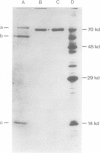
Abstract
The nucleotide sequence of the yeast chromosomal gene coding for the 70-kd protein of the mitochondrial outer membrane was determined. The deduced amino acid sequence of the protein agrees with the experimentally determined size and amino acid composition of the purified protein and correctly predicts the fragments obtained by cleaving the protein at its single tryptophan residue. The deduced NH2-terminal sequence features an uninterrupted stretch of 28 uncharged amino acids flanked on both sides by basic amino acids. By sequencing a truncated version of the gene it was found that the corresponding polypeptide product lacks the 203 carboxy-terminal amino acids of the authentic 70-kd protein. As shown in the accompanying paper, this protein fragment still becomes attached to the mitochondrial outer membrane in vivo.
Full text
PDF
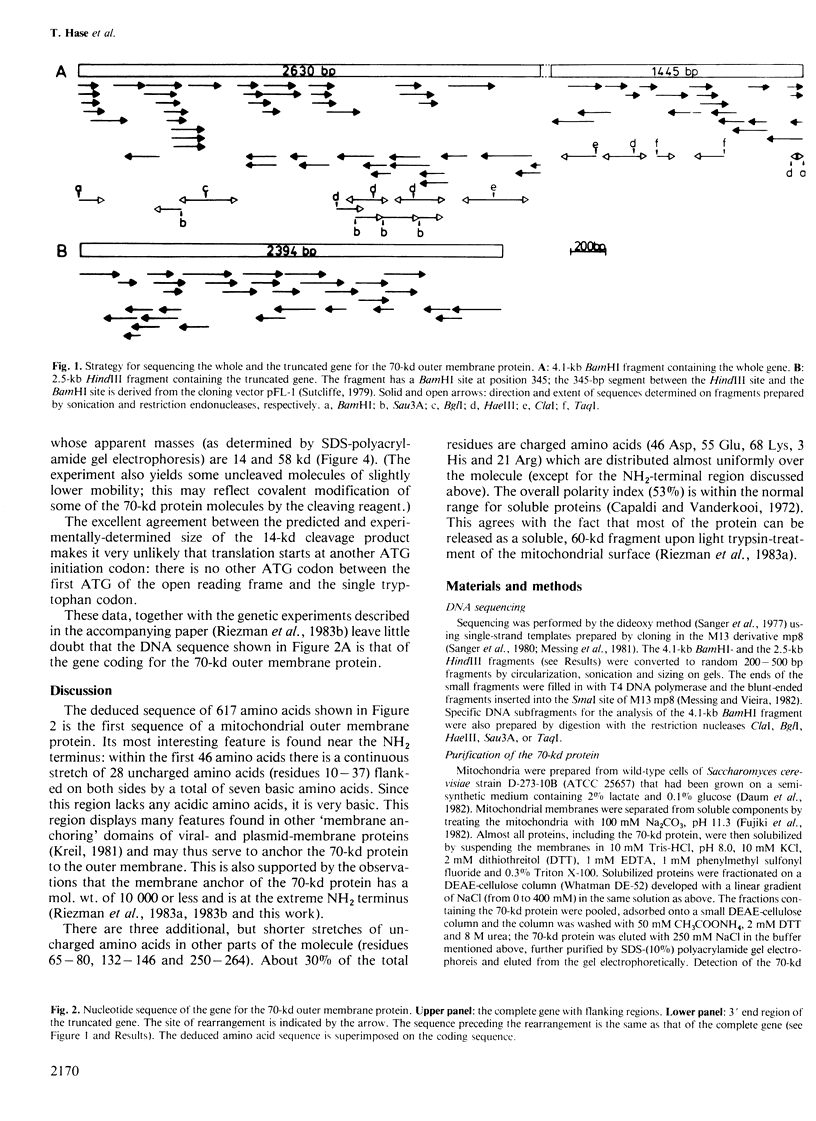


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Battey J., Clayton D. A. The transcription map of mouse mitochondrial DNA. Cell. 1978 May;14(1):143–156. doi: 10.1016/0092-8674(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennicke A. Mitochondrial DNA from Oenothera berteriana: PURIFICATION AND PROPERTIES. Plant Physiol. 1980 Jun;65(6):1207–1210. doi: 10.1104/pp.65.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence of subunit 2 of yeast cytochrome oxidase. J Biol Chem. 1979 Sep 25;254(18):9324–9330. [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Faye G., Simon M. Analysis of a yeast nuclear gene involved in the maturation of mitochondrial pre-messenger RNA of the cytochrome oxidase subunit I. Cell. 1983 Jan;32(1):77–87. doi: 10.1016/0092-8674(83)90498-1. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Five TGA "stop" codons occur within the translated sequence of the yeast mitochondrial gene for cytochrome c oxidase subunit II. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6534–6538. doi: 10.1073/pnas.76.12.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Freitag H., Janes M., Neupert W. Biosynthesis of mitochondrial porin and insertion into the outer mitochondrial membrane of Neurospora crassa. Eur J Biochem. 1982 Aug;126(1):197–202. doi: 10.1111/j.1432-1033.1982.tb06766.x. [DOI] [PubMed] [Google Scholar]
- Fristensky B., Lis J., Wu R. Portable microcomputer software for nucleotide sequence analysis. Nucleic Acids Res. 1982 Oct 25;10(20):6451–6463. doi: 10.1093/nar/10.20.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Fowler S., Shio H., Hubbard A. L., Lazarow P. B. Polypeptide and phospholipid composition of the membrane of rat liver peroxisomes: comparison with endoplasmic reticulum and mitochondrial membranes. J Cell Biol. 1982 Apr;93(1):103–110. doi: 10.1083/jcb.93.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Schatz G. Import of proteins into mitochondria. In vitro studies on the biogenesis of the outer membrane. J Biol Chem. 1983 Mar 25;258(6):3427–3430. [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mihara K., Blobel G., Sato R. In vitro synthesis and integration into mitochondria of porin, a major protein of the outer mitochondrial membrane of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7102–7106. doi: 10.1073/pnas.79.23.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Hase T., van Loon A. P., Grivell L. A., Suda K., Schatz G. Import of proteins into mitochondria: a 70 kilodalton outer membrane protein with a large carboxy-terminal deletion is still transported to the outer membrane. EMBO J. 1983;2(12):2161–2168. doi: 10.1002/j.1460-2075.1983.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederoff R. R., Levings C. S., Timothy D. H., Hu W. W. Evolution of DNA sequence organization in mitochondrial genomes of Zea. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5953–5957. doi: 10.1073/pnas.78.10.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lund E., Smithies O., Blattner F. R. Hybridization of labeled RNA to DNA in agarose gels. Nucleic Acids Res. 1975 Oct;2(10):1911–1929. doi: 10.1093/nar/2.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens G. J., Buse G. Studies on cytochrome c oxidase, IV[1--3]. Primary structure and function of subunit II. Hoppe Seylers Z Physiol Chem. 1979 Apr;360(4):613–619. [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]