Abstract
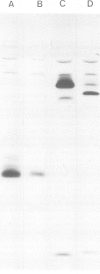
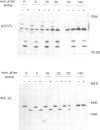
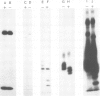
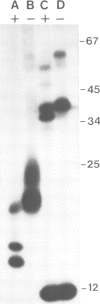
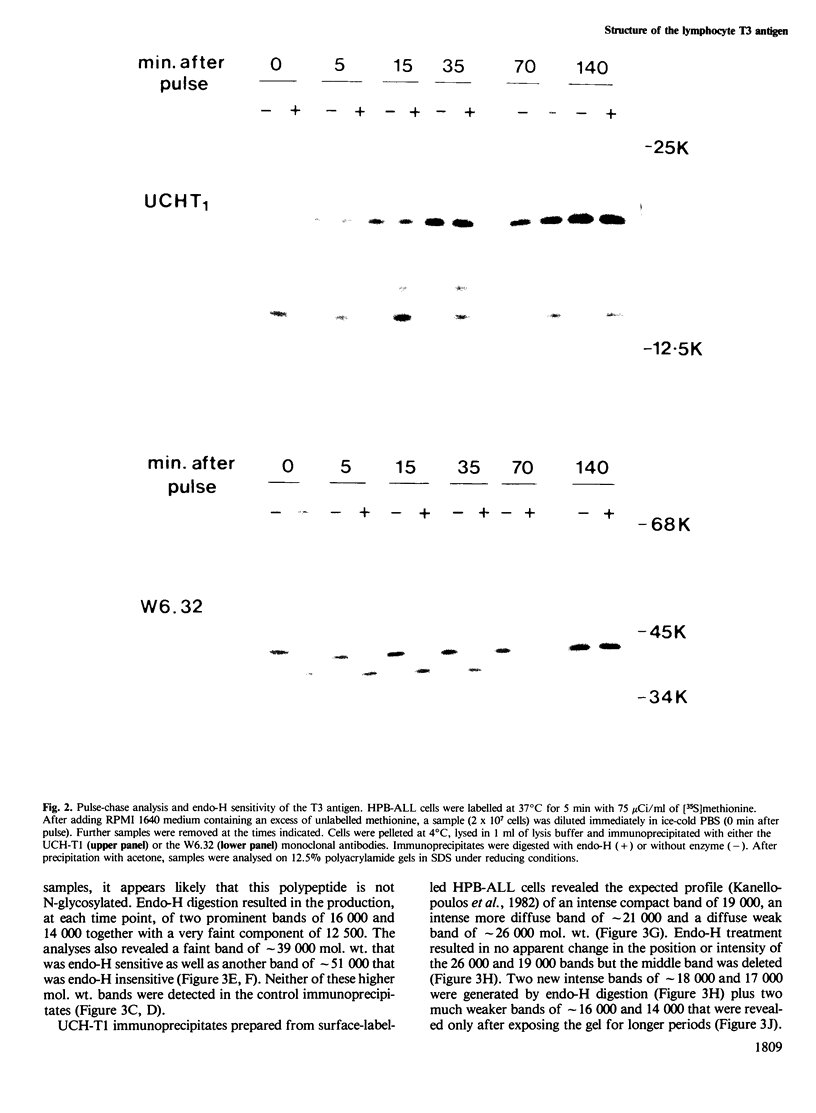
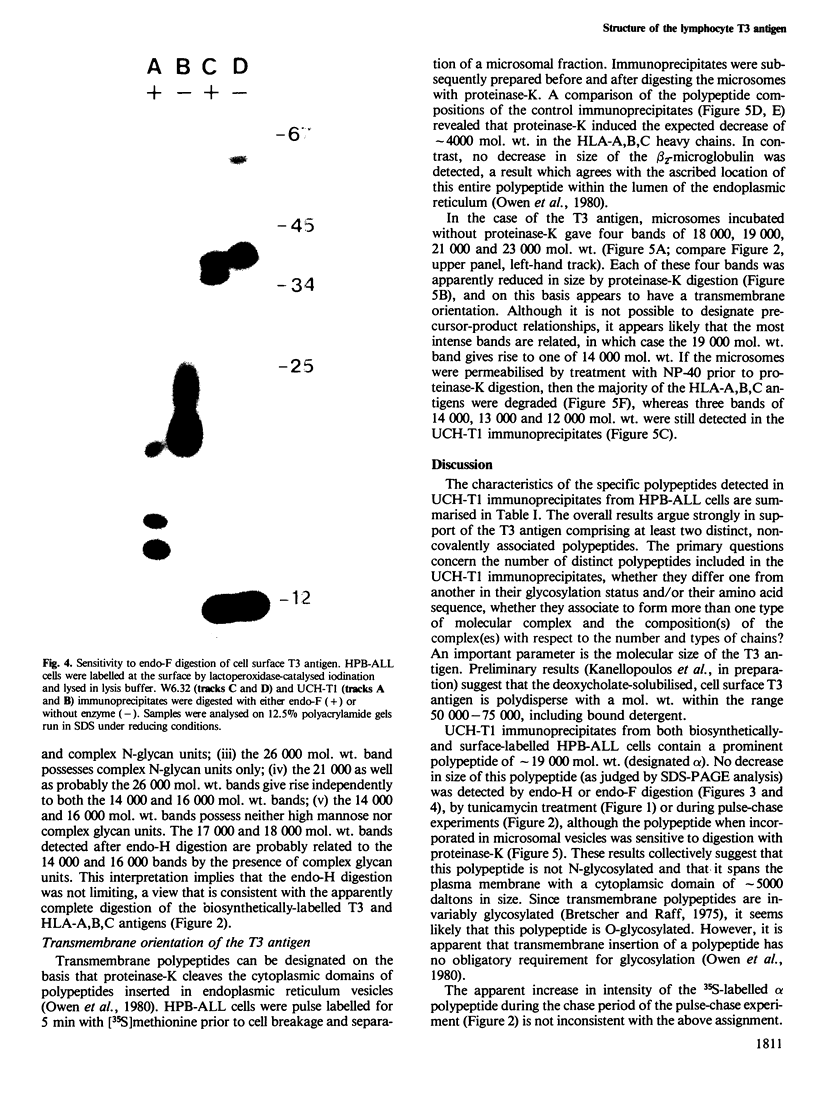
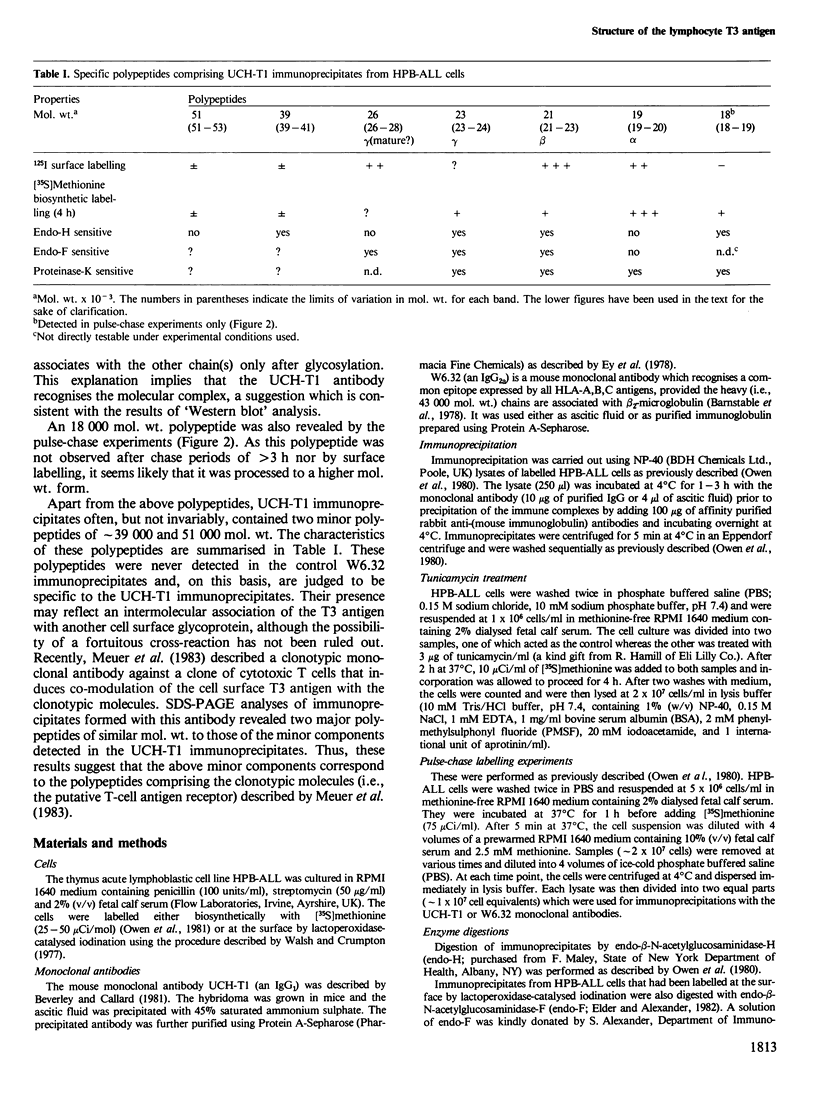
Immunoprecipitates of the T3 antigen prepared from HPB-ALL cells by using the monoclonal antibody UCH-T1 were analysed by SDS-polyacrylamide gel electrophoresis. Cells which had been biosynthetically labelled for up to 4 h gave a major polypeptide of mol. wt. 19 000 plus two weaker, more diffuse bands of mol. wts. 21 000 and 23 000, whereas surface labelled cells gave a prominent band of mol. wt. 19 000, a major band of 21 000 and a weaker diffuse band of approximately 26 000. As judged from their sensitivity to proteinase-K digestion, all the above polypeptides possess a transmembrane orientation. Digestion with endoglycosidases H and F (endo-H and endo-F), and tunicamycin treatment indicate that all the polypeptides, except that of 19 000 mol. wt. are N-glycosylated. The 21 000 and 23 000 mol. wt. chains possess both immature and mature oligosaccharide units, whereas the 26 000 mol. wt. band apparently has mature units only. Pulse chase experiments combined with digestion by endo-F and endo-H suggest that the N-glycosylated polypeptides are derived from two polypeptides of mol. wts. 14 000 and 16 000. It is concluded that the T3 antigen is derived from three different non-glycosylated polypeptides two of which are subsequently N-glycosylated to give the 21 000, 23 000 and 26 000 forms. The cell surface T3 antigen most probably comprises at least two distinct, non-covalently associated polypeptides, but the number and types of polypeptides giving rise to the whole molecule and whether different complexes exist is at present unclear.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Rao P. E., Talle M. A., Goldstein G., Shaw S. Possible involvement of the OKT4 molecule in T cell recognition of class II HLA antigens. Evidence from studies of cytotoxic T lymphocytes specific for SB antigens. J Exp Med. 1982 Oct 1;156(4):1065–1076. doi: 10.1084/jem.156.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J., Prendiville M. A., Terhorst C. Complexity of the human T lymphocyte-specific cell surface antigen T3. J Immunol. 1982 Apr;128(4):1560–1565. [PubMed] [Google Scholar]
- Burns G. F., Boyd A. W., Beverley P. C. Two monoclonal anti-human T lymphocyte antibodies have similar biologic effects and recognize the same cell surface antigen. J Immunol. 1982 Oct;129(4):1451–1457. [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Snary D. Preparation and properties of lymphocyte plasma membrane. Contemp Top Mol Immunol. 1974;3:27–56. doi: 10.1007/978-1-4684-2838-4_2. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Hodgdon J. C., Hercend T., Schlossman S. F., Reinherz E. L. Surface structures involved in target recognition by human cytotoxic T lymphocytes. Science. 1982 Oct 29;218(4571):471–473. doi: 10.1126/science.6981845. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F., Crumpton M. J. Biosynthesis and maturation of HLA-DR antigens in vivo. J Biol Chem. 1981 Sep 10;256(17):8987–8993. [PubMed] [Google Scholar]
- Reinherz E. L., Hussey R. E., Schlossman S. F. A monoclonal antibody blocking human T cell function. Eur J Immunol. 1980 Oct;10(10):758–762. doi: 10.1002/eji.1830101006. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- van Agthoven A., Terhorst C., Reinherz E., Schlossman S. Characterization of T cell surface glycoproteins T 1 and T 3 present on all human peripheral T lymphocytes and functionally mature thymocytes. Eur J Immunol. 1981 Jan;11(1):18–21. doi: 10.1002/eji.1830110105. [DOI] [PubMed] [Google Scholar]