrook et al found that following platelet activation, ERp72 is released by platelets and relocated to the platelet surface.2 In this study, we found that ERp72-null platelets have defective platelet responses, including aggregation and secretion. The addition of recombinant ERp72 protein recovers the aggregation and secretion defects, implying the primary site of action of ERp72 is the platelet surface. The decreased P-selectin expression and ATP release in ERp72-null platelets suggests that ERp72 also regulates platelet secretion reactions. P-selectin and ATP are markers of α and dense granule secretion and have a role in platelet function. While αIIbβ3 may regulate platelet P-selectin de novo synthesis and thus indirectly regulate the amount expressed on the surface,30,31 in our study, ERp72 likely regulates P-selectin expression with platelet activation through a non-αIIbβ3 substrate.6 Moreover, binding of ERp72 to thrombin-activated and Mn2+-treated platelets is substantially reduced in the absence of integrin αIIbβ3 on platelets. Since Mn2+ directly mediates conformational changes in αIIbβ3, the major association of ERp72 with platelets is likely through a direct interaction with this integrin. Since most binding of ERp72 occurs when αIIbβ3 is activated, the increased binding of ERp72 to platelets appears dependent on conformational changes that occur in αIIbβ3 as it undergoes activation.
Most catalytic domains in the PDI family contain a conserved catalytic motif, CXYC, most commonly CGHC, and to date, 3 CGHC-containing disulfide isomerases (PDI, ERp57, and ERp5) have been implicated in thrombosis. ERp72 is the only member of the PDI family with 5 thioredoxin-like domains with an additional N-terminal catalytic domain a° to give an a°–a–b–b′–a′ architecture. This is the first study to our knowledge addressing the relative importance of the active sites of ERp72 in mammalian cells or a physiologic environment. We found the single active site mutants of the a or a′ CGHC motif of ERp72 inhibited aggregation and ATP release of human platelets, while ERp72(oo-ss-ss) potentiated aggregation to the same degree as wild-type ERp72 (Figure 3A). Moreover, ERp72(oo-ss-ss) containing intact a and a′ CGHC motifs fully recovered the decreased aggregation and ATP release of ERp72 null-platelets. These results demonstrate that the catalytic activity of the a and a′ domains, but not a° domain, is important in platelet function. Since ERp72 interacts with αIIbβ3 on platelets and ERp72(oo-ss-ss) potentiates aggregation, the a and a′ active sites of ERp72 may catalyze disulfide cleavage or rearrangement in αIIbβ3 facilitating formation of the high affinity form of this integrin and platelet aggregation.
The absence of a role of the a° active site of ERp72 in thrombosis provides additional evidence showing that the function of each CGHC domain in PDIs is not identical. We recently reported that the C-terminal CGHC motifs of PDI and ERp57 are critical for thrombosis, but their N-terminal CGHC motif is not.5,6 While some mobility between the domains of the PDIs allows them to accommodate a wide range of substrates, some restriction in mobility is also needed to enhance cooperativity between domains.32 The noncatalytic bb′ domains of ERp72 form a relatively rigid spacer between the catalytic a and a′ domains, providing some stability in the positions of the a and a′ CGHC motifs.14 Further investigation will be required to determine how the a and a′ active sites cooperate in the regulation of platelet function.
Four members of the PDI family (PDI, ERp57, ERp5, and now ERp72) have been implicated in hemostasis and thrombosis. Each has a role in platelet aggregation and interacts with αIIbβ3.3,4,6,26 There are up to 80 000 molecules of αIIbβ3 on the platelet33 but relatively few molecules of PDI.34 While the stoichiometry of the interaction with αIIbβ3 is not well defined, each of these 4 PDI family members appears to interact with αIIbβ3 and facilitate formation of the high-affinity fibrinogen-binding conformation.6 In the endoplasmic reticulum, large disulfide-rich proteins such as laminin or thyroglobulin can be found as disulfide-linked substrates with multiple members of the PDI family,8,9 with each PDI family member catalyzing reactions in different regions of the substrate.9 We found the aggregation defect in ERp72-, PDI-, or ERp57-null mice was only recovered by the specific PDI that was missing, implying that different members of the PDI family target different cysteines in αIIbβ3 to fully activate this receptor.
There are 2.6 thiol/mol of inactivated αIIbβ3 and 4.4 thiol/mol of activated αIIbβ3.35 However, using biotin-HPDP, we identified a total of 12 and 14 labeled cysteine residues in the nonactivated and activated forms of αIIbβ3, respectively, suggesting that each of the labeled cysteines was a free thiol in only a fraction of αIIbβ3 molecules. This suggests that the position of the thiol varies through thiol-disulfide exchange and that multiple conformations exist in αIIbβ3. These findings are consistent with previous reports suggesting a role for thiol-disulfide exchange in activation of αIIbβ3.16,35-37 The catalytic activity of ERp72 and the other PDIs may have a role in the allosteric changes relayed between the integrin transmembrane domains and ligand-binding head of αIIbβ3.
We also found that ERp72 is important in fibrin formation at the sites of injury. We and others have previously reported that extracellular PDI, ERp57, and ERp5 regulate coagulation.5,26,38,39 Potential substrates for PDI in coagulation include tissue factor,39-41 factor XI,42,43 and factor V.44 Since in vivo coagulation activation mechanisms involve the participation of multiple coagulation factors and cofactors, the preferential substrates of ERp72 and the specific reactions it catalyzes will need further investigation.
In conclusion, we provide the first evidence demonstrating that ERp72 regulates platelet function and coagulation, and the a and a′ domains are critical in the processes. ERp72, PDI, and ERp57 act by separate mechanisms on αIIbβ3. Our new observations underscore the complexity of the PDI family in thrombosis while providing fundamental knowledge of the hemostatic proteins involved in platelet function and coagulation. Identification of the functional active sites in ERp72 in thrombosis may help design new antithrombotic agents.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL118526 (D.W.E.) and P01HL110860 (M.P.) and the Priority Academic Program Development of Jiangsu Higher Education Institutions, Natural Science Foundation of China (grants 81670133, 81270592, 31201058, and 91539122). The mass spectrometry study was funded in part by National Institutes of Health, National Institute of Neurological Disorders and Stroke grant P30NS046593 to the Neuroproteomics Core Facility (H.L.). The method development of the biotin-HPDP labeling of free thiols was supported in part by National Institutes of Health, National Institute of General Medical Sciences grant R01GM112415.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.W. designed the project, supervised and performed research, analyzed the data, and wrote the manuscript; J.Z., F.C., and L.W. performed research and collected and analyzed data; L.R., V.M.H., and M.P. assisted with the in vivo hemostasis and thrombosis models and helped with study design and interpretation; T.L. and H.L. performed the mass spectrometry experiments; J.L. provided the β3-null mice and helped set up the initial mesenteric artery experiments; and D.W.E. designed the project, supervised the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Wu, The Cyrus Tang Hematology Center, Jiangsu Institute of Hematology, First Affiliated Hospital, Soochow University, 199 Ren-Ai Rd, Suzhou, 215123, China; e-mail: yiwu99@gmail.com; and David W. Essex, Temple University School of Medicine, Room 204 MRB, 3420 N Broad St, Philadelphia, PA 19140; e-mail: david.essex@temple.edu.
References
- 1.Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11(11):2807-2850. [DOI] [PubMed] [Google Scholar]
- 2.Holbrook LM, Watkins NA, Simmonds AD, Jones CI, Ouwehand WH, Gibbins JM. Platelets release novel thiol isomerase enzymes which are recruited to the cell surface following activation. Br J Haematol. 2010;148(4):627-637. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Wu Y, Zhou J, et al. Platelet-derived ERp57 mediates platelet incorporation into a growing thrombus by regulation of the αIIbβ3 integrin. Blood. 2013;122(22):3642-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K, Hahm E, Li J, et al. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122(6):1052-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Wu Y, Wang L, et al. The disulfide isomerase ERp57 is required for fibrin deposition in vivo. J Thromb Haemost. 2014;12(11):1890-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Wu Y, Wang L, et al. The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis. J Clin Invest. 2015;125(12):4391-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharda A, Kim SH, Jasuja R, et al. Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome. Blood. 2015;125(10):1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessop CE, Watkins RH, Simmons JJ, Tasab M, Bulleid NJ. Protein disulphide isomerase family members show distinct substrate specificity: P5 is targeted to BiP client proteins. J Cell Sci. 2009;122(Pt 23):4287-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Jeso B, Morishita Y, Treglia AS, et al. Transient covalent interactions of newly synthesized thyroglobulin with oxidoreductases of the endoplasmic reticulum. J Biol Chem. 2014;289(16):11488-11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walczak CP, Tsai B. A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J Virol. 2011;85(5):2386-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue T, Dosey A, Herbstman JF, Ravindran MS, Skiniotis G, Tsai B. ERdj5 reductase cooperates with protein disulfide isomerase to promote simian virus 40 endoplasmic reticulum membrane translocation. J Virol. 2015;89(17):8897-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh M, Shimada A, Kashiwai A, Saga S, Hosokawa M. Differential cooperative enzymatic activities of protein disulfide isomerase family in protein folding. Cell Stress Chaperones. 2005;10(3):211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzarella RA, Srinivasan M, Haugejorden SM, Green M. ERp72, an abundant luminal endoplasmic reticulum protein, contains three copies of the active site sequences of protein disulfide isomerase. J Biol Chem. 1990;265(2):1094-1101. [PubMed] [Google Scholar]
- 14.Kozlov G, Määttänen P, Schrag JD, et al. Structure of the noncatalytic domains and global fold of the protein disulfide isomerase ERp72. Structure. 2009;17(5):651-659. [DOI] [PubMed] [Google Scholar]
- 15.Kramer B, Ferrari DM, Klappa P, Pöhlmann N, Söling HD. Functional roles and efficiencies of the thioredoxin boxes of calcium-binding proteins 1 and 2 in protein folding. Biochem J. 2001;357(Pt 1):83-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Essex DW, Li M, Miller A, Feinman RD. Protein disulfide isomerase and sulfhydryl-dependent pathways in platelet activation. Biochemistry. 2001;40(20):6070-6075. [DOI] [PubMed] [Google Scholar]
- 17.Essex DW, Li M. Redox control of platelet aggregation. Biochemistry. 2003;42(1):129-136. [DOI] [PubMed] [Google Scholar]
- 18.Manickam N, Sun X, Hakala KW, Weintraub ST, Essex DW. Thiols in the alphaIIbbeta3 integrin are necessary for platelet aggregation. Br J Haematol. 2008;142(3):457-465. [DOI] [PubMed] [Google Scholar]
- 19.Manickam N, Ahmad SS, Essex DW. Vicinal thiols are required for activation of the αIIbβ3 platelet integrin. J Thromb Haemost. 2011;9(6):1207-1215. [DOI] [PubMed] [Google Scholar]
- 20.Reheman A, Yang H, Zhu G, et al. Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor. Blood. 2009;113(8):1809-1817. [DOI] [PubMed] [Google Scholar]
- 21.Ni H, Denis CV, Subbarao S, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106(3):385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Reheman A, Spring CM, et al. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest. 2014;124(10):4281-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni H, Papalia JM, Degen JL, Wagner DD. Control of thrombus embolization and fibronectin internalization by integrin alpha IIb beta 3 engagement of the fibrinogen gamma chain. Blood. 2003;102(10):3609-3614. [DOI] [PubMed] [Google Scholar]
- 24.Litvinov RI, Nagaswami C, Vilaire G, Shuman H, Bennett JS, Weisel JW. Functional and structural correlations of individual alphaIIbbeta3 molecules. Blood. 2004;104(13):3979-3985. [DOI] [PubMed] [Google Scholar]
- 25.Jasuja R, Furie B, Furie BC. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood. 2010;116(22):4665-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passam FH, Lin L, Gopal S, et al. Both platelet- and endothelial cell-derived ERp5 support thrombus formation in a laser-induced mouse model of thrombosis. Blood. 2015;125(14):2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho J, Kennedy DR, Lin L, et al. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of β3 integrins. Blood. 2012;120(3):647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira J, Soto M, Palomo I, et al. Platelet aging in vivo is associated with activation of apoptotic pathways: studies in a model of suppressed thrombopoiesis in dogs. Thromb Haemost. 2002;87(5):905-909. [PubMed] [Google Scholar]
- 29.Bode AP, Orton SM, Frye MJ, Udis BJ. Vesiculation of platelets during in vitro aging. Blood. 1991;77(4):887-895. [PubMed] [Google Scholar]
- 30.Yang H, Lang S, Zhai Z, et al. Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression. Blood. 2009;114(2):425-436. [DOI] [PubMed] [Google Scholar]
- 31.Merten M, Thiagarajan P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation. 2000;102(16):1931-1936. [DOI] [PubMed] [Google Scholar]
- 32.Kozlov G, Azeroual S, Rosenauer A, et al. Structure of the catalytic a(0)a fragment of the protein disulfide isomerase ERp72. J Mol Biol. 2010;401(4):618-625. [DOI] [PubMed] [Google Scholar]
- 33.Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88(3):907-914. [PubMed] [Google Scholar]
- 34.Burgess JK, Hotchkiss KA, Suter C, et al. Physical proximity and functional association of glycoprotein 1balpha and protein-disulfide isomerase on the platelet plasma membrane. J Biol Chem. 2000;275(13):9758-9766. [DOI] [PubMed] [Google Scholar]
- 35.Yan B, Smith JW. A redox site involved in integrin activation. J Biol Chem. 2000;275(51):39964-39972. [DOI] [PubMed] [Google Scholar]
- 36.O’Neill S, Robinson A, Deering A, Ryan M, Fitzgerald DJ, Moran N. The platelet integrin alpha IIbbeta 3 has an endogenous thiol isomerase activity. J Biol Chem. 2000;275(47):36984-36990. [DOI] [PubMed] [Google Scholar]
- 37.Zhu G, Zhang Q, Reddy EC, et al. The integrin PSI domain has an endogenous thiol isomerase function and is a novel target for antiplatelet therapy. Blood. 2017;129(13):1840-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118(3):1123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhardt C, von Brühl ML, Manukyan D, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118(3):1110-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahamed J, Versteeg HH, Kerver M, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103(38):13932-13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langer F, Spath B, Fischer C, et al. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121(12):2324-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannakopoulos B, Gao L, Qi M, et al. Factor XI is a substrate for oxidoreductases: enhanced activation of reduced FXI and its role in antiphospholipid syndrome thrombosis. J Autoimmun. 2012;39(3):121-129. [DOI] [PubMed] [Google Scholar]
- 43.Zucker M, Seligsohn U, Yeheskel A, Mor-Cohen R. An allosteric disulfide bond is involved in enhanced activation of factor XI by protein disulfide isomerase. J Thromb Haemost. 2016;14(11):2202-2211. [DOI] [PubMed] [Google Scholar]
- 44.Stopa JD, Neuberg D, Puligandla M, Furie B, Flaumenhaft R, Zwicker JI. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight. 2017;2(1):e89373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.