Abstract
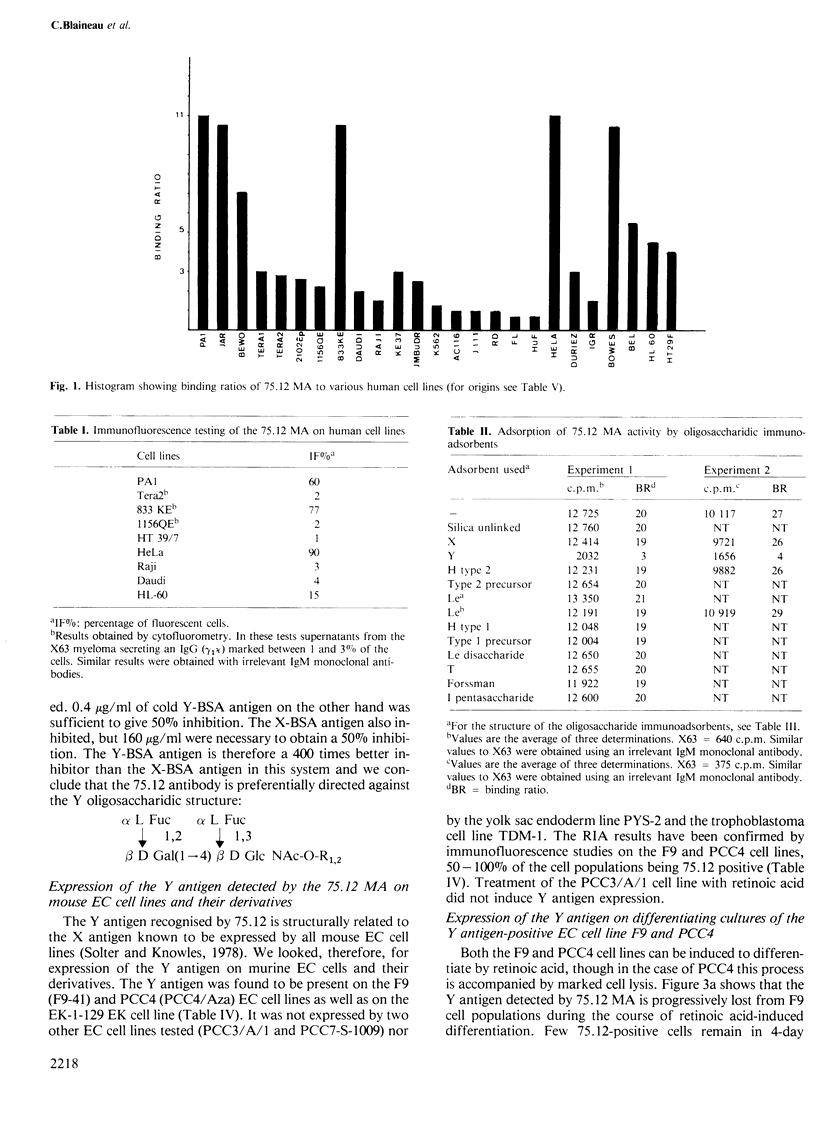
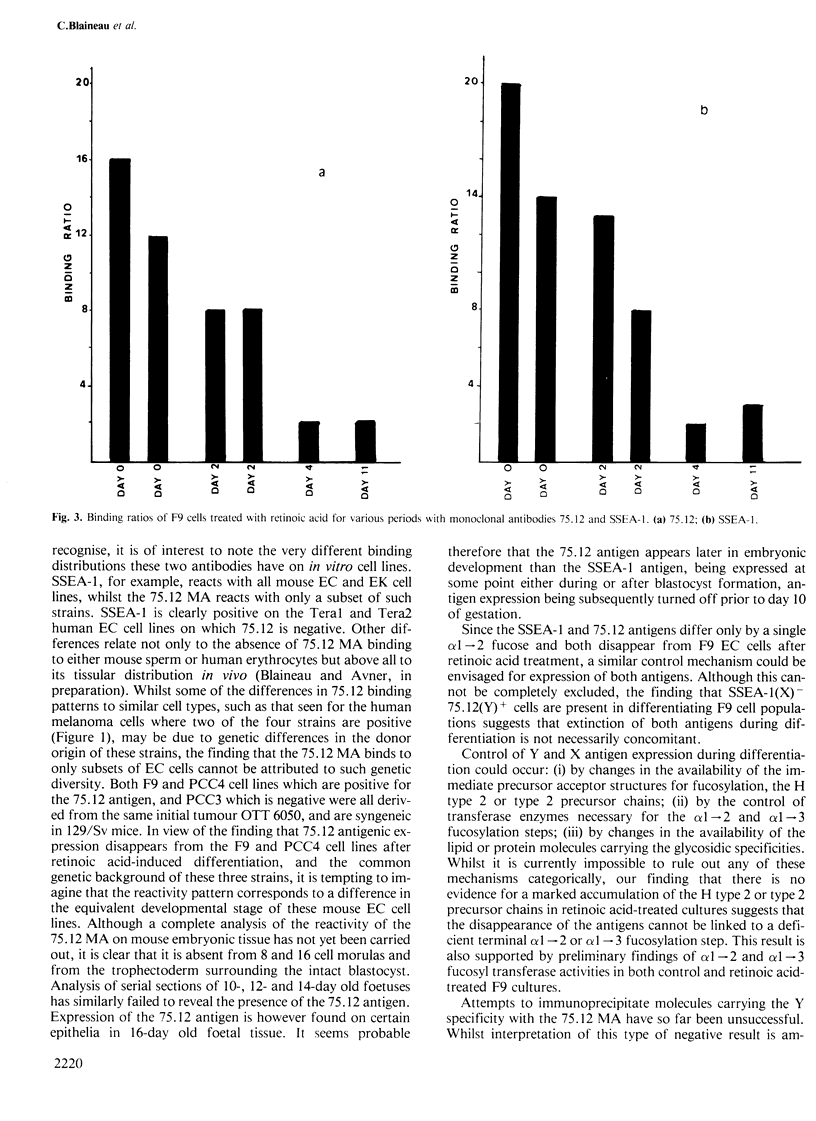
Monoclonal antibody 75.12 raised against the human ovarian teratocarcinoma cell line PA1 detects a 'Y' or iso-leb glycosidic structure. Using the 75.12 antibody we have established that the Y antigen is expressed on some but not all mouse embryonal carcinoma (EC) lines. The Y or 75.12 antigen-positive EC cell lines F9 and PCC4 cease to express the antigen after differentiation induced with retinoic acid and this decreased expression parallels the morphological differentiation of the EC cells. These results support not only the idea that carbohydrate structures present on embryonic cells undergo marked alteration during differentiation, but also that established mouse EC cells may differ in their differentiation states.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono R., Cartron J. P., Mulet C., Avner P., Fellous M. Selective expression of blood group antigens on human teratocarcinoma cell lines. Rev Fr Transfus Immunohematol. 1981 Feb;24(1):97–107. doi: 10.1016/s0338-4535(81)80030-x. [DOI] [PubMed] [Google Scholar]
- Bronson D. L., Andrews P. W., Solter D., Cervenka J., Lange P. H., Fraley E. E. Cell line derived from a metastasis of a human testicular germ cell tumor. Cancer Res. 1980 Jul;40(7):2500–2506. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- FOGH J., LUND R. O. Continuous cultivation of epithelial cell strain (FL) from human amniotic membrane. Proc Soc Exp Biol Med. 1957 Mar;94(3):532–537. doi: 10.3181/00379727-94-23003. [DOI] [PubMed] [Google Scholar]
- Fellous M., Gachelin G., Buc-Caron M. H., Dubois P., Jacob F. Similar location of an early embryonic antigen on mouse and human spermatozoa. Dev Biol. 1974 Dec;41(2):331–337. doi: 10.1016/0012-1606(74)90310-8. [DOI] [PubMed] [Google Scholar]
- Fellous M., Günther E., Kemler R., Wiels J., Berger R., Guenet J. L., Jakob H., Jacob F. Association of the H-Y male antigen with beta2-microglobulin on human lymphoid and differentiated mouse teratocarcinoma cell lines. J Exp Med. 1978 Jul 1;148(1):58–70. doi: 10.1084/jem.148.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Levinson J. R., Williams V. E., 2nd, McDevitt H. O. Monoclonal antibodies reacting with murine teratocarcinoma cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):377–380. doi: 10.1073/pnas.76.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi H. C., Feizi T., Kapadia A., Knowles B. B., Solter D., Evans M. J. Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature. 1981 Jul 9;292(5819):156–158. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- Hindsgaul O., Norberg T., Le Pendu J., Lemieux R. U. Synthesis of type 2 human blood-group antigenic determinants. The H, X, and Y haptens and variations of the H type 2 determinant as probes for the combining site of the lectin I of Ulex europaeus. Carbohydr Res. 1982 Nov 1;109:109–142. doi: 10.1016/0008-6215(82)84034-2. [DOI] [PubMed] [Google Scholar]
- Kapadia A., Feizi T., Evans M. J. Changes in the expression and polarization of blood group I and i antigens in post-implantation embryos and teratocarcinomas of mouse associated with cell differentiation. Exp Cell Res. 1981 Jan;131(1):185–195. doi: 10.1016/0014-4827(81)90418-3. [DOI] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lipinski M., Parks D. R., Rouse R. V., Herzenberg L. A. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5147–5150. doi: 10.1073/pnas.78.8.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- McAllister R. M., Melnyk J., Finkelstein J. Z., Adams E. C., Jr, Gardner M. B. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969 Sep;24(3):520–526. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Miyauchi T., Yonezawa S., Takamura T., Chiba T., Tejima S., Ozawa M., Sato E., Muramatsu T. A new fucosyl antigen expressed on colon adenocarcinoma and embryonal carcinoma cells. Nature. 1982 Sep 9;299(5879):168–169. doi: 10.1038/299168a0. [DOI] [PubMed] [Google Scholar]
- Monday L. A., Lemieux B. Etude audiovestibulaire dans l'ataxie de Friedreich. J Otolaryngol. 1978 Oct;7(5):415–423. [PubMed] [Google Scholar]
- Muramatsu H., Muramatsu T., Avner P. Biochemical properties of the high-molecular-weight glycopeptides released from the cell surface of human teratocarcinoma cells. Cancer Res. 1982 May;42(5):1749–1752. [PubMed] [Google Scholar]
- Muramatsu T., Avner P., Fellous M., Gachelin G., Jacob F. Distinctive properties of fucosyl glycopeptides on human teratoma cells. Somatic Cell Genet. 1979 Nov;5(6):753–761. doi: 10.1007/BF01542639. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Gachelin G., Nicolas J. F., Condamine H., Jakob H., Jacob F. Carbohydrate structure and cell differentitation: unique properties of fucosyl-glycopeptides isolated from embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2315–2319. doi: 10.1073/pnas.75.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. F., Avner P., Gaillard J., Guenet J. L., Jakob H., Jacob F. Cell lines derived from teratocarcinomas. Cancer Res. 1976 Nov;36(11 Pt 2):4224–4231. [PubMed] [Google Scholar]
- Nicolas J. F., Dubois P., Jakob H., Gaillard J., Jacob F. Tératocarcinome de la souris: différenciation en culture d'une lignée de cellules primitives a potentialités multiples. Ann Microbiol (Paris) 1975 Jan;126(1):3–22. [PubMed] [Google Scholar]
- OSGOOD E. E., BROOKE J. H. Continuous tissue culture of leukocytes from human leukemic bloods by application of gradient principles. Blood. 1955 Oct;10(10):1010–1022. [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Pattillo R. A., Gey G. O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968 Jul;28(7):1231–1236. [PubMed] [Google Scholar]
- Preud'homme J. L., Gonnot M., Fellous M., Seligmann M. Receptors for IgM on human lymphoblastoid B and T cell lines. Scand J Immunol. 1979;10(3):207–211. doi: 10.1111/j.1365-3083.1979.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Schlom J., Wunderlich D., Teramoto Y. A. Generation of human monoclonal antibodies reactive with human mammary carcinoma cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6841–6845. doi: 10.1073/pnas.77.11.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D., Shevinsky L., Knowles B. B., Strickland S. The induction of antigenic changes in a teratocarcinoma stem cell line (F9) by retinoic acid. Dev Biol. 1979 Jun;70(2):515–521. doi: 10.1016/0012-1606(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Stern P. L., Willison K. R., Lennox E., Galfrè G., Milstein C., Secher D., Ziegler A. Monoclonal antibodies as probes for differentiation and tumor-associated antigens: a Forssman specificity on teratocarcinoma stem cells. Cell. 1978 Aug;14(4):775–783. doi: 10.1016/0092-8674(78)90333-1. [DOI] [PubMed] [Google Scholar]
- Wang N., Trend B., Bronson D. L., Fraley E. E. Nonrandom abnormalities in chromosome 1 in human testicular cancers. Cancer Res. 1980 Mar;40(3):796–802. [PubMed] [Google Scholar]
- Willison K. R., Karol R. A., Suzuki A., Kundu S. K., Marcus D. M. Neutral glycolipid antigens as developmental markers of mouse teratocarcinoma and early embryos: an immunologic and chemical analysis. J Immunol. 1982 Aug;129(2):603–609. [PubMed] [Google Scholar]
- Zeuthen J., Nørgaard J. O., Avner P., Fellous M., Wartiovaara J., Vaheri A., Rosén A., Giovanella B. C. Characterization of a human ovarian teratocarcinoma-derived cell line. Int J Cancer. 1980 Jan 15;25(1):19–32. doi: 10.1002/ijc.2910250104. [DOI] [PubMed] [Google Scholar]


