Abstract
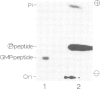
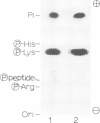
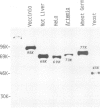
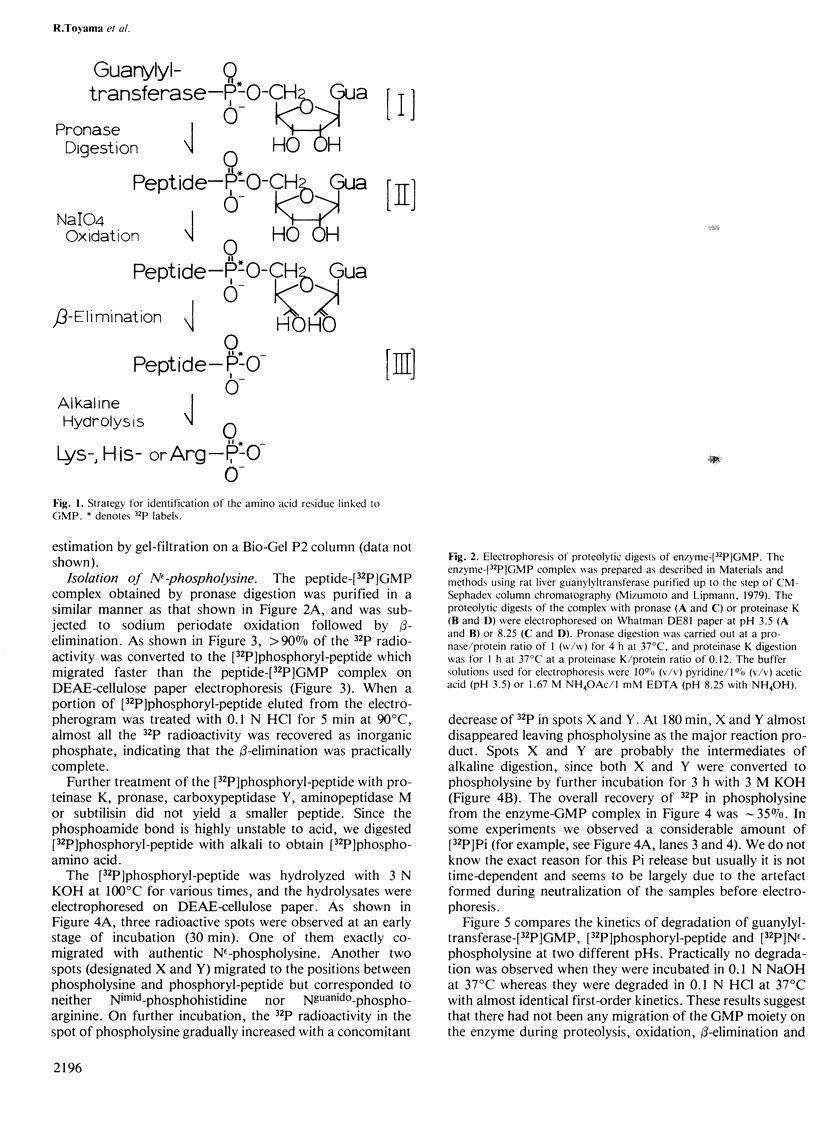
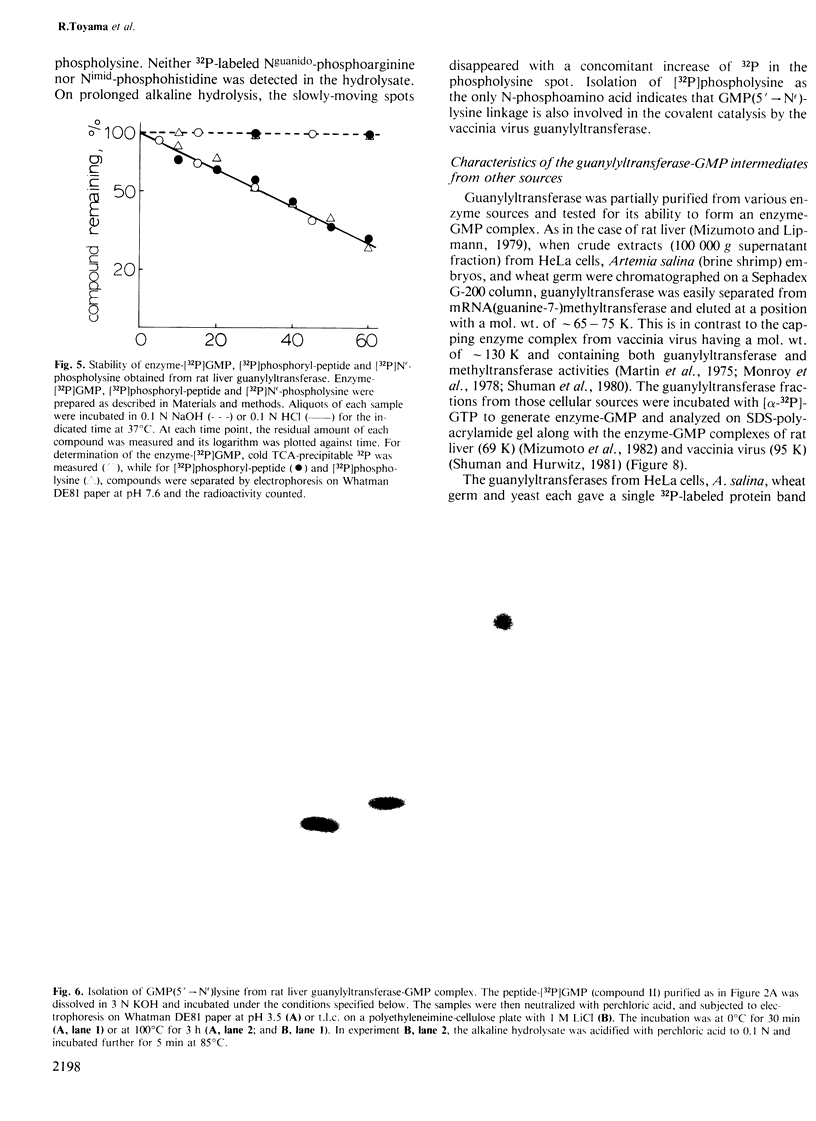
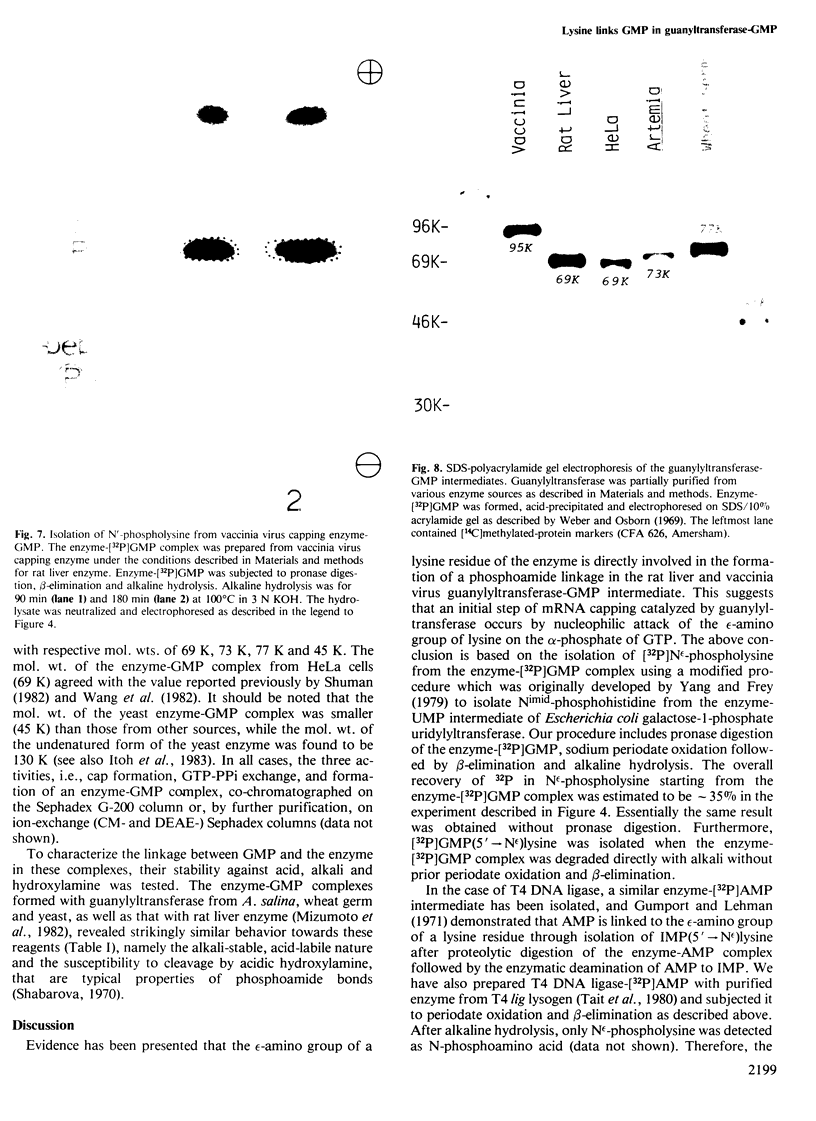
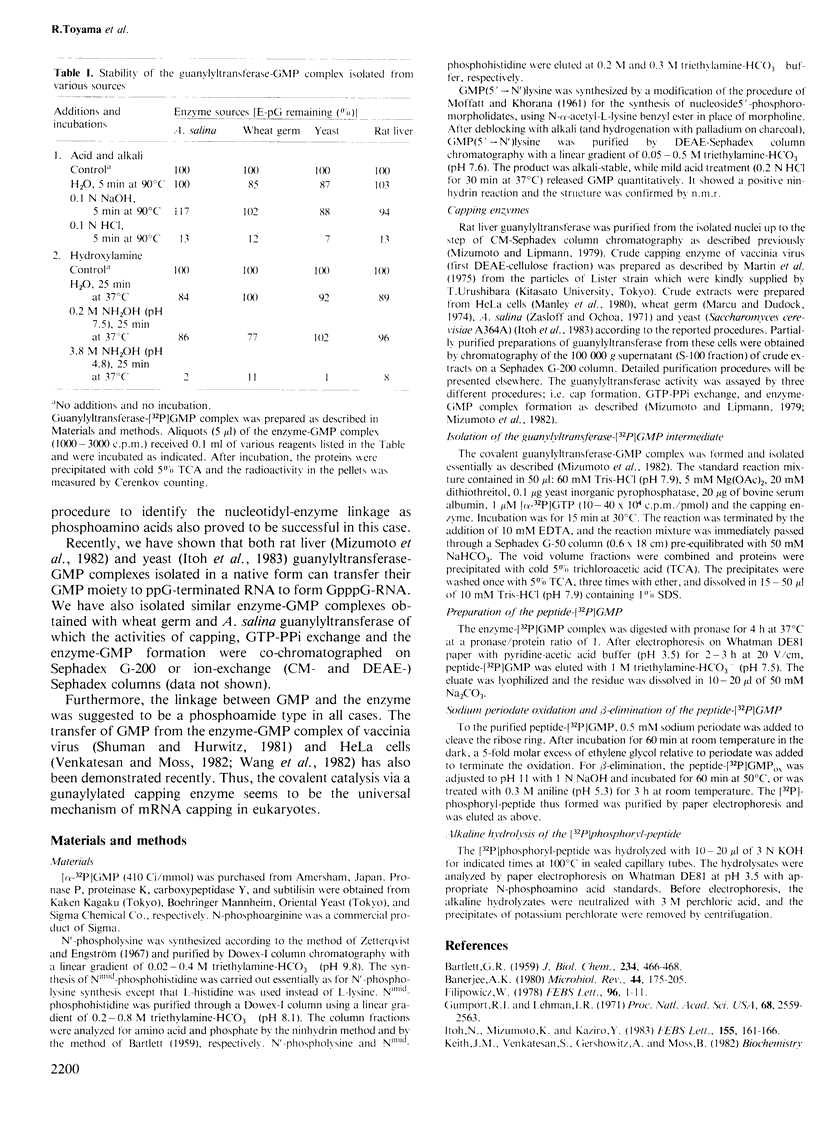
The mRNA capping reaction catalyzed by rat liver mRNA guanylyltransferase proceeds through an enzyme-GMP intermediate in which GMP is linked to the enzyme by a phosphoamide linkage. The studies described here show that GMP is bound to the epsilon-amino group of lysine of rat liver guanylyltransferase. The enzyme-[32P]GMP intermediate was digested with pronase to a [32P]GMP-peptide which was then converted to [32P]phosphoryl-peptide through periodate oxidation followed by beta-elimination. After alkaline hydrolysis of the [32P]phosphoryl-peptide, the major radioactive product co-electrophoresed with the authentic N epsilon-phospholysine on DEAE-cellulose paper. Neither [32P]Nimid-phosphohistidine nor Nguanido-phosphoarginine was detected in the hydrolysates. Furthermore, formation of N epsilon-guanylyl-lysine linkage on the enzyme was more directly shown by isolation of [32P]GMP(5' leads to N epsilon)lysine when the steps of periodate oxidation and beta-elimination were omitted. The results indicate that the nucleophile in the guanylyltransferase to which the guanylyl residue is linked is the epsilon-amino group of a lysine residue. [32P]Phosphoryl-lysine was also isolated from the vaccinia virus capping enzyme-[32P]GMP intermediate. Guanylyltransferase from HeLa cells, wheat germ, Artemia salina and yeast also formed the enzyme-GMP complex and, from the stability of the complex, the linkage between the enzyme and GMP was suggested to be a phosphoamide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Itoh N., Mizumoto K., Kaziro Y. Partial purification and characterization of mRNA guanylyltransferase from Saccharomyces cerevisiae. FEBS Lett. 1983 May 2;155(1):161–166. doi: 10.1016/0014-5793(83)80231-2. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Mizumoto K., Kaziro Y., Lipmann F. Reaction mechanism of mRNA guanylyltransferase from rat liver: isolation and characterization of a guanylyl-enzyme intermediate. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1693–1697. doi: 10.1073/pnas.79.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Lipmann F. Transmethylation and transguanylylation in 5'-RNA capping system isolated from rat liver nuclei. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4961–4965. doi: 10.1073/pnas.76.10.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy G., Spencer E., Hurwitz J. Purification of mRNA guanylyltransferase from vaccinia virions. J Biol Chem. 1978 Jun 25;253(12):4481–4489. [PubMed] [Google Scholar]
- Nishikawa Y., Chambon P. Purification of mRNA guanylyltransferase from calf thymus. EMBO J. 1982;1(4):485–492. doi: 10.1002/j.1460-2075.1982.tb01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabarova Z. A. Synthetic nucleotide-peptides. Prog Nucleic Acid Res Mol Biol. 1970;10:145–182. doi: 10.1016/s0079-6603(08)60564-4. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shuman S., Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci U S A. 1981 Jan;78(1):187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S. RNA capping by HeLa cell RNA guanylyltransferase. Characterization of a covalent protein-guanylate intermediate. J Biol Chem. 1982 Jun 25;257(12):7237–7245. [PubMed] [Google Scholar]
- Shuman S., Surks M., Furneaux H., Hurwitz J. Purification and characterization of a GTP-pyrophosphate exchange activity from vaccinia virions. Association of the GTP-pyrophosphate exchange activity with vaccinia mRNA guanylyltransferase . RNA (guanine-7-)methyltransferase complex (capping enzyme). J Biol Chem. 1980 Dec 10;255(23):11588–11598. [PubMed] [Google Scholar]
- Tait R. C., Rodriguez R. L., West R. W., Jr The rapid purification of T4 DNA ligase from a lambda T4 lig lysogen. J Biol Chem. 1980 Feb 10;255(3):813–815. [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Purification and characterization of mRNA guanylyltransferase from HeLa cell nuclei. J Biol Chem. 1980 Apr 10;255(7):2829–2834. [PubMed] [Google Scholar]
- Venkatesan S., Moss B. Eukaryotic mRNA capping enzyme-guanylate covalent intermediate. Proc Natl Acad Sci U S A. 1982 Jan;79(2):340–344. doi: 10.1073/pnas.79.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Furuichi Y., Shatkin A. J. Covalent guanylyl intermediate formed by HeLa cell mRNA capping enzyme. Mol Cell Biol. 1982 Aug;2(8):993–1001. doi: 10.1128/mcb.2.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yang S. L., Frey P. A. Nucleophile in the active site of Escherichia coli galactose-1-phosphate uridylyltransferase: degradation of the uridylyl-enzyme intermediate to N3-phosphohistidine. Biochemistry. 1979 Jul 10;18(14):2980–2984. doi: 10.1021/bi00581a011. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ochoa S. A supernatant factor involved in initiation complex formation with eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3059–3063. doi: 10.1073/pnas.68.12.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterqvist O., Engström L. Isolation of N-e-[32P]phosphoryl-lysine from rat-liver cell sap after incubation with [32P]adenosine triphosphate. Biochim Biophys Acta. 1967 Aug 29;141(3):523–532. doi: 10.1016/0304-4165(67)90181-x. [DOI] [PubMed] [Google Scholar]