Abstract
Prion diseases in humans and animals are invariably fatal. Prions are composed of a disease-causing isoform (PrPSc) of the normal host prion protein (PrPC) and replicate by stimulating the conversion of PrPC into nascent PrPSc. We report here that tricyclic derivatives of acridine and phenothiazine exhibit half-maximal inhibition of PrPSc formation at effective concentrations (EC50) between 0.3 μM and 3 μM in cultured cells chronically infected with prions. The EC50 for chlorpromazine was 3 μM, whereas quinacrine was 10 times more potent. A variety of 9-substituted, acridine-based analogues of quinacrine were synthesized, which demonstrated variable antiprion potencies similar to those of chlorpromazine and emphasized the importance of the side chain in mediating the inhibition of PrPSc formation. Thus, our studies show that tricyclic compounds with an aliphatic side chain at the middle ring moiety constitute a new class of antiprion reagents. Because quinacrine and chlorpromazine have been used in humans for many years as antimalarial and antipsychotic drugs, respectively, and are known to pass the blood–brain barrier, we suggest that they are immediate candidates for the treatment of Creutzfeldt–Jakob disease and other prion diseases.
Prion diseases are uniquely manifest as spontaneous, inherited, and infectious maladies. These diseases include Gerstmann–Sträussler–Scheinker (GSS) disease, fatal insomnia, and Creutzfeldt–Jakob disease (CJD). Most cases of CJD are sporadic with 10–15% being inherited (1). Although the infectious human prion diseases are most notorious, they account for less than 1% of all prion disorders (2). Concern about these infectious disorders has been heightened by the identification of more than 100 young adults and teenagers who have developed new variant CJD (nvCJD) in Europe after exposure to bovine prions from cattle with bovine spongiform encephalopathy (BSE; refs. 3 and 4). Other infectious prion diseases include kuru, which is found among New Guinea natives and is caused by ritualistic cannibalism, and iatrogenic CJD, which is caused by prion-contaminated cadaveric growth hormone and dura mater grafts (2, 5, 6).
A wealth of experimental data indicates that prions are composed solely of a misfolded prion protein (PrP) isoform (PrPSc) of a glycolipid-anchored host protein (PrPC; refs. 7 and 8). Unlike all other infectious agents, prions are devoid of nucleic acid (9, 10). For years, the existence of prion strains caused many investigators to argue that a small nucleic acid encodes prion diversity (11). Eventually, convincing data accumulated arguing that prion diversity is enciphered in the conformation of PrPSc (3, 12, 13).
Patients with CJD and other prion diseases develop progressive neurologic dysfunction. Prion diseases are invariably fatal, and death frequently occurs in less than 1 year after the first symptoms appear (2). No effective therapy exists for prion diseases in humans or animals (14).
Many compounds have been identified that inhibit prion propagation when administered at the time of inoculation in rodents (15–18). Treatment with these same compounds administered immediately before or during the onset of neurologic dysfunction has proven ineffective. Other compounds that inhibit PrPSc formation, including Congo red, have been identified by using scrapie-infected cultured cells (19–21). Some of these compounds have been examined in rodents but none have been effective when given around the time that neurologic signs appear (22).
In a search for compounds that might prove effective in treating prion diseases, we have used scrapie-infected neuroblastoma (ScN2a) cells to screen for inhibition of nascent PrPSc formation as well as the clearance of preexisting PrPSc. Based on the demonstration that PrPSc formation occurs in cholesterol-rich microdomains, inhibitors of cholesterol biosynthesis were examined for their ability to inhibit the conversion of PrPC into PrPSc (23, 24). Although statin drugs were found to inhibit PrPSc formation in cultured cells, the level of cholesterol depletion required does not permit such an approach to be used in animals. In contrast, dominant negative inhibition of PrPSc formation has been found in sheep and humans (25, 26). This approach was the basis of rational drug design strategy that identified several lead compounds, which mimic a discontinuous epitope on the surface of PrPC (27). In another study, we identified antibody fragments (Fabs) that bind to PrPC on the surface of cells and inhibit PrPSc formation (D. Peretz, R. A. Williamson, K. Kaneko, J. Vergara, E. Leclerc, I. R. Mehlhorn, G. Legname, M. R. Wormald, P. M. Rudd, R. A. Dwek, et al., unpublished data). Treatment of cells with these Fab fragments also resulted in the clearance of preexisting PrPSc. Branched polyamines also were found to promote the clearance of PrPSc from scrapie-infected cultured cells (28).
Because the blood–brain barrier (BBB) restricts the access of many molecules to the central nervous system, we decided to screen a variety of drugs that are known to penetrate the BBB for their ability to inhibit PrPSc formation. We report here on tricyclic acridine and phenothiazine derivatives. Phenothiazines are compounds with a tricyclic scaffold and a side chain extending from the middle ring moiety that have been used to treat psychoses for nearly 50 years (29). Effective concentrations for half-maximal inhibition (EC50) of PrPSc formation were determined in ScN2a cells. Chlorpromazine inhibited PrPSc formation at 3 μM, whereas quinacrine, a structural antecedent of the phenothiazines, was 10 times more potent. A variety of quinacrine derivatives were synthesized and demonstrated potencies similar to chlorpromazine, emphasizing the importance of the aliphatic side chain in mediating the inhibition of PrPSc formation. Our findings define a new class of antiprion compounds consisting of a tricyclic scaffold with an aliphatic side chain extending from the middle ring moiety.
While preparing our manuscript, we learned of three earlier reports describing the potent inhibition of PrPSc formation by acridine and phenothiazine derivatives. Chlorpromazine administered repeatedly to mice s.c. before and after intracerebral inoculation of scrapie prions resulted in a prolongation of the incubation period by 1 month, but no change in the incubation period was seen if the prions were inoculated i.p. (30). In another study, 50 μM of chlorpromazine was added to hamster brain fractions enriched for membranes and the suspension was exposed to UV irradiation (31). The prion titers of the nonirradiated controls (exposed only to chlorpromazine) were reduced by ≈10-fold, whereas the titers of the irradiated samples were reduced >100-fold. In a third study, quinacrine and other lysomotrophic reagents added to ScN2a cells resulted in the clearance of PrPSc (32).
Because quinacrine has been used in humans as an antimalarial drug for over 60 years and is known to cross the BBB, it is an immediate candidate for the treatment of patients dying of CJD and other prion diseases. Because humans can tolerate 100–1,000 mg of quinacrine administered orally on a daily schedule, such doses are likely to find an immediate place in attempts to treat patients with clinical signs of prion disease. As an alternative or even an adjunct to quinacrine therapy, phenothiazine derivatives effective in PrPSc inhibition may be used to treat humans, despite their weaker antiprion potency in our ScN2a cell assay, because they may cross the BBB more effectively. It will be important to treat patients dying of prion diseases within the context of well designed clinical trials to establish as rapidly as possible whether quinacrine or phenothiazine derivatives can provide effective treatment.
Materials and Methods
Compounds were purchased from Sigma and Aldrich. Drugs were dissolved in PBS whenever possible (chlorpromazine and quinacrine are soluble in PBS), otherwise in 100% DMSO and then diluted into a stock solution of 10% (vol/vol) DMSO/PBS. Final DMSO concentration was, in no case, greater than 0.1%, and most often less than 0.01%. Stock solutions were filtered although a 0.2-μm syringe filter, kept at 4°C during the time of the experiment, and made freshly for each separate experiment.
Synthesis of 9-Substituted Acridines.
The appropriate amine (0.35 mM) was reacted with 6,9-dichloro-2-methoxyacridine (0.35 mM) in phenol (500 mg) by heating at 120°C for 2 h. The reaction mixture was cooled to room temperature, diluted with 5 M aqueous sodium hydroxide (50 ml), and extracted with ether (3 × 75 ml). The combined organics were extracted with 10% (vol/vol) aqueous HCl (3 × 75 ml), and the pooled aqueous phases made alkaline to pH ≈11 with 10% (vol/vol) aqueous potassium hydroxide. The aqueous phase then was extracted with ether (3 × 75 ml), and the pooled organics were dried (MgSO4), filtered, and evaporated to give the desired 9-substituted acridine in 53–78% yield. The structure of the acridine analogs was confirmed by 1H, 13C NMR (CD3OD) and high-resolution electrospray MS.
PrPSc Inhibition Assay in ScN2a Cells.
Neuroblastoma cells were infected with the RML strain of mouse-adapted scrapie prions and subcloned (33). A confluent 10-cm dish was split and a drop of cells was pipetted into a 60-mm dish of 4 ml of MEM containing 10% (vol/vol) FCS, penicillin-streptomycin, and nonessential amino acids. Medium was exchanged every second day, together with the drug. Cells were lysed (lysis buffer: 10 mM Tris, pH 8.0/150 mM NaCl/0.5% Nonidet P-40/0.5% deoxycholate) on the 7th day, having achieved 80% confluency.
Western Blotting and Densitometry.
Cell lysates were digested with proteinase K at 20 μg/ml for 30 min at 37°C. The reaction was stopped with 2 mM PMSF, and the lysates were centrifuged for 45 min at 100,000 × g. Pellets were resuspended in sample buffer and SDS/PAGE/immunoblotting was performed according to standard techniques. Immunoblots were incubated with the recombinant Fab fragment D13 (34), a secondary horseradish peroxidase-labeled Ab, and developed with the enhanced chemiluminescence (ECL) system (Amersham Pharmacia). Densitometry was performed with National Institutes of Health image software, computing at least three independent experiments.
Results
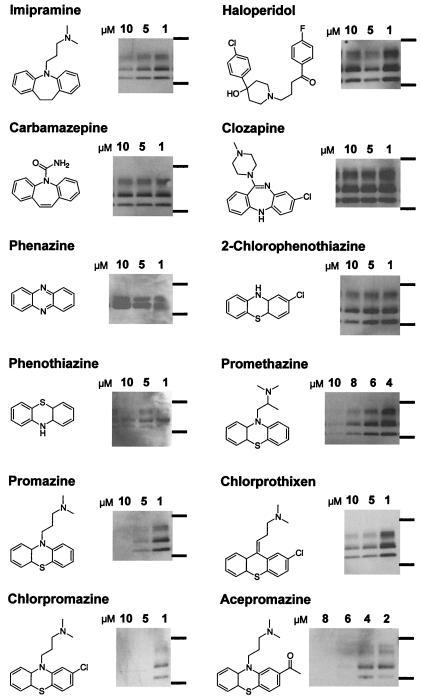
At concentrations in the range of 2–10 μM, treatment with promazine, chlorpromazine, and acepromazine, but not with phenothiazine, 2-chlorphenothiazine, or phenazine, led to the disappearance of PrPSc in ScN2a cells after 6-day treatments (Fig. 1). These effects were already visible 2 days after the beginning of treatment (data not shown).
Figure 1.
PrPSc-inhibiting effects of phenothiazine derivatives and other psychopharmacological substances. Anti-PrP immunoblots of protease-digested ScN2a cell lysates treated with the respective substance for 6 days at the concentration indicated. Molecular mass markers at the right of the immunoblot correspond to 37 kDa (upper bands) and 20 kDa (lower bands). The characteristic PrP immunostain depicted corresponds to the amount of PrPSc and prion infectivity present in the neuroblastoma cells after treatment with the concentration indicated above. The schematic molecular structure of the compound used is depicted on the left side of each immunoblot.
Because the structurally unrelated high potency antipsychotic drugs haloperidol and clozapine did not inhibit PrPSc formation, it is likely that inhibition of PrPSc formation by phenothiazine derivatives is not mediated through dopamine antagonism and thus, the antipsychotic and antiprion effects of these substances are dissociable. Moreover, addition of dopamine to cultures of ScN2a cells grown in the presence of chlorpromazine did not reverse the inhibition of PrPSc production (data not shown).
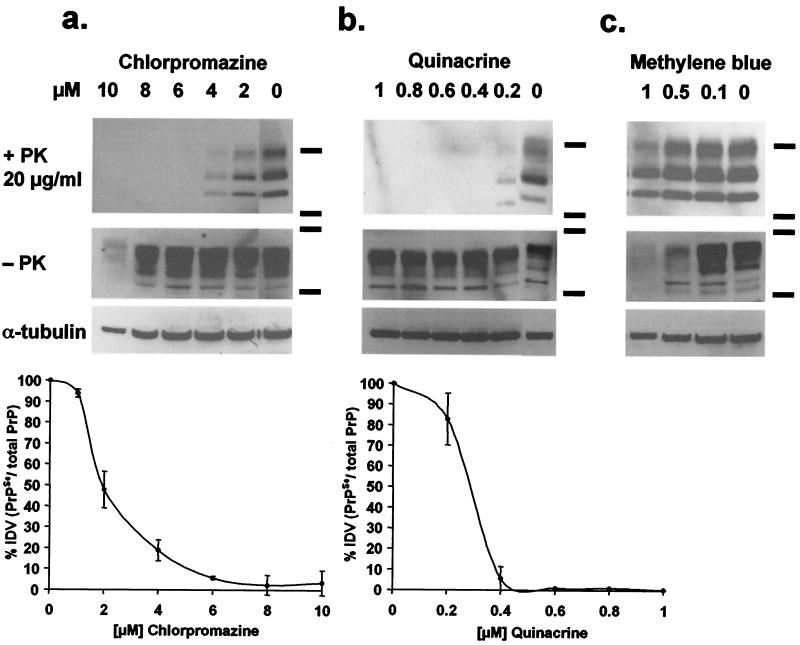
For chlorpromazine, a clear relationship could be established between dose and PrPSc inhibition after 7 days of treatment of ScN2a cells (Fig. 2a). The EC50 concentration, at which 50% of PrPSc production is eliminated, was 2 μM and the EC90 was 5 μM (Fig. 2a Bottom). At 10 μM, chlorpromazine was cytotoxic; the total cell number was decreased by 50%, and tubulin in the cell lysate was reduced compared with controls (Fig. 2a).
Figure 2.
Dose-response relationship of ScN2a cells treated with chlorpromazine, quinacrine, and methylene blue. Anti-PrP immunoblots of the same ScN2a cell lysates treated with proteinase K (1st row), without proteinase K (2nd row), anti-tubulin immunoblot of the same lysate (3rd row), and average immunoblot densitometry values (IDV) of three independent immunoblots (Bottom; bars represent standard error). ScN2a cells were treated for 6 days with chlorpromazine (a), quinacrine (b), or methylene blue (c). EC50 levels are ≈2 μM for chlorpromazine and ≈250 nM for quinacrine. Methylene blue does not inhibit PrPSc formation and is cytotoxic at concentrations >500 nM (see text for discussion).
We observed cytotoxicity with many phenothiazines, at doses between 8 and 50 μM or higher. For the perazines and phenazines, however, cytotoxicity at concentrations >6 μM made reliable measurements of dose-response relationships for these drugs questionable, although we did observe PrPSc inhibition. It is noteworthy that methylene blue did not inhibit PrPSc formation, even at cytotoxic concentrations (Fig. 2c).
Our data suggest that the tricyclic scaffold alone is not sufficient for PrPSc inhibition because phenazine, phenothiazine, and 2-chlorphenothiazine do not inhibit PrPSc formation (Fig. 1). A side-chain substituent on the central ring was necessary for inhibition of PrPSc: promazine, chlorpromazine, and acepromazine, all of which have a dimethylaminopropyl side chain extending from the ring nitrogen at the 9 position of the tricyclic scaffold, displayed EC50 concentrations of 5 μM. Promethazine, with a dimethylamino-2-methylpropyl side chain, was slightly less active with an EC50 of ≈8 μM.
The nitrogen at the 9 position of the tricyclic scaffold increased the antiprion potency. Chlorprothixene, with a carbon substituted for the nitrogen and a dimethylaminopropyliden side chain, had an EC50 of ≈10 μM, demonstrating that it is not as efficient in inhibiting PrPSc formation as its chlorpromazine counterpart. The tricyclic antidepressant imipramine, with a dibenzoazepine scaffold and the same side chain as promazine, inhibited PrPSc formation at an EC50 concentration of ≈10 μM. Our findings argue that the tricyclic scaffold containing a nitrogen at the 9 position as well as the length and composition of the side chain mediate the antiprion effects of the phenothiazines.
To extend the structure–activity relationships described above, we examined the tricyclic compounds quinacrine and methylene blue. Quinacrine was 10-fold more potent than chlorpromazine, completely inhibiting PrPSc formation at 400 nM (Fig. 2b). Inhibition of PrPSc formation by quinacrine was evident at 200 nM, whereas cytotoxic effects were not observed below 5 μM (Fig. 2b). An EC50 value of 300 nM for quinacrine (Table 1) is similar to that observed by others investigators (32). Neither methylene blue nor other structurally related nontoxic dyes inhibited PrPSc formation (Fig. 2c).
Table 1.
Structure–activity relationship of quinacrine analogues on PrPSc inhibition
| Compound | Structure | EC50,* μM | Compound | Structure | EC50,* μM |
|---|---|---|---|---|---|
| Quinacrine | 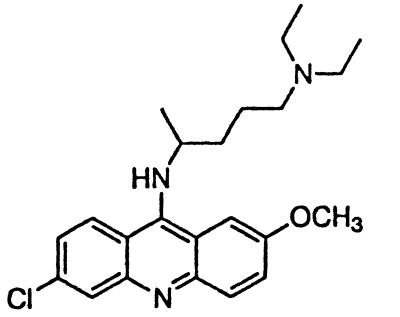 |
∼0.3 | Quinacrine mustard | 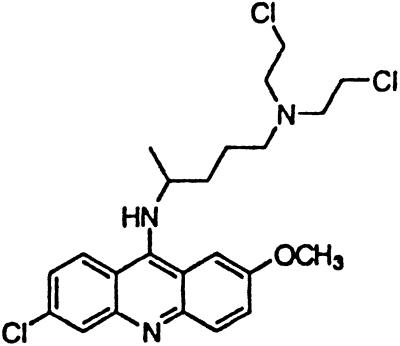 |
>5 |
| BM no. 51 | 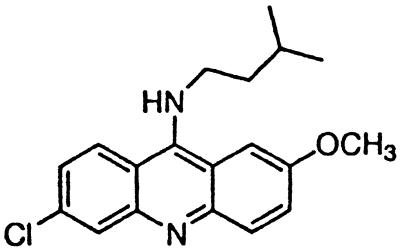 |
∼3 | BM no. 49 | 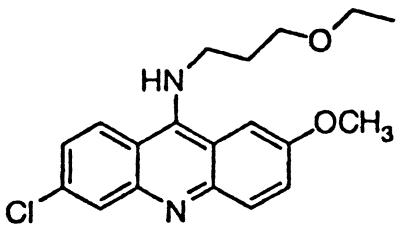 |
>5 |
| BM no. 47 | 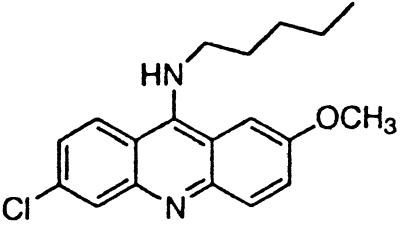 |
∼4 | BM no. 52 | 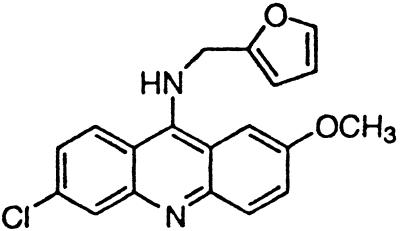 |
>5 |
| Pamaquine | 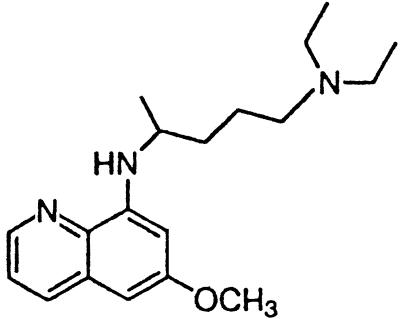 |
∼4 | Amsacrine | 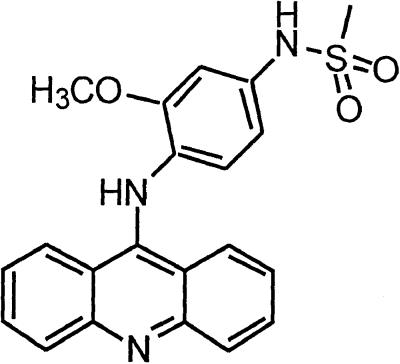 |
>5 |
| Chloroquine | 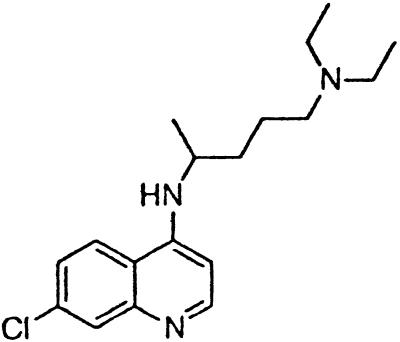 |
∼4 | BM no. 48 | 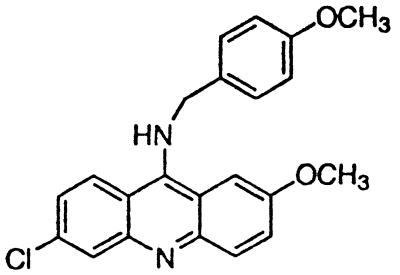 |
>5 |
| 9-Amino-acridine | 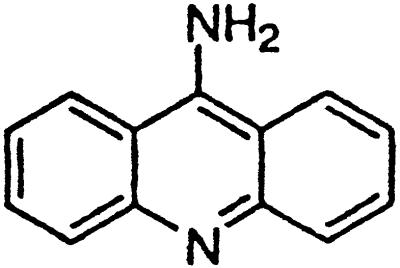 |
>5 | Methylene blue† | 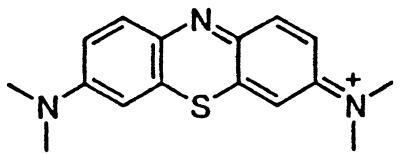 |
>1 |
EC50 measured in a dose-response curve for quinacrine as depicted in Fig. 2a and estimated semiquantitatively from visual inspection of Western blots for all other compounds.
Because of toxicity, no EC50 > 1 μM could be determined.
At a cellular level, the range between efficacious and toxic quinacrine concentrations defines a promising therapeutic index. These results suggest that quinacrine may be an even more potent antiprion substance than the phenothiazines and may represent a new lead compound for the development of such drugs. The specificity of the PrPSc-inhibiting effect was emphasized when structural relatives of quinacrine (quinacrine mustard, amsacrine, and 9-aminoacridine) as well as some synthesized derivatives (BM no. 49, BM no. 48, and BM no. 52) failed to demonstrate PrPSc inhibition at the same concentrations, and others had weaker antiprion efficacy (BM no. 47, BM no. 51, pamaquine, chloroquine; Table 1).
In contrast to the phenothiazines, quinacrine does not have a sulfur atom in the middle ring moiety of the tricyclic scaffold. Instead, it contains a carbon at the 9 position, to which the aliphatic side chain is attached (Table 1). Quinacrine has been used widely as an antimalarial drug and thus numerous analogs have been synthesized. Pamaquine and chloroquine contain the same side chain found in quinacrine but are anchored to a bicyclic quinoline scaffold. These two drugs inhibit PrPSc formation with an EC50 of ≈4 μM (Table 1).
Similar to the phenothiazine derivatives, the aliphatic side chain of quinacrine was found to modify antiprion activity. Neither 9-aminoacridine, lacking an aliphatic side chain, nor quinacrine mustard, with a bis-(2-chloroethyl) amine-substituted side chain, inhibited PrPSc formation as effectively as quinacrine. Of note, 9-aminoacridine resembles tacrine, an acridine proposed to be effective in the treatment of Alzheimer's disease (35); however, because it lacks the necessary side-chain, it was not an effective antiprion drug in the ScN2a cell assay (data not shown). To test further the effects of the side-chain substitution, we synthesized analogues of quinacrine to contain variations in the side chain linked to the acridine scaffold (Table 1). The 9-substituted acridines, BM no. 47, BM no. 48, BM no. 49, BM no. 51, and BM no. 52, were prepared by heating 6,9-dichloro-2-methoxyacridine with the corresponding amine in phenol (36). Compounds BM no. 48, BM no. 49, and BM no. 52, all containing major variations of the side chain by introducing a ring structure and/or oxygen atoms, were not effective inhibitors of PrPSc formation. Compounds BM no. 47 and BM no. 51 contained minor variations in the side chain and were 10 times less effective than quinacrine as inhibitors of PrPSc formation; these compounds exhibited EC50 values of ≈3 to 4 μM (Table 1). Our results argue that the aliphatic side chain carries major determinants that modify the potency of PrPSc inhibition. By comparing the side-chain variations and the EC50 values, we found that both the number of carbon atoms and the diethylamino bifurcation determine the antiprion activity of acridine derivatives.
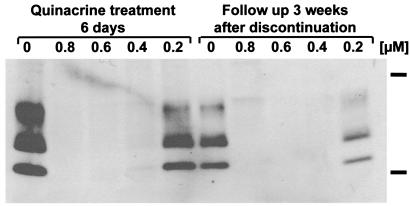
Quinacrine treatment seems to cure the cells of prion infection. ScN2a cells were treated with 0.2–0.8 μM quinacrine for 6 days. After removal of the quinacrine, the cells were cultured for an additional 3 weeks. As judged by Western blotting, PrPSc did not reappear during the 3 weeks after discontinuation of quinacrine when the concentration of quinacrine was initially >0.4 μM (Fig. 3).
Figure 3.
Quinacrine treatment permanently cures ScN2a cells. Anti-PrP immunoblots of protease-digested ScN2a cell lysates treated with 0.2–0.8 μM quinacrine for 6 days (left) and then left without treatment and weekly splitting for 3 weeks. The lack of reappearance of protease-resistant PrPSc after discontinuation of treatment demonstrates that quinacrine permanently cures ScN2a cells. Molecular mass markers at the right of the immunoblot correspond to 37 kDa (upper band) and 20 kDa (lower band).
Discussion
In the studies reported here, we have identified a class of compounds with a tricyclic ring structure and an aliphatic chain extending from the 9 position that inhibits PrPSc formation and possibly enhances clearance from ScN2a cells. Because some of these compounds have been used for the chronic treatment of diseases in humans, they are candidates for immediate treatment of CJD and other prion diseases, all of which are invariably fatal.
Some of the earliest studies of tricyclic drugs in humans are the investigations of Guttmann and Ehrlich (37), who pioneered the use of methylene blue as an antimalarial. Quinacrine was derived from methylene blue and was introduced in the 1930s as a drug with improved antimalarial properties (38, 39). The phenothiazines were discovered as a by-product of a search for antimalarial substances less toxic than quinacrine (40).
Quinacrine, commonly referred to as atabrine or mepacrine, was used widely during World War II as an antimalarial agent (41). Although newer antimalarial drugs have replaced quinacrine, it is approved for the treatment of giardiasis, producing cure rates of greater than 90%. Quinacrine is known to exert its antitrypanosomal effects by inhibiting trypanothione reductase from Trypanosoma cruzi (36), but no mammalian reductase has been shown to bind to quinacrine.
Dosage of quinacrine is recommended to be 100 mg orally for a few days for the treatment of giardiasis, whereas the drug has been administered orally for treatment of malaria in daily doses from 100 mg to 1,000 mg for up to many months (42–44). Quinacrine distributes slowly and achieves steady-state levels only 4 weeks after the start of therapy. Some of the lowest concentrations in the body are measured in the central nervous system (CNS). In low oral doses, few side effects are described; higher oral doses and parenteral administration increase the occurrence of serious side effects in the cardiovascular system, skin, gastrointestinal system, and CNS. A toxic psychosis from quinacrine has been reported in as many as 0.5% of recipients (42, 44).
Chlorpromazine has been used in the treatment of schizophrenia and other psychotic conditions since the 1950s (29). Daily oral dosage is usually between 100–600 mg. Side effects of chlorpromazine at doses used clinically include the tardive dyskinesias and occasionally agranulocytosis.
For the inhibition of PrPSc formation, the preferred effective composition of the aliphatic side chain for phenothiazine and acridine derivatives includes at least three or four carbon atoms, respectively, between two nitrogen atoms. The last nitrogen atom of the aliphatic side chain for phenothiazine and acridine derivatives has two methyl and alkyl side chains, respectively. The structure–activity relationships for inhibition of PrPSc formation described here should be useful in developing even more potent tricyclic compounds for treating prion diseases. The structures of chlorpromazine and quinacrine are quite different from those of the small molecules that were designed for dominant negative inhibition of prion formation (27). These tricyclic compounds are also quite different from other known inhibitors of PrPSc formation including Congo red, heparins, polysulfated glucosaminoglycans, branched polyamines, and lovastatin. Congo red is a multicyclic anionic molecule that binds to PrP amyloid plaques (45) and, more generally, to β-sheet structures of amyloidogenic proteins. It has been suggested that Congo red binds to PrPSc and thereby sequesters the infectious template (19, 20). Other polyanionic molecules, such as the sulfated polyanions, also are thought to act nonspecifically on the PrPSc template (18, 46, 47). We have no evidence that phenothiazine derivatives or quinacrine bind to PrPSc because in applying an assay (28) in which purified mouse prion rods were preincubated with these substances for 2 hours, no decrease in the amount of protease-resistant PrPSc was observed (data not shown). Lovastatin is thought to act by depleting cells from cholesterol and thereby destroying the raft compartments where the conversion of PrPC into PrPSc occurs (24). The inability of highly charged polyanions to cross the BBB and reach target cells, as well as the toxicity of lovastatin, make these substances unlikely therapeutics.
The mechanism by which quinacrine and chlorpromazine inhibit PrPSc formation and possibly enhance clearance is unknown. In contrast to the branched polyamines, both quinacrine and chlorpromazine require a few days of treatment before PrPSc in ScN2a cells disappear. The branched polyamines require only a few hours of treatment before the levels of preexisting PrPSc decline (28).
The enormous experience with the chronic administration of quinacrine as an antimalarial presents a situation in which this drug can be used immediately to treat humans dying of prion disease. Determining the best regimen will undoubtedly be performed in humans but such studies need to be carefully controlled so that the results will be meaningful. For example, it will be critical to determine whether the candidate patients actually have prion disease before receiving quinacrine. Because no reliable blood or cerebrospinal fluid test for prion disease in humans has been developed, brain biopsies will be essential unless the electroencephalograms and MRIs show patterns characteristic of prion disease (2). If quinacrine can be shown to cure prion disease in symptomatic humans, then prophylactic treatment for carriers of PrP gene mutations and for people previously exposed to prions should be considered. At the same time, it will be reasonable to consider treating animals exposed to prions; such a possibility might provide a much-needed rational approach to managing the problems currently plaguing European livestock industries.
Because the treatment of people with prion disease requires the drug to cross the BBB, it is unclear whether quinacrine or chlorpromazine will be more effective. It is possible that chlorpromazine, with a higher EC50 in the ScN2a cells than quinacrine, may prove more efficacious clinically if it crosses the BBB much more readily. It is well known that the phenothiazines exhibit large differences in their distribution between plasma and brain (48), arguing that drugs, like the perazines or thioridazine, which could not be reliably examined because of their cytotoxicity to ScN2a cells, might be more potent antiprion drugs than chlorpromazine or even quinacrine. Alternatively, very high doses of phenothiazines assuring high concentrations in the brain might be given to produce a state described at the beginning of the chlorpromazine therapy era as “artificial hibernation” (49). Still another possibility is the use of chlorpromazine in combination with quinacrine. Whether or not the two drugs act synergistically remains to be established.
The structure–activity relationships reported here provide a platform from which to synthesize many new compounds, some of which will undoubtedly have superior antiprion potencies as well as higher penetration of the BBB. Whether these new compounds will be useful in dissecting the etiologies of such psychotic disorders as the schizophrenias remains unclear. It has been suggested that the schizophrenias might be disorders of protein processing and thus are etiologically similar to many other neurodegenerative diseases (14, 50).
Acknowledgments
The authors thank Gerold Schmitt-Ulms for discussions. This work was supported by National Institutes of Health Grants AG02132, NS14069, and AG10770, as well as by a gift from the G. Harold and Leila Y. Mathers Foundation.
Abbreviations
- PrP
prion protein
- PrPC
normal cellular isoform
- PrPSc
disease-causing isoform
- CJD
Creutzfeldt–Jakob disease
- BBB
blood–brain barrier
- ScN2a
scrapie-infected neuroblastoma
References
- 1.Gambetti P, Peterson R B, Parchi P, Chen S G, Capellari S, Goldfarb L, Gabizon R, Montagna P, Lugaresi E, Piccardo P, Ghetti B. In: Prion Biology and Diseases. Prusiner S B, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 509–583. [Google Scholar]
- 2.Will R G, Alpers M P, Dormont D, Schonberger L B, Tateishi J. In: Prion Biology and Diseases. Prusiner S B, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 465–507. [Google Scholar]
- 3.Scott M R, Will R, Ironside J, Nguyen H-O B, Tremblay P, DeArmond S J, Prusiner S B. Proc Natl Acad Sci USA. 1999;96:15137–15142. doi: 10.1073/pnas.96.26.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Will R G, Cousens S N, Farrington C P, Smith P G, Knight R S G, Ironside J W. Lancet. 1999;353:979. doi: 10.1016/s0140-6736(99)01160-5. [DOI] [PubMed] [Google Scholar]
- 5.Alpers M P. In: The Central Nervous System: Some Experimental Models of Neurological Diseases. Bailey O T, Smith D E, editors. Baltimore: Williams & Wilkins; 1968. pp. 234–251. [Google Scholar]
- 6.Gajdusek D C. Science. 1977;197:943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- 7.Prusiner S B. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 8.Pan K-M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R J, Cohen F E, Prusiner S B. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alper T, Cramp W A, Haig D A, Clarke M C. Nature (London) 1967;214:764–766. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- 10.Kellings K, Prusiner S B, Riesner D. Philos Trans R Soc London B. 1994;343:425–430. doi: 10.1098/rstb.1994.0039. [DOI] [PubMed] [Google Scholar]
- 11.Bruce M E, Dickinson A G. J Gen Virol. 1987;68:79–89. doi: 10.1099/0022-1317-68-1-79. [DOI] [PubMed] [Google Scholar]
- 12.Bessen R A, Marsh R F. J Virol. 1994;68:7859–7868. doi: 10.1128/jvi.68.12.7859-7868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Telling G C, Parchi P, DeArmond S J, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner S B. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 14.Prusiner S B. N Engl J Med. 2001;344:1516–1526. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 15.Kimberlin R H, Walker C A. Arch Virol. 1983;78:9–18. doi: 10.1007/BF01310854. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson A G, Fraser H, Outram G W. Nature (London) 1975;256:732–733. doi: 10.1038/256732a0. [DOI] [PubMed] [Google Scholar]
- 17.Diringer H, Ehlers B. J Gen Virol. 1991;72:457–460. doi: 10.1099/0022-1317-72-2-457. [DOI] [PubMed] [Google Scholar]
- 18.Ehlers B, Diringer H. J Gen Virol. 1984;65:1325–1330. doi: 10.1099/0022-1317-65-8-1325. [DOI] [PubMed] [Google Scholar]
- 19.Caughey B, Race R E. J Neurochem. 1992;59:768–771. doi: 10.1111/j.1471-4159.1992.tb09437.x. [DOI] [PubMed] [Google Scholar]
- 20.Caspi S, Sasson S B, Taraboulos A, Gabizon R. J Biol Chem. 1998;273:3484–3489. doi: 10.1074/jbc.273.6.3484. [DOI] [PubMed] [Google Scholar]
- 21.Priola S A, Raines A, Caughey W S. Science. 2000;287:1503–1506. doi: 10.1126/science.287.5457.1503. [DOI] [PubMed] [Google Scholar]
- 22.Ingrosso L, Ladogana A, Pocchiari M. J Virol. 1995;69:506–508. doi: 10.1128/jvi.69.1.506-508.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorodinsky A, Harris D A. J Cell Biol. 1995;129:619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taraboulos A, Scott M, Semenov A, Avrahami D, Laszlo L, Prusiner S B. J Cell Biol. 1995;129:121–132. doi: 10.1083/jcb.129.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneko K, Vey M, Scott M, Pilkuhn S, Cohen F E, Prusiner S B. Proc Natl Acad Sci USA. 1997;94:2333–2338. doi: 10.1073/pnas.94.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zulianello L, Kaneko K, Scott M, Erpel S, Han D, Cohen F E, Prusiner S B. J Virol. 2000;74:4351–4360. doi: 10.1128/jvi.74.9.4351-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrier V, Wallace A C, Kaneko K, Safar J, Prusiner S B, Cohen F E. Proc Natl Acad Sci USA. 2000;97:6073–6078. doi: 10.1073/pnas.97.11.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Supattapone S, Nguyen H-O B, Cohen F E, Prusiner S B, Scott M R. Proc Natl Acad Sci USA. 1999;96:14529–14534. doi: 10.1073/pnas.96.25.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delay J, Deniker P, Harl J-M. Ann Med Psychol (Paris) 1952;110:267–273. [PubMed] [Google Scholar]
- 30.Roikhel V M, Fokina G I, Pogodina V V. Acta Virol. 1984;28:321–324. [PubMed] [Google Scholar]
- 31.Dees C, Wade W F, German T L, Marsh R F. J Gen Virol. 1985;66:845–849. doi: 10.1099/0022-1317-66-4-845. [DOI] [PubMed] [Google Scholar]
- 32.Doh-ura K, Iwaki T, Caughey B. J Virol. 2000;74:4894–4897. doi: 10.1128/jvi.74.10.4894-4897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosque P J, Prusiner S B. J Virol. 2000;74:4377–4386. doi: 10.1128/jvi.74.9.4377-4386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peretz D, Williamson R A, Matsunaga Y, Serban H, Pinilla C, Bastidas R B, Rozenshteyn R, James T L, Houghten R A, Cohen F E, et al. J Mol Biol. 1997;273:614–622. doi: 10.1006/jmbi.1997.1328. [DOI] [PubMed] [Google Scholar]
- 35.Knapp M J, Knopman D S, Solomon P R, Pendlebury W W, Davis C S, Gracon S I. J Am Med Assoc. 1994;271:985–991. [PubMed] [Google Scholar]
- 36.Bonse S, Santelli-Rouvier C, Barbe J, Krauth-Siegel R L. J Med Chem. 1999;42:5448–5454. doi: 10.1021/jm990386s. [DOI] [PubMed] [Google Scholar]
- 37.Guttmann P, Ehrlich P. Berl Klin Wochenschr. 1891;39:953–956. [Google Scholar]
- 38.Schulemann W. Br Med J. 1932;1:100–101. [Google Scholar]
- 39.Green R. Lancet. 1932;1:826–829. [Google Scholar]
- 40.Gilman H, van Ess P R, Shirley D A. J Am Chem Soc. 1944;66:1214–1216. [Google Scholar]
- 41.Goodman L S, Gilman A. The Pharmacological Basis of Therapeutics. New York: Macmillan; 1975. [Google Scholar]
- 42.Goodman L S, Gilman A. The Pharmacological Basis of Therapeutics: A Textbook of Pharmacology, Toxicology, and Therapeutics for Physicians and Medical Students. New York: Macmillan; 1970. [Google Scholar]
- 43.Hardman J G, Limbird L L, Molinoff P B, Ruddon R W, Gilman A G. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw–Hill; 1996. [Google Scholar]
- 44.Findlay G M. Recent Advances in Chemotherapy. Philadelphia: Blakiston; 1951. [Google Scholar]
- 45.Prusiner S B, McKinley M P, Bowman K A, Bolton D C, Bendheim P E, Groth D F, Glenner G G. Cell. 1983;35:349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- 46.Kimberlin R H, Walker C A. Antimicrob Agents Chemother. 1986;30:409–413. doi: 10.1128/aac.30.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caughey B, Raymond G J. J Virol. 1993;67:643–650. doi: 10.1128/jvi.67.2.643-650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svendsen C N, Hrbek C C, Casendino M, Nichols R D, Bird E D. Psychiatry Res. 1988;23:1–10. doi: 10.1016/0165-1781(88)90029-7. [DOI] [PubMed] [Google Scholar]
- 49.Laborit H, Huguenard P. Presse Med. 1952;60:1455–1456. [PubMed] [Google Scholar]
- 50.Lieberman J A. Biol Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]