Abstract
High levels of angiogenesis are associated with poor prognosis and a highly invasive phenotype in esophageal squamous carcinoma. C-C chemokine receptor type 7 (CCR7) is overexpressed in multiple tumor types and has been suggested to act as an oncogene and pro-angiogenic factor. This study aimed to elucidate the effect of CCR7 on the angiogenic capacity of esophageal squamous carcinoma cells in vitro. Expression of CCR7 in esophageal squamous carcinoma cell lines and normal human esophageal epithelial cell line was examined by western blotting and quantitative real-time PCR. CCR7 was stably overexpressed or transiently knocked down in esophageal squamous carcinoma cell lines. Overexpressing CCR7 enhanced the capacity of esophageal squamous carcinoma cell conditioned media to induce human umbilical vein endothelial cells (HUVEC) proliferation and migration and neovascularization in the chicken chorioallantoic membrane (CAM) assay. While silencing CCR7 caused an opposite outcome. Moreover, we demonstrated that CCR7 activated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling and regulated its targets, including vascular endothelial growth factor A (VEGF-A), VEGF-C, tumor necrosis factor-α (TNFα), interleukin (IL)-6, IL-8 and transforming growth factor-β (TGF-β) expression. Additionally, CCR7 down-regulation reduced tumor volume and weight in xenograft mouse model, and significantly decreased NF-κB signaling pathway. This study suggests that CCR7 plays an important pro-angiogenic role in esophageal squamous carcinoma via a mechanism linked to activation of the NF-κB pathway; CCR7 may represent a potential target for anti-angiogenic therapy in esophageal squamous carcinoma.
Keywords: CCR7, esophageal squamous carcinoma, angiogenesis, VEGF
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most aggressive tumors and is also the most common cause of esophagus cancer deaths worldwide [1]. Most of individuals presenting with ESCC are diagnosed with advanced disease, due to the late emergence of clinical symptoms. In particular, the presence of regional invasion, distant metastasis and tumor-induced angiogenesis indicate highly malignant potential in esophageal carcinoma patients [2]. Although these patients may benefit from primary tumor surgery or chemotherapy, the prognosis is still quite poor. Plenty of researches demonstrate that tumor induced angiogenesis is required to maintain malignant ESCC growth and metastasis, and constitutes an important hallmark of ESCC progression [3]. Tumor angiogenesis is the complex processes by generation of a new network of blood vessels develop from an existing vasculature that penetrates into the neoplastic tissue to supply the nutrients and oxygen required to maintain and enable tumor growth and invasion [4]. Consequently, application of specific strategy, including monoclonal antibody therapeutics and tyrosine kinase inhibitor that may block tumor angiogenesis could prevent the formation of tumor blood vessels and combat ESCC [5].
Tumor angiogenesis is a consequence of an imbalance between pro-angiogenic factors, such as the vascular endothelial growth factor (VEGF) family and inhibitors of angiogenesis, including endostatin, angiostatin and other related molecules [6]. VEGF family comprises several isotypes, including VEGF-A (vascular permeability factor), VEGF-B, VEGF-C and VEGF-D, as numerous splice variant isoforms [7]. VEGF-A regulates the sprouting and proliferation of endothelial cells and can stimulate tumor angiogenesis. A number of currently-used anti-angiogenesis drugs function by inhibiting pro-angiogenic factors, for example the monoclonal antibody bevacizumab binds to VEGF-A and prevents it from binding to the VEGF receptors, and sunitinib and sorafenib are small molecules that attach to VEGF-R and inhibit the binding of VEGF-A [8]. However, serious side effects, such as hypertension, bleeding and gastrointestinal perforation, have been associated with currently available anti-VEGF agents, limiting their chronic use. However, our knowledge of the precise regulation and mechanisms of tumor angiogenesis is clearly limited and the identification of other novel targets of angiogenesis is urgently required to improve the treatment outcomes and extend survival for cancer patients [9].
Chemokines belong to the small molecule chemo-attractive cytokine family and are grouped into CXC chemokines and CC chemokines on the basis of the characteristic presence of four conserved cysteine residues [10]. Chemokines mediate their chemical effect on target cells through G-protein-coupled receptors, which are characterized structurally by seven transmembrane spanning domains and are involved in the attraction and activation of mononuclear and polymorphonuclear leukocytes. Chemokines and their receptors play important roles in metastasis and tumor growth, however, the role of chemokine receptors in angiogenesis has only recently been explored [11]. As a member of chemokine receptor family, C-C chemokine receptor type 7 (CCR7) is mainly located on the membrane of mature dendritic and T cells, and it could induce the “homing” of dendritic and T cells to the lymph node by binding with its specific ligands CCL19 and CCL21, which are highly expressed in the endothelium of lymphatic vessels and secondary lymph nodes [12]. Interestingly, studies have identified the up-regulation of CCR7 in various types of malignant tumors, such as breast cancer, gastric cancer, and prostate cancer, and have revealed its function in promoting lymph node metastasis. Chemokines and their receptors are induced by inflammatory stimuli in the tumor microenvironment, which are often potent activators of nuclear factor-κB (NF-κB) and activator protein 1 (AP1) transcription factors, thereby linking chronic inflammation to cancer progression [13]. Accumulating evidences suggest that poor therapeutic effect and the dismal survival rate of ESCC are associated with aberrantly activated signaling pathways, including NF-κB signaling. It has been reported that the constitutive activation of NF-κB signaling plays key roles in the development and progression of ESCC and contributes to characteristics of the malignant phenotype in ESCC [14]. For instance, activated NF-κB signaling results in ESCC cell resistance to chemotherapy and promotes cell survival, while inhibition of NF-κB signaling dramatically reduces the proliferation of oral squamous cell carcinoma. In addition, NF-κB signaling is involved in the EGF-induced epithelial-mesenchymal transition (EMT) and is positively associated with lymph node metastasis of ESCC [15]. Based on these findings, CCR7 has been suggested to function as an oncogene in multiple tumor types. However, the effect of CCR7 on tumor angiogenesis in esophageal squamous carcinoma has not yet been elucidated.
To clarify the role of CCR7 in esophageal ESCC, we investigated the CCR7 expression using western blotting, RT-PCR and performed Cancer Genome Atlas (TCGA) analysis. Additionally, ectopic overexpression of CCR7 enhanced the angiogenic capacity of esophageal squamous carcinoma cells in vitro and also up-regulated VEGF-A and VEGF-C, whereas knock-down CCR7 had the opposite effects. This study also demonstrates that CCR7 may promote angiogenesis and the expression of VEGF in esophageal squamous carcinoma cells by activating the NF-κB signaling pathway; therefore, CCR7 may have potential as a therapeutic target in ESCC.
Methods
Cells and treatments
The normal human esophageal epithelial cell line, HEEC and HUVEC were purchased from and cultured as recommended by the American Type Culture Collection (Manassas, VA, USA) and were cultured in 1640 or M199 with 10% FBS, and 1% Penicillin/Streptomycin mix and maintained at 37°C in a humidified atmosphere containing 5% CO2. The esophageal squamous carcinoma cell lines ECA109, TE-1, TE-2, TE-3, TE-8 and TE-12 were purchased from the ATCC and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) and 100 U penicillin-streptomycin (Invitrogen) in a humidified incubator at 37°C in 5% CO2.
Vectors, retrovirus infection and transfection
The CCR7 expression construct was generated by sub-cloning PCR-amplified full-length human CCR7 cDNA into pMSCV-retro-puro using the forward primer 5’-CCTCCGGTGCTGCCTCAAGCCCTTGG-3’ and reverse primer 5’-TTAGGGCCAGAAAGTGGTTGTGCTCT-3’. To knockdown CCR7, a siRNA sequence targeting human CCR7 (The sequences of siCCR7 and was: CCR7, 5’-GCGUCAACCCUUUCUUGUATT-3’) was cloned into pSuper-retro-puro (Promega) to generate pSuper-retro-CCR7-RNAi (referred to as CCR7-Ri). The esophageal squamous carcinoma cells were transiently transfected using Lipofectamine 2000 reagent (Invitrogen) according the manufacturer’s instructions.
Cell viability assay
Briefly, cells (3 × 104 cells per well) were seeded in 96-well plates and cultured for the indicated time. Cell viability was measured by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay and three independent experiments with triplicate were carried out.
Wound healing assay
We examined the migration of human umbilical vein endothelial cells (HUVEC) using a wound-healing assay. Briefly, cells were each grown on 3.5-cm plates with M199. After the growing cell layers had reached confluence, we inflicted a uniform wound in each plate using a pipette tip, and washed the wounded layers with PBS to remove all cell debris. Then, cells were cultured with culture supernatants from the indicated cells. We evaluated the closure at 24 h using bright-field microscopy [16].
Quantitative real-time RT-PCR
Total cellular RNA was extracted using TRIzol reagent (Invitrogen) and 2 μg of RNA was subjected to cDNA synthesis using random hexamers. Quantitative real-time RT-PCR (qRT-PCR) was performed using an Applied Biosystems 7500 Sequence Detection system with an initial denaturation step at 95°C for 10 min, followed by 28 cycles of denaturation at 95°C for 60 sec, primer annealing at 58°C for 30 sec and primer extension at 72°C for 30 sec, with a final extension step at 72°C for 5 min. Target gene expression was calculated using the threshold cycle (Ct) values and the formula 2-[(Ct of Genes) - (Ct of GAPDH)] relative to the internal control gene GAPDH. PCR primers were designed using Primer Express version 2.0 (Applied Biosystems, Foster City, CA, USA) and were as follows: CCR7 forward: 5’-CCCTTGGGTGTCAAAGGTAAA-3’ and reverse: 5’-AAACTGATGCGTGAAGTGCTG-3’; GAPDH forward: 5’-GACTCATGACCACAGTCCATGC-3’ and reverse, 5’-AGAGGCAGGGATGATGTTCTG-3’.
Western blotting
Total cellular protein was extracted and the samples were heated at 100°C for 5 min. Samples containing 20 μg protein were separated by SDS-PAGE, electro-blotted onto PVDF membranes (Millipore, Billerica, MA, USA), blocked in non-fat milk, probed with polyclonal rabbit anti-CCR7 (Abcam, Cambridge, MA, USA), anti-IKK, anti-phosphorylated-IKK (p-IKK), anti-IκBα or anti-p-IκBα (p-IκBα; all Cell Signaling, Danvers, MA, USA), VEGF-A (Cell Signaling), VEGF-C (Cell Signaling), TNF-α (Cell Signaling), IL-6 (Cell Signaling), IL-8 (Cell Signaling), and TGF-β (Cell Signaling). The membranes were stripped and re-probed using anti-α-Tubulin (Cell Signaling) as a loading control.
Chicken chorioallantoic membrane assay
The chicken chorioallantoic membrane (CAM) assay was performed using eight-day-old fertilized chicken eggs. A 1 cm diameter window was created in the shell of each egg and the surface of the dermic sheet was removed to expose the CAM. A 0.5 cm diameter filter paper was placed on top of the CAM, and 100 μl culture supernatants harvested from the indicated esophageal squamous carcinoma cells placed on the center of the filter paper. The eggs were incubated at 37°C at 80-90% relative humidity for 48 h, then the windows in the shell were closed using sterilize bandages. Following fixation with stationary solution (1:1 vol/vol mixture of methanol and acetone) for 15 min, the CAM was excised and imaged using a digital camera. The number of second- and third-order vessels in the test groups was expressed relative to that of CAM treated with culture supernatants from the indicated cells [17].
Luciferase reporter assay of NF-κB transcriptional activity
The pNF-κB-luciferase reporter and control plasmids (Clontech, Mountain View, CA, USA) were used examine NF-κB transcriptional activity. Approximately 1.5 × 104 esophageal squamous carcinoma cells were seeded in triplicate in 24-well plates, allowed to adhere, and co-transfected with 100 ng of the NF-κB luciferase reporter plasmid or control luciferase plasmid and 1 ng of pRL-TK Renilla plasmid (Promega) using Lipofectamine 2000 reagent (Invitrogen). The luciferase and Renilla signals were measured 48 h after transfection using the Dual Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol.
ELISA
Cells (7 × 105) were plated in six-well dishes and treated for 24 h. Supernatants were collected and ELISA for TNF-α, IL-6, IL-8, and TGF-β following the manufacturer’s instructions [18].
Mouse Xenografts
All animal procedures were approved by the ethical commission of the Institutional Animal Care and Use Committee (IACUC) of Third Military Medical University. Eca109 (5 × 106 cells per mouse) were injected s.c. into the right posterior flanks of 7-wk-old immunodeficient NOD/SCID female mice (6 mice per group). Tumor volume and mice body weight were measured every 3 days. Tumor volume was calculated as mm3 = 0.5 × length × width (mm)2. After sacrificing mice on day 37, tumor tissues were assessed with specific antibodies using western blotting analysis [19]. All animal experiments were carried out in compliance with the Guidelines for the Institutional Animal Care and Use Committee (IACUC) of Third Military Medical University.
Statistical analysis
All experimental data are presented as the mean ± SD of three independent biological replicates. Statistical analyses were performed using SPSS 13.0 (IBM, Armonk, NY, USA). Analysis of variance (ANOVA) was used to evaluate the significance of the differences between two groups. P-values ≤ 0.05 were considered statistically significant.
Results
CCR7 is up-regulated in esophageal squamous carcinoma
To determine whether the expression of CCR7 in esophageal squamous carcinoma is altered, Oncomine analysis of neoplastic vs. normal tissue from TCGA dataset (https://tcga-data.nci.nih.gov/tcga) proved that CCR7 was significantly over-expression in esophageal squamous carcinoma (Figure 1A). To further assess the expression level of CCR7 in esophageal squamous carcinoma, western blotting analyses were performed to examine CCR7 protein expression in esophageal squamous carcinoma cell lines. Our data showed CCR7 protein expression was significantly higher in all five esophageal squamous carcinoma cell lines tested compared with the normal human esophageal epithelial cell line, HEEC, which expressed low levels of CCR7 (Figure 1B). Consistently, qRT-PCR revealed that CCR7 was significantly overexpressed in g esophageal squamous carcinoma cell lines at mRNA level, compared with HEEC (Figure 1C). Taken together, these data suggest that CCR7 is up-regulated in esophageal squamous carcinoma cells.
Figure 1.
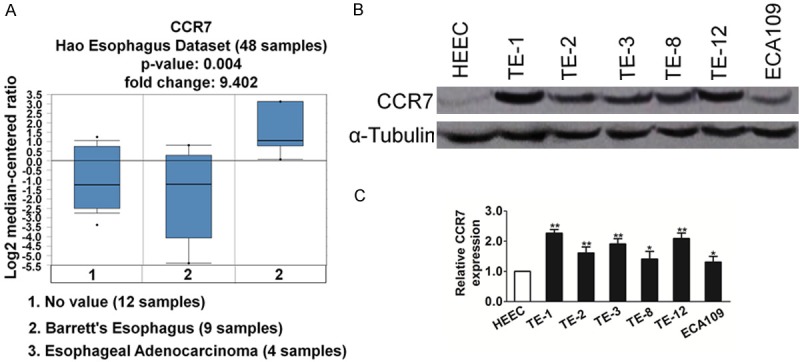
CCR7 is up-regulated in esophageal squamous carcinoma. A. The expression of CCR71 in esophageal squamous carcinoma versus paired non-tumorous esophageal epithelial tissues using datasets deposited in the TCGA datasets (Reporter ID: IMAGE: 2345206). B. Western blotting analysis of CCR7 expression in the normal human esophageal epithelial cell line and six cultured esophageal squamous carcinoma cell lines; α-Tubulin was used as a loading control. C. mRNA expression of CCR7 was assessed by qRT-PCR in normal cell line and six cultured ESCC cell lines. Transcript levels were normalized to GAPDH and expressed relative to HEEC cells. Data is mean ± SD of three independent experiments; for indicated comparisons, *P < 0.05, **P < 0.01.
CCR7 promotes the angiogenic capacity and expression of VEGFC
The esophageal squamous carcinoma cell line ECA109 expressed moderate levels of CCR7 and was used to create stable cell line overexpressing CCR7. Overexpression of CCR7 in the stable cell lines was verified by western blotting (Figure 2A). As analyzed by MTT assay and shown in Figure 2B and 2C, CCR7 over-expression significantly increased the cell viability of ECA109 cells, as compared with their corresponding control cells. Firstly, the effect of CCR7 on the ability of ECA109 cells to induce angiogenesis was investigated using the HUVEC proliferation assay. HUVECs were seeded in culture supernatants harvested from CCR7-overexpressing ECA109 cells. Culture supernatants derived from CCR7-transduced cells significantly increased the growth of HUVEC cells compared to culture supernatants from vector control cells (Figure 2D). Moreover, culture supernatants from CCR7-overexpressing ECA109 cells significantly increased the migration of HUVEC cells in the migration assay (Figure 2E). Furthermore, ectopic over-expression of CCR7 in ECA109 cells enhanced the ability of culture supernatants to induce the formation of vessels in the CAM assay (Figure 2F). As neovessel formation is closely associated with VEGF, we examined the expression of VEGF-A and VEGF-C in CCR7-overexpressing and vector control ECA109 cells using western blotting analysis. Both VEGF-A and VEGF-C protein expression was significantly up-regulated in the CCR7-overexpressing ECA109 cells (Figure 2G). Taken together; these results suggest that CCR7 enhanced the capacity of esophageal squamous carcinoma cells to induce neovessel formation in vitro.
Figure 2.
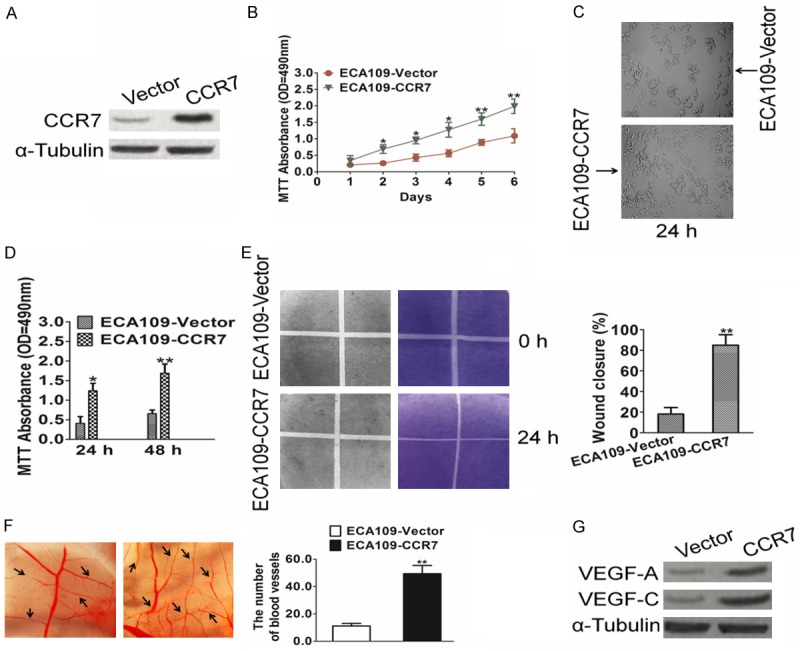
CCR7 enhances the angiogenic capacity of esophageal squamous carcinoma cells in vitro. A. Western blotting analysis of CCR7 protein expression in ECA109-vector, and ECA109-CCR7 cells; α-Tubulin was used as a loading control. B. Cell viability was determined by MTT proliferation assay after ECA109-vector and ECA109-CCR7 cells cultured for the indicated days. C. Representative images of cell morphology in ECA109 cells were transfected with vector or CCR7. D. HUVEC viability was determined by MTT proliferation assay after cells after incubation in culture supernatants derived from the indicated cells. E. Representative images (left) and quantification (right) of the number of migrated HUVEC cells after incubation in culture supernatants derived from the indicated cells in the migration assay. F. Representative images (left) and quantification (right) of capillaries formed in the CAM assay when stimulated by culture supernatants derived from the indicated cells. G. Western blotting analysis of VEGF-A and VEGF-C expression in the indicated cells. Data is mean ± SD of three independent experiments; for indicated comparisons, *P < 0.05, **P < 0.01.
Silencing CCR7 reduces the angiogenic capacity and expression of VEGFC
To further confirm the effect of CCR7 on angiogenesis during the progression of esophageal squamous carcinoma, stable Eca109 cell line in which CCR7 was silenced were established; knockdown of CCR7 in these cells was confirmed by western blotting (Figure 3A). Compared to culture supernatants from control cells, culture supernatants from CCR7-silenced cells inhibited Eca109 proliferation (Figure 3B and 3C); suggesting that knockdown of endogenous CCR7 reduced the proliferation ability of esophageal squamous carcinoma cells. Moreover, culture supernatan-ts from CCR7-silenced Eca109 cells inhibited HUVEC growth (Figure 3D), migration (Figure 3E) and decreased the formation of vessels in the CAM assay (Figure 3F). In parallel with these results, knockdown of CCR7 significantly reduced VEGF-A and VEGF-C protein expression (Figure 3G) in Eca109 cell line. These results confirmed that CCR7 enhances the angiogenic capacity of esophageal squamous carcinoma cells.
Figure 3.
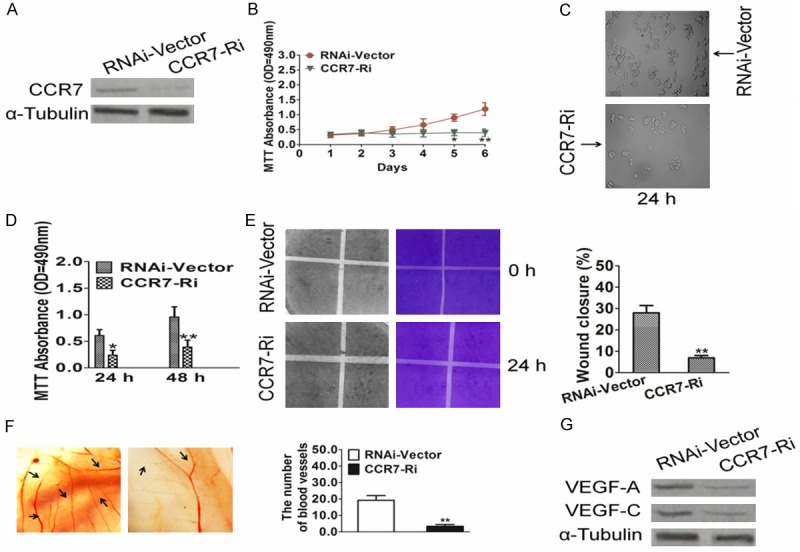
Knockdown of CCR7 reduces the angiogenic capacity of esophageal squamous carcinoma cells in vitro. A. Western blotting analysis of CCR7 protein expression in CCR7-siRNA-transduced Eca109 cell line (shown as CCR7-RNAi) and the corresponding control cells (shown as RNAi-Vector); α-Tubulin was used as a loading control. B. Cell viability was determined by MTT proliferation assay after CCR7-siRNA-transduced Eca109 and Vector-siRNA-transduced Eca109 cells cultured for the indicated days. C. Representative images of cell morphology in Eca109 cells were transfected with Vector-siRNA or siRNA-CCR7. D. HUVEC viability was determined by MTT proliferation assay after cells after incubation in culture supernatants derived from the indicated cells. E. Representative images (left) and quantification (right) of the number of migrated HUVEC cells when incubated in culture supernatants derived from the indicated cells in the Transwell migration assay. F. Representative images (left) and quantification (right) of neovessels formed in the CAM assay when stimulated by culture supernatants derived from the indicated cells. G. Western blotting analysis of VEGF-A and VEGF-C expression in the indicated cells. Data is mean ± SD of three independent experiments; for indicated comparisons, *P < 0.05, **P < 0.01.
CCR7 promotes the angiogenic capacity of esophageal squamous carcinoma cells via NF-κB signaling pathway
As VEGF has been reported to be a downstream target of the NF-κB pathway, we explored effect of CCR7 on NF-κB signaling activity. Luciferase reporter assays demonstrated that overexpression of CCR7 enhanced the transcriptional activity of a NF-κB reporter gene, while knockdown of CCR7 suppressed NF-κB transcriptional activity (Figure 4A). Western blotting showed that over-expression of CCR7 increased the levels of phosphorylated IKK and phosphorylated IκBα but did not significantly change the total protein level of IKK or IκBα (Figure 4B). In addition, the levels of number of NF-κB target genes, including TNF-α, IL-6, IL-8, and TGF-β were up-regulated in CCR7-overexpressing cells and down-regulated in CCR7-silenced esophageal squamous carcinoma cells (Figure 4C and 4D). Taken together, these results indicated that the NF-κB pathway may underlie the pro-angiogenic effect of CCR7 in esophageal squamous carcinoma.
Figure 4.
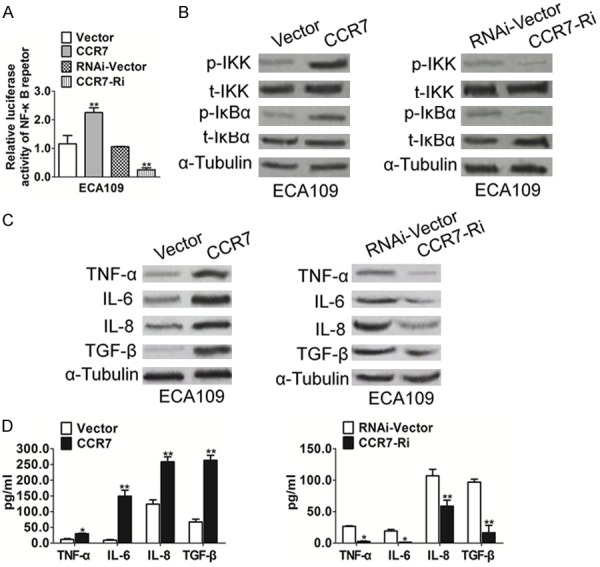
CCR7 promotes NF-κB transcriptional activity. A. Luciferase reporter assay of NF-κB transcriptional activity in CCR7-overexpressing or silenced cells expressed relative to the respective control cells. B. Western blotting analysis of the expression of phosphorylated IKK (p-IKK), total IKK, phosphorylated IκBα (p-IκBα) and total IκBα; α-Tubulin was used as a loading control. C. Western blotting analysis of the expression of genes downstream of NF-κB in the indicated cells; transcript levels were normalized to α-Tubulin. D. ELISA assay was conducted to evaluate the TNF-α, IL-6, IL-8, and TGF-β secretion in the indicated cells. Data is mean ± SD of three independent experiments; for indicated comparisons, *P < 0.05, **P < 0.01.
Down-regulation CCR7 inhibits esophageal squamous carcinoma cells growth in vivo
In view of these findings in vitro, we tested the efficacy of siRNA targeting CCR7 in a nude mouse xenograft model, which were vaccinated subcutaneously 1 × 107 Eca109 cells after CCR7 siRNA. As shown in Figure 5A and 5B, control siRNA group did not affect the growth of tumor, whereas mean tumor volume in mice injected with CCR7 siRNA was significantly decreased compared that in mice treated with control siRNA. In addition, compared with the control groups, no significant body weight change or other toxicity was observed in the treatment groups (data not shown). To explore the molecule mechanisms of suppressing expression of CCR7 leading to tumor growth inhibition in the nude mouse xenograft, the tumor protein expression of VEGF-A, VEGF-C, TNF-α, IL-6, IL-8, TGF-β and NF-κB were detected by western blotting (Figure 5C). In accordance with the results in vitro, after the expression of CCR7 was suppressed, the levels of VEGF-A, VEGF-C, TNF-α, IL-6, IL-8 and TGF-β were simultaneously significantly decreased and NF-κB signaling pathway was blocked.
Figure 5.
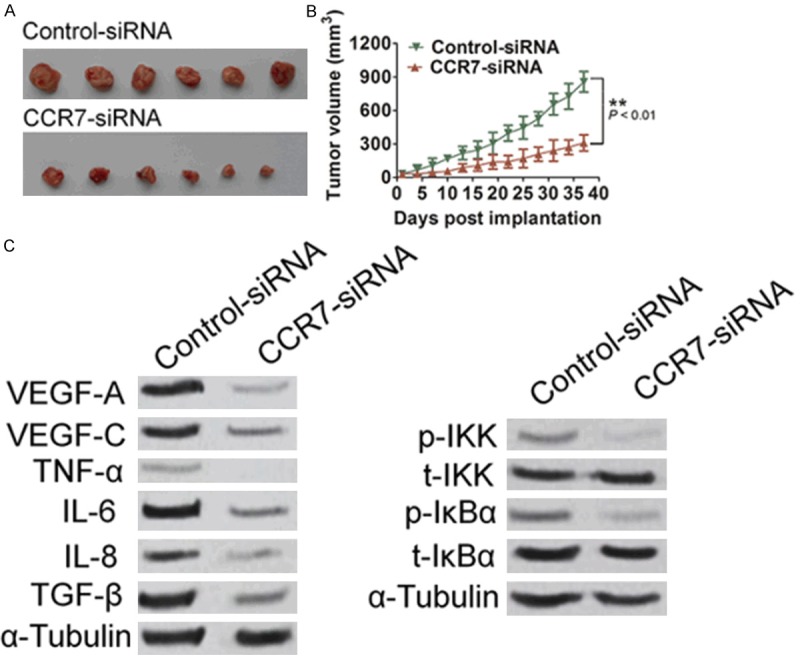
CCR7 siRNA inhibits esophageal squamous carcinoma cells growth in the nude mouse xenograft model. Representative images of tumor-bearing mice (A) and tumor volume from all of the mice in each group (B). n = 6, Data is mean±SD of three independent experiments; for indicated comparisons, *P<0.05, **P<0.01. (C) Effect of CCR7 siRNA on the expression of VEGF-A, VEGF-C, TNF-α, IL-6, IL-8, TGF-β expression and NF-κB phosphorylation in the nude mouse xenograft was detected by western blotting with specific antibodies.
Discussion
Even though the mortality and incidence rate for esophageal squamous cell carcinoma (ESCC) have decreased over the last two decades, likely due to improved nutritional status and advancesin detection and treatment. ESCC is however, still one of the most lethal types of digestive tract malignancy in China [20]. Surgical resection remains chief curative therapy, but tumor angiogenesis allows poor prognosis. Angiogenesis is essential for ESCC progression in order to supply nutrients and oxygen, and remove metabolic waste [21]. Therefore, inhibiting the angiogenic process may be used as a potential therapy to prevent tumor growth and metastasis. Among the angiogenic factors, vascular endothelial growth factors (VEGF) are positively associated with the proliferation, migration and tube formation of endothelial cells. Upon VEGF binding to VEGF receptor (VEGFR), the main functional receptor tyrosine kinases of VEGF are activated, and a cascade of events are initiated, including the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and RAF/MEK/ERK signaling pathways, which stimulate endothelial cell proliferation, migration and tube formation [22]. Most anti-angiogenic therapies currently being evaluated in clinical trials target the VEGF pathway; however, the tumor vasculature can acquire resistance to VEGF-targeted therapy by shifting to other angiogenesis mechanisms. Therefore, better strategies of treatment will ultimately require understanding the molecular mechanisms of the angiogenesis step of tumor and identifying and specifically targeting the critical signaling effectors.
CCR7 can promote the growth and survival of esophageal squamous carcinoma cells and was the first gene identified to be up-regulated in the presence of HBxAg, indicating CCR7 may potentially play a role in the progression of esophageal squamous carcinoma [23]. Although several studies have indicated that CCR7 may act as an oncogene in various tumor types, the exact function and molecular mechanism of actions of CCR7 on angiogenesis in esophageal squamous carcinoma has not yet been precisely elucidated. In the present study, we demonstrate for the first time that over-expression of CCR7 increased the proliferation in HUVEC cells and significantly increased the migration of HUVEC cells, and enhanced the ability to induce the formation of vessels of CAM. Therefore, all of the results indicate CCR7 may exert a number of functions during the development and angiogenesis of esophageal squamous carcinoma and should be considered as a potential novel therapeutic target for esophageal squamous carcinoma. In combination with the ability of CCR7 to promote the angiogenic capacity of esophageal squamous carcinoma cells, VEGF-A and VEGF-C were markedly up-regulated in CCR7-overexpressing cells, indicating that an association exists between CCR7 and VEGF.
VEGF is one of the target genes downstream of the NF-κB pathway [24]. Luciferase reporter assays showed overexpression of CCR7 significantly enhanced the transcriptional activity of NF-κB, suggesting NF-κB plays an essential role in the CCR7-induced angiogenic capacity of esophageal squamous carcinoma cells. NF-κB has been widely studied as a transcription factor that regulates inflammatory and immune responses, as well as range of other physiological and pathological processes including the development and progression of cancer. NF-κB mediates a range of biological processes in cancer cells by transcriptionally activating numerous target genes [25]. Activation of NF-κB signaling is negatively regulated by the IκBs, which bind and sequester NF-κB in the cytoplasm in an inactive state. IκBs are phosphorylated by IKKs, which leads to ubiquitin-mediated degradation of the IκBs and consequently enables the release and translocation of NF-κB to the nucleus. Consistent with these well-studied processes, the present study demonstrated that over-expression of CCR7 up-regulated the level of p-IKK and p-IκBα and ultimately enhanced the activation of NF-κB. These findings indicate that CCR7 promotes the angiogenic capacity of esophageal squamous carcinoma cells - at least in part - by activating the NF-κB/VEGF signaling pathway.
Additionally, overexpression of CCR7 up-regulated a number of genes downstream of the NF-κB signaling pathway: TNF-α, IL-6, IL-8 and TGF-β. TNF-α is well-recognized to promote angiogenesis and drive remodeling of blood vessels in vivo; interleukin-6 increases the expression of VEGF and can promote angiogenesis; IL-8 has been shown to play an important role in tumor angiogenesis; and TGF-β plays an essential role in vasculogenesis and angiogenesis during the development and progression of various types of cancer. It would be interesting to explore whether TNF, IL-6, IL-8 or TGF-β play a role in angiogenesis and disease progression in esophageal squamous carcinoma, and explore the correlation between the expression of these genes and NF-κB/VEGF pathway. The regulatory mechanism by which up-regulation of CCR7 modulates the NF-κB/VEGF pathway and enhances the angiogenic capacity of esophageal squamous carcinoma cells remains to be elucidated and should be investigated further. In conclusion, this study demonstrates that CCR7 is upregulated in esophageal squamous carcinoma cell lines and enhances the angiogenic capacity of esophageal squamous carcinoma cells via activation of the NF-κB signaling pathway. The major findings in this study can be illustrated in Figure 6. These results may provide new insight into the mechanisms that regulate angiogenesis in esophageal squamous carcinoma; targeting CCR7 may represent a promising therapeutic strategy for esophageal squamous carcinoma.
Figure 6.
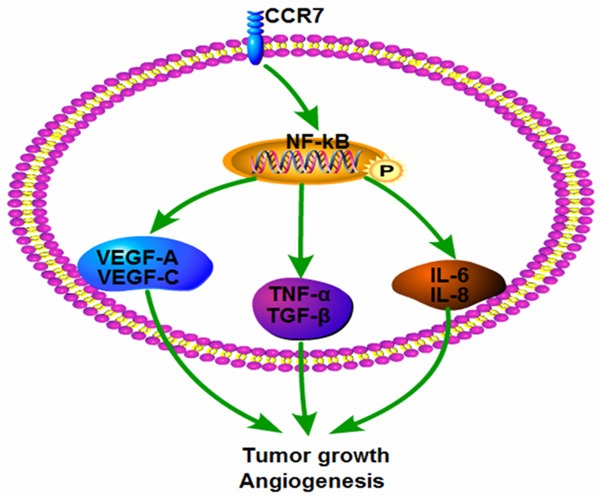
Proposed model by which CCR7 regulated NF-κB/VEGF pathway and induced angiogenesis in esophageal squamous carcinoma cells.
Acknowledgements
This project was supported by a grant from the National Natural Sciences Foundation of China (No. [2013]81301730) and Science and Technology Foundation of Guizhou [2013] No. 2325.
Disclosure of conflict of interest
None.
References
- 1.Fan T, Chen J, Zhang L, Gao P, Hui Y, Xu P, Zhang X, Liu H. Bit1 knockdown contributes to growth suppression as well as the decreases of migration and invasion abilities in esophageal squamous cell carcinoma via suppressing FAK-paxillin pathway. Mol Cancer. 2016;15:23. doi: 10.1186/s12943-016-0507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao SG, Liu RM, Zhao YG, Wang P, Ward DG, Wang GC, Guo XQ, Gu J, Niu WB, Zhang T, Martin A, Guo ZP, Feng XS, Qi YJ, Ma YF. Integrative topological analysis of mass spectrometry data reveals molecular features with clinical relevance in esophageal squamous cell carcinoma. Sci Rep. 2016;6:21586. doi: 10.1038/srep21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji C, Liu H, Xiang M, Liu J, Yue F, Wang W, Chu X. Deregulation of decorin and FHL1 are associated with esophageal squamous cell carcinoma progression and poor prognosis. Int J Clin Exp Med. 2015;8:20965–20970. [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W, Li M, Chen X, Zhang D, Wei L, Zhang Z, Wang S, Meng L, Zhu S, Li B. MicroRNA-373 promotes migration and invasion in human esophageal squamous cell carcinoma by inhibiting TIMP3 expression. Am J Cancer Res. 2016;6:1–14. [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Q, Jin B, Zhang Y, Shi Y, Zhang C, Luo D, Wang P, Duan C, Song H, Li X, Deng X, Chen Z, Wang Z, Jiang H, Liu Y. Secreted recombinant human IL-24 protein inhibits the proliferation of esophageal squamous cell carcinoma Eca-109 cells in vitro and in vivo. Oncol Rep. 2016;35:2681–2690. doi: 10.3892/or.2016.4633. [DOI] [PubMed] [Google Scholar]
- 6.Shen WB, Zhu SC, Gao HM, Li YM, Su JW, Li J, Liu ZK. Analysis of failure patterns in patients with resectable esophageal squamous cell carcinoma receiving chemoradiotherapy. J Cancer Res Ther. 2016;12:62–68. doi: 10.4103/0973-1482.146128. [DOI] [PubMed] [Google Scholar]
- 7.Huang JX, Yao J, Lin MS, Lin M, Xiao W, Yu H, Chen P, Qian RY. Evaluation of tumor metastasis-associated markers for molecular classification in patients with esophageal squamous cell carcinoma. Int J Clin Exp Med. 2015;8:15920–15929. [PMC free article] [PubMed] [Google Scholar]
- 8.Cui Y, Dong C, Wu BQ, Duan XC, Shi G, Gong M, Wang TY. Expression of cyclooxygenase-2, vascular endothelial growth factor, and epidermal growth factor receptor in Chinese patients with esophageal squamous cell carcinoma. J Cancer Res Ther. 2015;11(Suppl 1):C44–48. doi: 10.4103/0973-1482.163838. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Wang Z, Liu X, Jiang W, Shi M. CCR7 and VEGF-C: molecular indicator of lymphatic metastatic recurrence in pN0 esophageal squamous cell carcinoma after Ivor-Lewis esophagectomy? Ann Surg Oncol. 2012;19:3606–3612. doi: 10.1245/s10434-012-2419-y. [DOI] [PubMed] [Google Scholar]
- 10.Irino T, Takeuchi H, Matsuda S, Saikawa Y, Kawakubo H, Wada N, Takahashi T, Nakamura R, Fukuda K, Omori T, Kitagawa Y. CCChemokine receptor CCR7: a key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer. 2014;14:291. doi: 10.1186/1471-2407-14-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi BJ, Du CL, Fu YF, Zhang YN, Wang RW. Silencing of CCR7 inhibits the growth, invasion and migration of prostate cancer cells induced by VEGFC. Int J Clin Exp Pathol. 2015;8:12533–12540. [PMC free article] [PubMed] [Google Scholar]
- 12.Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K, Jin Y, Jansson M, Alexander JS, Nelson CM, Jakobsson L, Betsholtz C, Sund M, Karlsson MC, Fuxe J. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene. 2016;35:748–760. doi: 10.1038/onc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma H, Gao L, Li S, Qin J, Chen L, Liu X, Xu P, Wang F, Xiao H, Zhou S, Gao Q, Liu B, Sun Y, Liang C. CCR7 enhances TGF-beta1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget. 2015;6:24348–24360. doi: 10.18632/oncotarget.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi M, Chen D, Yang D, Liu XY. CCL21-CCR7 promotes the lymph node metastasis of esophageal squamous cell carcinoma by up-regulating MUC1. J Exp Clin Cancer Res. 2015;34:149. doi: 10.1186/s13046-015-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paccez JD, Duncan K, Vava A, Correa RG, Libermann TA, Parker MI, Zerbini LF. Inactivation of GSK3beta and activation of NFkappaB pathway via Axl represents an important mediator of tumorigenesis in esophageal squamous cell carcinoma. Mol Biol Cell. 2015;26:821–831. doi: 10.1091/mbc.E14-04-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Li X, Dong W, Sun C, Guo D, Zhang L. Mmu-miR-1894-3p Inhibits Cell Proliferation and Migration of Breast Cancer Cells by Targeting Trim46. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li MY, Lv YC, Tong LJ, Peng T, Qu R, Zhang T, Sun YM, Chen Y, Wei LX, Geng MY, Duan WH, Xie H, Ding J. DW10075, a novel selective and small-molecule inhibitor of VEGFR, exhibits antitumor activities both in vitro and in vivo. Acta Pharmacol Sin. 2016;37:398–407. doi: 10.1038/aps.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao HH, Wang YC, Chen MC, Tsai HY, Lin J, Chen ST, Tsay GJ, Cheng SL. Downregulation of granulocyte-macrophage colonystimulating factor by 3C-like proteinase in transfected A549 human lung carcinoma cells. BMC Immunol. 2011;12:16. doi: 10.1186/1471-2172-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiue YW, Lu CC, Hsiao YP, Liao CL, Lin JP, Lai KC, Yu CC, Huang YP, Ho HC, Chung JG. Casticin Induced Apoptosis in A375. S2 Human Melanoma Cells through the Inhibition of NF-[Formula: see text] B and Mitochondria-Dependent Pathways In Vitro and Inhibited Human Melanoma Xenografts in a Mouse Model In Vivo. Am J Chin Med. 2016:1–25. doi: 10.1142/S0192415X1650035X. [DOI] [PubMed] [Google Scholar]
- 20.Lu C, Xie C. Radiation-induced autophagy promotes esophageal squamous cell carcinoma cell survival via the LKB1 pathway. Oncol Rep. 2016;35:3559–65. doi: 10.3892/or.2016.4753. [DOI] [PubMed] [Google Scholar]
- 21.Kumagai Y, Sobajima J, Higashi M, Ishiguro T, Fukuchi M, Ishibashi K, Mochiki E, Yakabi K, Kawano T, Tamaru J, Ishida H. Coexpression of COX-2 and iNOS in Angiogenesis of Superficial Esophageal Squamous Cell Carcinoma. Int Surg. 2015;100:733–743. doi: 10.9738/INTSURG-D-14-00234.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao ZH, Tian Y, Yang JP, Zhou J, Chen KS. RhoC, vascular endothelial growth factor and microvascular density in esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:905–912. doi: 10.3748/wjg.v21.i3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu L, Chen W, Guo W, Wang J, Tian Y, Shi D, Zhang X, Qiu H, Xiao X, Kang T, Huang W, Wang S, Deng W. Berberine Targets AP-2/hTERT, NF-kappaB/COX-2, HIF-1alpha/VEGF and Cytochrome-c/Caspase Signaling to Suppress Human Cancer Cell Growth. PLoS One. 2013;8:e69240. doi: 10.1371/journal.pone.0069240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo M, Hou L, Li J, Shao S, Huang S, Meng D, Liu L, Feng L, Xia P, Qin T, Zhao X. VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-kappaB and beta-catenin. Cancer Lett. 2016;373:1–11. doi: 10.1016/j.canlet.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Katanov C, Lerrer S, Liubomirski Y, Leider-Trejo L, Meshel T, Bar J, Feniger-Barish R, Kamer I, Soria-Artzi G, Kahani H, Banerjee D, Ben-Baruch A. Regulation of the inflammatory profile of stromal cells in human breast cancer: prominent roles for TNF-alpha and the NFkappaB pathway. Stem Cell Res Ther. 2015;6:87. doi: 10.1186/s13287-015-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


