Abstract
Purpose of review
Hematopoietic stem cells (HSCs) predominantly reside either in direct contact or in close proximity to the vascular endothelium throughout their lifespan. From the moment of HSC embryonic specification from hemogenic endothelium, endothelial cells (ECs) act as a critical cellular-hub that regulates a vast repertoire of biological processes crucial for HSC maintenance throughout its lifespan. In this review, we will discuss recent findings in endothelial niche-mediated regulation of HSC function during development, aging and regenerative conditions.
Recent findings
Studies employing genetic vascular models have unequivocally confirmed that ECs provide the essential instructive cues for HSC emergence during embryonic development as well as adult HSC maintenance during homeostasis and regeneration. Aging of ECs may impair their ability to maintain HSC function contributing to the development of aging-associated hematopoietic deficiencies. These findings have opened up new avenues to explore the therapeutic application of ECs. ECs can be adapted to serve as an instructive platform to expand bona fide HSCs and also utilized as a cellular therapy to promote regeneration of the hematopoietic system following myelosuppressive and myeloablative injuries.
Summary
ECs provide a fertile niche for maintenance of functional HSCs throughout their lifecycle. An improved understanding of the EC-HSC cross-talk will pave the way for development of EC-directed strategies for improving HSC function during aging.
Keywords: endothelial cells, hematopoietic stem cells, bone marrow microenvironment, niche, aging
Introduction
Endothelial cells (ECs) are specialized cells lining blood vessels and that supply tissues with oxygen and nutrients required for homeostasis (1). ECs are an adaptable life-support system essential for promoting organ growth through their ability to extend and remodel the vascular network (2–4). However, ECs are not only passive channels supplying nutrients and fulfilling metabolic demands, but also serve as instructive scaffolds that provide the essential paracrine signaling cues to promote tissue maintenance and regeneration (5–7)*.
From the initial birth of a hematopoietic precursor and subsequent emergence of a definitive hematopoietic stem cell (HSC) during development, to the functional decline of the HSC during physiological aging, the surrounding microenvironment or the niche serve as cellular hubs responsible for the proper balance of HSC self-renewal, expansion, and lineage-directed differentiation. In this review, we will discuss the recent advances that have led to a better understanding of the inherent complexity of HSC niches. The review will focus on the role of ECs in the emergence of HSC activity during development and how ECs support HSC homeostasis and regeneration during adulthood. We will also explore emerging data that suggest that physiological aging of the endothelial niche, and the bone marrow (BM) microenvironment as a whole, can promote functional defects observed in the aged hematopoietic compartment. Lastly, we will discuss the therapeutic application of ECs in the treatment of hematological diseases and how transplantation of ECs can augment hematopoietic regeneration following hematopoietic insults.
Emergence of definitive hematopoietic stem cells
Hematopoietic and vasculogenic precursors originate in the yolk sac as early as day 7 of murine development. At this time, the first primitive hematopoietic cells and ECs are derived from the extraembryonic mesoderm (8). Later during development, definitive hematopoiesis first appears through the production of erythroid and myeloid progenitors followed by the emergence of definitive HSCs that are capable of long-term, multi-lineage engraftment. The emergence of the HSC during embryogenesis occurs in parallel to cardiac output and the onset of blood flow (9–11). The regions of definitive hematopoiesis are known as the para-aortic splanchnopleura (P-Sp) between 8.5 and 10 days of gestation, and as the aorta-gonad-mesonephros (AGM) from day 10.5 to day 12 (12, 13). The P-Sp/AGM contains multipotent progenitors as early as day 8.5 (14–16). Furthermore, transplantation studies have shown that long-term multi-lineage repopulating HSCs (LT-HSCs) initially develop within the P-Sp/AGM, likely serving as a primary source for seeding the fetal liver, where LT-HSC expansion occurs for the remainder of development (17, 18). Notably, ECs that form the ventral surface of the aorta are also derived from the P-Sp/AGM (19). P-Sp/AGM hematopoietic cells are found closely adhering to the ECs on the floor of the dorsal aorta (20). This close temporal and spatial development of hematopoiesis and vasculogenesis suggests not only a potential common cellular origin, but also that constitutive cellular interaction between these two populations promotes the maturation and specification of HSCs. Indeed, a small and unique subset of vascular hemogenic endothelium found in the dorsal aorta, umbilical arteries, and the placenta has been demonstrated to have the ability to acquire hematopoietic potential that allow for the emergence of definitive HSCs during development (21–29). Recent data has also suggested that ECs derived from the somite endotome migrates to the location of the hemogenic endothelium, making up the dorsal aorta and eliciting inductive signals that drive the emergence of the LT-HSC (30)**. Lastly, it has been shown that ECs derived from AGM can recapitulate the developmental in vivo endothelial-to-HSC transition in an in vitro setting (31)**. These data strongly suggest that the surrounding supportive endothelium of AGM can provide the essential educational cues necessary to induce the emergence, maintenance, and amplification of functional HSCs from hematopoietic precursors. Taken together, the current data demonstrates that vascular endothelium is not only the source of the first definitive LT-HSC, but also suggests that neighboring ECs can serve as an instructional guide mediating endothelial-to-hematopoietic transition during embryonic development.
Regulation of HSC maintenance by the endothelial niche
The body must produce about a trillion new blood cells each day to replenish daily losses. This massive production is tightly regulated via coordinated cell-fate decisions made by the HSC. The postnatal HSC is a remarkable somatic cell that is defined by its ability to undergo self-renewal and maintain the capacity to generate all of the mature hematopoietic cell types within the blood and immune system for the life of the organism (32). These unique qualities make the HSC clinically useful in bone marrow (BM) transplantation settings for the treatment of a wide variety of hematological diseases.
Maintenance of HSC function is dependent upon their cell-intrinsic properties, as well as the extrinsic cues from the BM microenvironment (33). To date, most studies have primarily focused on the intrinsic regulation of the HSC. However, research has emerged over the past two decades demonstrating that the BM microenvironment is critical in maintaining the HSC pool and its functional output, with one of the first experiments suggesting that the HSC is dependent on microenvironmental cues from non-hematopoietic BM stromal cells and that these niche cells were capable of maintaining the HSC in vitro (34, 35). In 1978 Raymond Schofield was the first to formally suggest that a specialized microenvironment was responsible to maintain stem cell function (36), with his “niche” concept laying the ground work for all subsequent studies related to the microenvironmental control of the HSC.
Within the BM microenvironment there exists a vast array of diverse cellular components that comprise the HSC niche (6, 7, 37–41), including vascular endothelial cells, perivascular stromal cells, osteoblasts, sympathetic nerves, and cells part of the hematopoietic hierarchy such as macrophages and megakaryocytes. Although most of these niche components elicit a positive effect on HSC maintenance, some cell-types such as adipocytes have been shown to impose a negative effect by interfering with homeostatic and regenerative hematopoiesis (42–81)*, **. The BM is a densely vascularized tissue in which the blood vessels encompass a vast surface area, making vascular endothelial cells one of the most abundant niche cells within the BM microenvironment. The endothelial niche is made up of vascular ECs that form monolayers lining the lumen of blood vessels and consists of arteries, veins, and a large network of capillaries that connect the arterial and venous systems (82). The BM endothelial network is comprised of two main endothelial niches, the arteriolar niche identified with a VEcadherin+CD31+Endomucin+/−SCA1highVEGFR3− cell surface phenotype and the sinusoidal niche which is identified by a VEcadherin+ CD31+ Endomucin+ SCA1low VEGFR3+ cell surface phenotype (Figure 1) (61, 63). Although recent studies have suggested another endothelial subset known as Type-H endothelium defined as CD31highEndomucinhigh (83, 84), when processing ECs from the BM of adult mice with an intravital stain with VEcadherin and excluding Lineage+, CD45+, and Ter119+ cells, both arterioles and sinusoids are CD31high and all VEGFR3+ sinusoids and a subset of arterioles are Endomucinhigh, suggesting that Type-H endothelium may be more widespread and long-lived than previously thought (Figure 1). Nonetheless, Type-H ECs are suggested to play a major role in driving growth of the bone vasculature and in regulating the metabolic state of the BM microenvironment (83–85)*.
Figure 1. VEGFR3 and intravital VEcadherin identify distinct immunophenotypic arteriole and sinusoidal BMEC niches.
Mice expressing a Vegfr3::YFP BAC transgene reporter were intravitally-labeled using an EC-specific antibody (VEcadherin). A) Representative flow cytometry contour plots of enzymatically-dissociated and hematopoietic lineage+ cell-depleted whole bone marrow, stained with antibodies raised against CD45, TER119, CD31, VEcadherin, and Endomucin. Gating strategies (dashed line) and percentage of parent populations are indicated. Note: VEGFR3highVEcadherinhighCD31+ sinusoid bone marrow endothelial cells (BMECs) (green) and VEGFR3low/−VEcadherinlowCD31+ arteriole BMECs (red) can be distinguished using intravital VEcadherin staining in combination with hematopoietic exclusion (lineage−CD45−TER119−). B) Representative histogram of Endomucin stained VEGFR3highVEcadherinhighCD31+ sinusoid BMECs (green) and VEGFR3low/−VEcadherinlowCD31+ arteriole BMECs (red). (C) Representative confocal images of femoral trabecular and diaphysis regions demonstrating VEGFR3−VEcadherin+CD31+Endomucin+ (yellow arrow) and VEGFR3low/−VEcadherin+CD31+Endomucin− (white arrow) arteriole BMECs. Scale bar = 100 μm.
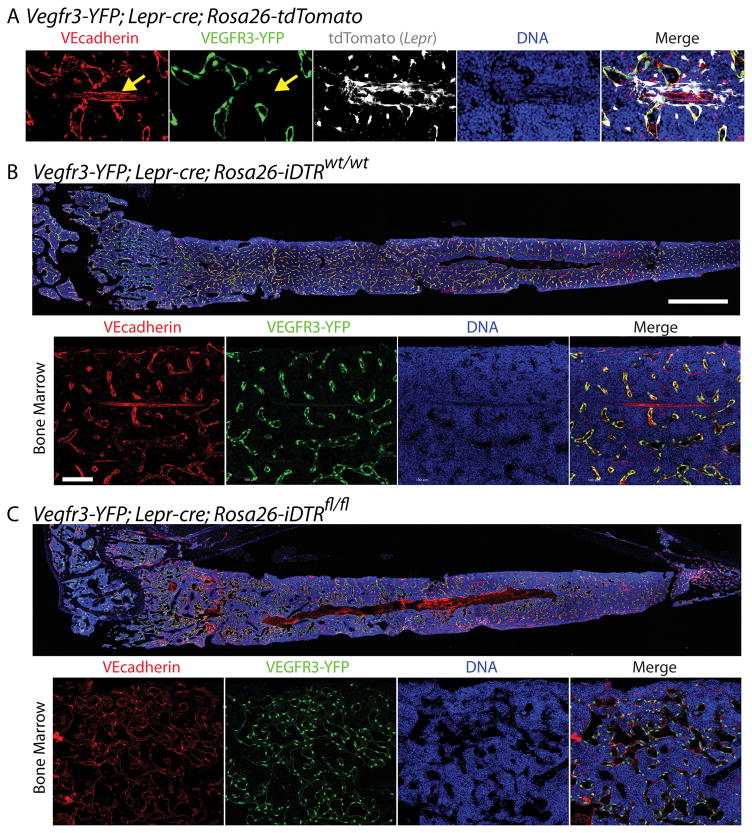
Prior work from our group has demonstrated that signaling pathways within the endothelial niche can dictate HSC cell fate; with AKT signaling supporting HSC self-renewal and activation of endothelial MAPK signaling driving HSC differentiation into hematopoietic progenitors and terminally differentiated cells (86). AKT-activated ECs promote the self-renewal of HSCs via the expression of HSC supportive paracrine factors, including KITL, CXCL12 and JAGGED-1. Additionally, we have demonstrated that inhibition of canonical NF-kB signaling in endothelium results in a profound increase in HSC self-renewal and regenerative potential (65). Subsequent in vivo studies have proven that EC-specific deletion of Kitl, Cxcl12, and Jag1 impaired the maintenance of the HSC at homeostasis (53, 54, 64). Additionally, conditional deletion of Jag1 from ECs resulted in profound defects in hematopoietic regeneration following myelosuppressive injury (64). It is also important to note that many of the EC-derived paracrine factors are also expressed in other BM niche constituents. In particular, there has been extensive data demonstrating that deficiency of factors in multiple niche cells, such as KITL and CXCL12, results in defects in homeostatic hematopoiesis. More importantly, depending on the cellular source of some of these factors, there could be differential regulation of the HSC and its progeny. For instance, it has been shown that deletion of Cxcl12 in ECs (53, 57) and NG2+, LEPR+, or PRX1+ perivascular cells results in defects in HSC maintenance, as well as localization of the HSC within the BM microenvironment (53, 57, 71). However, osteoblast-specific deletion of Cxcl12 resulted in defects in lymphopoiesis but not the HSC itself (53, 57). These findings have recently been extended to the cytokine IL-7 and IL-7+ mesenchymal cells, where conditional deletion of Il7 in ECs and mesenchymal progenitors affected B-lymphopoiesis and conditional deletion of Kitl and Cxcl12 in IL-7+ cells results in defects in the HSC and in multipotent progenitor cells (52). Taken together, these data suggest that the BM microenvironment is made up of multiple HSC niches that, depending on the type of growth factor and the cellular source producing that growth factor, regulates HSC function and its cell fate decisions. It is also important to take into account the possibility that the structural integrity and function of endothelial and perivascular niches are intimately dependent on one another to provide the instructional framework essential for governing HSC self-renewal and differentiation. It is likely that loss of factors such as KITL or CXCL12 in perivascular cells could have deleterious effects to the functionality of endothelium, and vice versa. Indeed, cKIT (87) and CXCR4 (88) are expressed on endothelium and deletion of their respective ligands, KITL and CXCL12, in perivascular cells could perturb integrity or function of neighboring ECs. Recent work by Zhou et. al. demonstrated that conditional deletion of Angpt1 from perivascular and hematopoietic cells resulted in increased vascular leakiness, suggestive of a cross-talk between the EC and perivascular components (89)*. One must also take caution when ablating niche cells to study their function, as the removal of specific niche cells can perturb the structural framework of the compound BM architecture leading to adverse effects on their neighboring niche cells. For instance, our lab has demonstrated that both sinusoidal and arteriole bone marrow endothelial cells (BMECs) are highly invested with LEPR+ perivascular cells (63) (Figure 2A) and when we ablate LEPR+ cells using an inducible diphtheria toxin system we find that the VEGFR3+ sinusoidal vasculature is more tortuous, dilated, and leaky (Figure 2B–C). Moving forward, it will be important to take into account the notion of a compound HSC niche and determine whether the alterations made on a particular niche sub-type directly influences hematopoietic function or causes indirect effects mediated via compound niche interactions. Currently, the lack of genetic tools and assays limit us from adequately testing these issues; at a minimum, the potential of off-target effects of gene deletion and cell ablation studies should be taken into consideration.
Figure 2. Perivascular LEPR+ cells support arteriole and sinusoidal BMECs.
Mice expressing Vegfr3-YFP; Lepr-cre transgenes were crossed with mice carrying either a Rosa26-expressing loxP-STOP-loxP tdTomato reporter or a Rosa26-expressing loxP-STOP-loxP inducible Diphtheria Toxin Receptor (iDTR) cell ablation cassette and intravitally-labeled with an Alex Fluor 647-conjugated antibody against VEcadherin. A) Representative confocal images of VEGFR3+VEcadherin+ sinusoid bone marrow endothelial cells (BMECs) (white arrow), VEGFR3−VEcadherin+ arteriole BMECs (yellow arrow), and sinusoid/arteriole BMEC-invested tdTOMATO+ Lepr-expressing cells (merged image). Scale bar = 50 μm. B–C) Representative confocal images of (B) wild type (wt) or (C) homozygous Rosa26-iDTR (flanked by loxP; fl) Vegfr3-YFP; Lepr-cre mice following diphtheria toxin administration. Dashed box indicates magnification. Note: Vascular BMECs appear dilated following LEPR cell ablation. Scale bar = 500 μm and 100 μm, respectively.
Essential role of the endothelial niche in hematopoietic regeneration
It has long been suggested that hematopoietic recovery is dependent upon the regeneration of the BM vasculature (90, 91). Utilizing in vivo genetic models, Heissig et. al. was the first to demonstrate that the BM endothelial niche was a supportive cellular hub for functional hematopoiesis (92). In this study, the authors demonstrated that mice lacking the soluble form of the pro-HSC factor KITL were unable to effectively regenerate the hematopoietic system following physiological insults as a result of their inability to properly position HSCs for self-renewal and directed differentiation. Notably, this landmark study was one of the first to coin and recognize the term “vascular niche”. Follow-up studies later determined that phenotypically marked HSCs were is close association with the vascular niche (93) and that conditional deletion of Vegfr2 in adult ECs (61) or infusion of neutralizing antibodies to VEcadherin (51, 94) interfered with the proper reassembly of the vascular niche and blocked hematopoietic recovery following hematopoietic insult. Conversely, endothelial-specific deletion of intrinsic drivers of apoptosis, Bak1 and Bax, confered a striking radio-protective effect and subsequently promotes rapid recovery of the hematopoietic system following insult, due to the preserved integrity of the BM endothelial niche (56). Additionally, recent studies have shown that endothelial-specific deletion of paracrine factors such as Jag1 (64), Ptn (60), and Egf (55), as well as vascular adhesion molecules such as E-selectin (66) can have deleterious effects to the regenerative capacity of the HSC. Taken together, these data demonstrate that the regeneration of the structural and functional integrity of the endothelial niche is a vital process in ensuring that the hematopoietic system returns back to homeostasis following hematopoietic insult.
The aging endothelial niche and its effect on HSC function
Most reports describing aging-related alterations in the hematopoietic compartment have focused on cell-intrinsic alterations within HSCs. These studies have shown that while the absolute number of immunophenotypically defined HSCs increases with age, aged HSCs exhibit a decrease in their long-term reconstitution abilities (95–99). Furthermore, aged HSCs exhibit a significant myeloid bias at the expense of lymphopoiesis (96, 100–102), leading to a predisposition towards the development of myeloid neoplasms. While these studies show that cell-intrinsic changes that include defects in cellular polarity, altered epigenetic landscape, and impaired genomic integrity and DNA damage largely contributes to the aging of the hematopoietic system, the role of the aged microenvironment is largely unexamined. As previously discussed, there is an ever-increasing body of evidence demonstrating essential interactions between the HSC and its niche, suggesting that both local and systemic factors regulate HSC function (42–81). Indeed, it has been demonstrated that transplanting young HSCs into aged recipients can, in part, can lead to reduced hematopoietic clonality (103) and can lead to myeloid skewing which is driven by the inflammatory cytokine CCL5 (104). Even more recently, a study by Guidi et. al. has demonstrated that stomal-derived osteopotin is decreased with age and that the infusion of thrombin-cleaved osteopontin has therapeutics effects in regards to rejuvenating aged HSC phenotypes (105)**. Our laboratory has determined that physiological aging results in defects in BMEC numbers and function. Analysis of the femurs in aged mice (24 months) revealed a tortuous and dilated vascular network when compared with young (3 month) counterparts (Figure 3A). Moreover, we observed a significant decrease in the frequency of functionally patent CD45−Ter119−CD31+VEcadherin+ BMECs (Figure 3B). In line with these findings, it has recently been demonstrated that aged BMECs have impaired niche function in regards to regulating hematopoietic niche cells and that it is possible to rejuvenate their functional readout. However, these studies were unable to restore the full functional capacity of the aged HSC (106)*. Taken together, these data suggest that changes in aged ECs and other BM niche constituents inefficiently sustain HSC homeostasis, potentially leading to aged-related hematopoietic disorders. Therefore, understanding the intimate relationship between the BM microenvironment and its resident HSCs during homeostasis and aging will provide a therapeutic opportunity to mitigate and potentially reverse the age-related functional decline observed in the hematopoietic system, including the development of hematologic malignancies.
Figure 3. Physiological aging alters BMEC function.
Endothelium from young and aged mice were intravitally-labeled using an Alex Fluor 647-conjugated antibody against VEcadherin. A) Representative confocal images of femoral trabecular region from young and aged mice. Scale bar = 200 μm. B) Quantification of hematopoietic lineage-depleted, CD45−TER119−VEcadherin+CD31+ BMECs from young and aged mice. Note: All quantifications were performed on enzymatically-disassociated and lineage+ cell-depleted femurs and gated on CD45−TER119−VEcadherin+CD31+ populations. Error bars represent mean ± SEM. Pairwise two-tailed comparisons using Student’s t-test were performed to determine significance. * P<0.05; ** P<0.01; *** P<0.001.
Therapeutic application of the endothelial niche
Despite advances in the understanding of HSC biology, the exact mechanisms that regulate the balance between self-renewal and lineage-specific differentiation are still unknown, which has limited the development of new in vivo and ex vivo HSC-based therapies. The necessity to build upon current HSC expansion strategies may potentiate the therapeutic use of HSCs, as well as ECs, for the treatment of hematological disorders. The use of endothelium to expand hematopoietic stem and progenitor cells (HSPCs) was first demonstrated by co-culturing human BMECs with CD34+ umbilical cord blood (107, 108). Subsequent studies showed that ECs isolated from fetal tissues, heart, and brain had the capacity to expand HSPCs (109–113). However, these studies were limited in that they relied on significant supplementation with serum and supraphysiological levels of growth factors to maintain ECs, causing adverse effects on HSPCs (114, 115). To study the role of ECs in the ex vivo expansion of HSCs, our group has developed an approach that allows for the maintenance of primary ECs by constitutively activating AKT-signaling, enabling EC survival for weeks under serum- and growth factor-free conditions, in part, by activating the downstream AKT signaling pathway mTOR (116). Additionally, AKT activation maintains the paracrine repertoire of the ECs without inducing immortalization or tumorgenic potential (116). Maintenance of primary mouse and human ECs without exogenous growth factors or serum allowed us to develop an unbiased EC-HSPC co-culture system, in which the co-culture of mouse HSCs (51) and human umbilical cord blood (UCB) hematopoietic stem and progenitor cells (HSPCs) (117) with ECs resulted in the expansion of bona fide repopulating HSCs which were able to engraft lethally irradiated primary and secondary recipients. This platform’s utility has also been extended to expand engraftable adult BM HSPCs and HSPCs derived from induced pluripotent stem cells (118, 119)*.
It has been over 3 decades since the first scientific experiments demonstrated that ECs are a critical part of the BM “stroma” (120, 121). These studies confirmed the notion that ECs may have instructive capabilities, sparking a line of seminal papers leading to the discovery that various sources of endothelium have the capacity to support many aspects of hematopoiesis, particularly in the ex vivo setting (51, 109, 122–124). The culmination of all of these experiments laid the foundation for all of the recent data confirming the essential role of ECs and the vascular niche in maintaining functional hematopoiesis, both in vivo and ex vivo. In addition to the expansion of repopulating HSCs, direct injection of various sources of allogeneic or syngeneic endothelium has been shown to enhance hematopoiesis following myelosuppression (62, 63, 65, 125–127). These data suggest that ECs, regardless of source, may be useful for acceleration of hematopoietic recovery. Although it appears that the source of ECs for therapeutic transplantation may not be a barrier in the clinical arena, our group (128) and others (2) have demonstrated that there is significant heterogeneity within the body’s vascular network, suggesting that each organ is endowed with a specialized endothelium with a unique paracrine signature that specifically supports their tissue-resident stem cell and their progeny. Notably, it has been shown that tissue specific ECs can enhance organ regeneration with other sources of ECs unable to elicit the same regenerative effect (129, 130). In line with this notion, we have demonstrated that transplantation of niche-specific BMECs can rapidly and efficiently enhance hematopoietic recovery following myeloablation (63, 65). Additionally, transplantation of BMECs resulted in the preservation of the BM vascular niche and the rapid regeneration of VEGFR3+ sinusoidal endothelium. It is important to note that we have attempted to transplant ECs isolated from other organs (i.e. BM-derived endothelial progenitor cells, lung ECs, and brain ECs) and found that only the niche-specific BMECs result in this profound protection of the hematopoietic system and the vascular niche following myeloablative insult (63, 65). These data suggest that BMECs are a specialized subtype of endothelium that can produce factors to supportive regenerative hematopoiesis and microenvironmental radioprotection (63). In summary, transplantation of ECs may create a more permissive microenvironment by protecting the structural and functional integrity of the endothelial niche following hematopoietic insults, as well as enhance the engraftment of HSPCs following BM transplantation. These finding have laid a scientific and therapeutic justification for the transplantation of endothelium to accelerate the rate of hematopoietic recovery following radiation or chemotherapeutic regimens and decreasing the morbidity/mortality resulting from associated pancytopenias.
Concluding Remarks
Understanding the intimate relationship between the endothelial niche and HSCs that maintains homeostatic hematopoiesis is critical to the future discovery of therapeutic pro-HSC paracrine factors that modulate self-renewal and expansion of adult HSCs and will aid in the development of strategies to enhance hematopoietic reconstitution in the clinical arena. However, there is still a great need to develop novel approaches to facilitate processes allowing for the unraveling of the complex BM microenvironment-HSPC interactions that are critical in providing the knowledge for the development of new HSC therapeutic applications. The field will need to be dedicated in understanding the role of tissue-specific ECs in establishing unique instructive vascular niche cells that produce the correct milieu and stoichiometry of paracrine factors to direct organ regeneration, in particular within the BM microenvironment. It is likely that the reconstitution of hematopoiesis along with endothelial transplantation will not only co-operatively augment both the stability and integrity of the newly formed vessels but also promote tissue repair and multi-organ regeneration via an inductive mechanism. At the field’s current pace, we will have a significant and positive impact on novel therapeutics aimed at decreasing the morbidity and mortality associated with life-threatening pancytopenias associated with hematopoietic disorders and myelosuppressive regimens.
Key points.
Endothelial cells serve as an instructive platform to nurture the emergence, maintenance, and regeneration of the HSC.
Improving our understanding of the intimate relationship between the HSC and its specialized niche by investigating the role of the BM microenvironment in supporting HSC function during aging may prove to be beneficial in reversing or preventing the age-related functional decline observed in the hematopoietic system.
Endothelial cells can be utilized as a stand-alone or adjuvant cellular therapy to temporize pancytopenia associated with myelosuppressive or myeloablative treatments.
Acknowledgments
Funding Sources: National Institutes of Health (NIH)
Leukemia & Lymphoma Society (LLS)
We would like to thank all members of the Butler lab for critically reading our review. We would like to particularly thank Michael C. Gutkin for all of his hard work and dedication in taking the images shown in Figures 1–3. We would also like to apologize to any of our distinguished colleagues whose work we inadvertently left out.
Financial support and sponsorship
The work presented in this Opinion was support by the National Cancer Institute (1R01CA204308), the National Heart, Lung, and Blood Institute (1R01HL133021), and Leukemia Lymphoma Society Quest for Cures.
Footnotes
Conflicts of interest
The authors declare no conflict of interests.
References
- 1.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circulation research. 2007;100(2):158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 3.Aird WC. Endothelium in health and disease. Pharmacological reports : PR. 2008;60(1):139–43. [PubMed] [Google Scholar]
- 4.Kliche K, Jeggle P, Pavenstadt H, Oberleithner H. Role of cellular mechanics in the function and life span of vascular endothelium. Pflugers Archiv : European journal of physiology. 2011;462(2):209–17. doi: 10.1007/s00424-011-0929-2. [DOI] [PubMed] [Google Scholar]
- 5.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nature reviews Cancer. 2010;10(2):138–46. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529(7586):316–25. doi: 10.1038/nature17040. In this review, Rafii et. al. highlight the vascular endothelial contributions to tissue development, maintainence, and regeneration through organ-specific paracrine signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasine JP, Yeo KT, Chute JP. Concise Review: Paracrine Functions of Vascular Niche Cells in Regulating Hematopoietic Stem Cell Fate. Stem Cells Transl Med. 2017;6(2):482–9. doi: 10.5966/sctm.2016-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113(3):891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 9.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nature immunology. 2008;9(2):129–36. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamo L, Garcia-Cardena G. The vascular origin of hematopoietic cells. Developmental biology. 2012;362(1):1–10. doi: 10.1016/j.ydbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Kaimakis P, Crisan M, Dzierzak E. The biochemistry of hematopoietic stem cell development. Biochim Biophys Acta. 2013;1830(2):2395–403. doi: 10.1016/j.bbagen.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lievre F, Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364(6432):67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- 13.Medvinsky AL, Samoylina NL, Muller AM, Dzierzak EA. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature. 1993;364(6432):64–7. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- 14.Godin I, Dieterlen-Lievre F, Cumano A. Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(3):773–7. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godin I, Garcia-Porrero JA, Dieterlen-Lievre F, Cumano A. Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. The Journal of experimental medicine. 1999;190(1):43–52. doi: 10.1084/jem.190.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8(6):761–9. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 17.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1(4):291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 18.Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7(3):335–44. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 19.Pardanaud L, Luton D, Prigent M, Bourcheix LM, Catala M, Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122(5):1363–71. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Porrero JA, Godin IE, Dieterlen-Lievre F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol (Berl) 1995;192(5):425–35. doi: 10.1007/BF00240375. [DOI] [PubMed] [Google Scholar]
- 21.Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circulation research. 2013;112(9):1272–87. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffredo T, Gautier R, Brajeul V, Dieterlen-Lievre F. Tracing the progeny of the aortic hemangioblast in the avian embryo. Developmental biology. 2000;224(2):204–14. doi: 10.1006/dbio.2000.9799. [DOI] [PubMed] [Google Scholar]
- 23.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125(22):4575–83. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell stem cell. 2008;2(3):252–63. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon-Keylock S, Sobiesiak M, Rybtsov S, Moore K, Medvinsky A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood. 2013;122(14):2338–45. doi: 10.1182/blood-2012-12-470971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. The EMBO journal. 2000;19(11):2465–74. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–11. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116–20. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 29.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112–5. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- **30.Nguyen PD, Hollway GE, Sonntag C, Miles LB, Hall TE, Berger S, et al. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature. 2014;512(7514):314–8. doi: 10.1038/nature13678. In this report, Nguyen et. al. describe how somite-derived endothelial precursors expressing meox1 provide inductive signaling cues for HSC specification within the dorsal aorta during development. [DOI] [PubMed] [Google Scholar]
- **31.Hadland BK, Varnum-Finney B, Poulos MG, Moon RT, Butler JM, Rafii S, et al. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. The Journal of clinical investigation. 2015;125(5):2032–45. doi: 10.1172/JCI80137. In this article, Hadland et. al. describe how an AGM-dervied endothelial cell ex vivo niche is sufficient to recapitulate in vivo embryonic HSC specification through NOTCH siganling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spangrude GJ, Smith L, Uchida N, Ikuta K, Heimfeld S, Friedman J, et al. Mouse hematopoietic stem cells. Blood. 1991;78(6):1395–402. [PubMed] [Google Scholar]
- 33.Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453(7193):306–13. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- 34.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91(3):335–44. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 35.Dexter TM, Wright EG, Krizsa F, Lajtha LG. Regulation of haemopoietic stem cell proliferation in long term bone marrow cultures. Biomedicine. 1977;27(9–10):344–9. [PubMed] [Google Scholar]
- 36.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 37.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nature medicine. 2014;20(8):833–46. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunisaki Y, Frenette PS. Influences of vascular niches on hematopoietic stem cell fate. International journal of hematology. 2014;99(6):699–705. doi: 10.1007/s12185-014-1580-4. [DOI] [PubMed] [Google Scholar]
- 40.Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125(17):2621–9. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157(1):41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nature medicine. 2014;20(11):1315–20. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behrens K, Triviai I, Schwieger M, Tekin N, Alawi M, Spohn M, et al. Runx1 downregulates stem cell and megakaryocytic transcription programs that support niche interactions. Blood. 2016;127(26):3369–81. doi: 10.1182/blood-2015-09-668129. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K, Suda T. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. The Journal of experimental medicine. 2015;212(12):2133–46. doi: 10.1084/jem.20150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nature medicine. 2014;20(11):1321–6. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 46.Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature medicine. 2013;19(4):429–36. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hur J, Choi JI, Lee H, Nham P, Kim TW, Chae CW, et al. CD82/KAI1 Maintains the Dormancy of Long-Term Hematopoietic Stem Cells through Interaction with DARC-Expressing Macrophages. Cell stem cell. 2016;18(4):508–21. doi: 10.1016/j.stem.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell stem cell. 2014;15(3):365–75. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Pierce H, Zhang D, Magnon C, Lucas D, Christin JR, Huggins M, et al. Cholinergic Signals from the CNS Regulate G-CSF-Mediated HSC Mobilization from Bone Marrow via a Glucocorticoid Signaling Relay. Cell stem cell. 2017 doi: 10.1016/j.stem.2017.01.002. Work from Pierce et. al. describes long-range hormonal signals originating in the central nervous system that modulate HSC mobilization in the BM microenvironment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 51.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell stem cell. 2010;6(3):251–64. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordeiro Gomes A, Hara T, Lim VY, Herndler-Brandstetter D, Nevius E, Sugiyama T, et al. Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity. 2016;45(6):1219–31. doi: 10.1016/j.immuni.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nature medicine. 2013;19(3):295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doan PL, Russell JL, Himburg HA, Helms K, Harris JR, Lucas J, et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31(2):327–37. doi: 10.1002/stem.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Himburg HA, Doan PL, Quarmyne M, Yan X, Sasine J, Zhao L, et al. Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms. Nature medicine. 2017;23(1):91–9. doi: 10.1038/nm.4251. In this article, Himburg et. al. describe paracrine cross-talk between lineage-restricted osterix+ stromal cells, endothelial cells, and HSCs within the BM microenvironment that regulate hematopietic regeneration following radiation-mediated injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Himburg HA, Harris JR, Ito T, Daher P, Russell JL, Quarmyne M, et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell reports. 2012;2(4):964–75. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nature medicine. 2010;16(4):475–82. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell stem cell. 2009;4(3):263–74. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li B, Bailey AS, Jiang S, Liu B, Goldman DC, Fleming WH. Endothelial cells mediate the regeneration of hematopoietic stem cells. Stem cell research. 2010;4(1):17–24. doi: 10.1016/j.scr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Poulos MG, Crowley MJ, Gutkin MC, Ramalingam P, Schachterle W, Thomas JL, et al. Vascular Platform to Define Hematopoietic Stem Cell Factors and Enhance Regenerative Hematopoiesis. Stem Cell Reports. 2015;5(5):881–94. doi: 10.1016/j.stemcr.2015.08.018. In this report, Poulos et. al. describe the potential of BM-derived endothelial cells as an ex vivo niche for HSC expansion and their theraputic application for augmenting hematopoietic regeneration following myelosuppressive insult. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poulos MG, Guo P, Kofler NM, Pinho S, Gutkin MC, Tikhonova A, et al. Endothelial jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell reports. 2013;4(5):1022–34. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **65.Poulos MG, Ramalingam P, Gutkin MC, Kleppe M, Ginsberg M, Crowley MJ, et al. Endothelial-specific inhibition of NF-kappaB enhances functional haematopoiesis. Nat Commun. 2016;7:13829. doi: 10.1038/ncomms13829. In this article, Poulos et. al. describe how inflammatory pathways within the endothelial microenvironment can be modulated to enhance homeostatic and regenerative HSC activity, highlighting the potential of targeted niche-directed hematopoietic therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nature medicine. 2012;18(11):1651–7. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- *67.Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526(7571):126–30. doi: 10.1038/nature15250. Using novel bone imaging techniques and CTNNAL1+cKIT+ demarcation of repopulating HSCs, Acar et. al. define the perivascular distribution of HSCs under homeostatic conditions in adult murine BM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *68.Chen JY, Miyanishi M, Wang SK, Yamazaki S, Sinha R, Kao KS, et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530(7589):223–7. doi: 10.1038/nature16943. Using a fluorescent reporter in the Hoxb5 gene that enriches for repopulating HSCs, Chen et. al. describe a direct association of HSCs with VE-cadherin+ endothelium in the adult BM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **71.Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nature cell biology. 2017;19(3):214–23. doi: 10.1038/ncb3475. In this report, Asada et. al. describe the differential paracrine contributions of perivascular NG2+ and LEPR+ stromal cells in their expression of Cxcl12 and Scf that regulate HSC localization and numbers in the adult BM microenvironment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 73.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 75.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–9. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 77.Ghode SS, Bajaj MS, Kulkarni RS, Limaye LS, Shouche YS, Kale VP. Neuropilin-1 Is an Important Niche Component and Exerts Context-Dependent Effects on Hematopoietic Stem Cells. Stem Cells Dev. 2017;26(1):35–48. doi: 10.1089/scd.2016.0096. [DOI] [PubMed] [Google Scholar]
- 78.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 80.Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nature medicine. 2013;19(6):695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **81.Smith-Berdan S, Nguyen A, Hong MA, Forsberg EC. ROBO4-mediated vascular integrity regulates the directionality of hematopoietic stem cell trafficking. Stem Cell Reports. 2015;4(2):255–68. doi: 10.1016/j.stemcr.2014.12.013. In this article, Smith-Berdan et. al. establish a mechanism in which HSCs are specifically targeted to the BM microenvironment through ROBO4- and VCAM1-depedent sinusoudal transendothleial cell migration, and ultimately maintained in the niche via vascular integrity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Augustin HG, Kozian DH, Johnson RC. Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. BioEssays : news and reviews in molecular, cellular and developmental biology. 1994;16(12):901–6. doi: 10.1002/bies.950161208. [DOI] [PubMed] [Google Scholar]
- 83.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–8. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376–80. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *85.Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532(7599):323–8. doi: 10.1038/nature17624. Itkin et. al. define discrete roles of BM vascular subtypes in HSPC maintainence and hematopoietic trafficking. Less permeable SCA1+ arterial endothleium establish local BM microenvironments with low reactive oxygen species (ROS) implicated in HSPC quiescence, while more permeable SCA1low sinusoids promote HSPC activation through high ROS states and increased hematopoietic migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature cell biology. 2010;12(11):1046–56. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. The Journal of biological chemistry. 2004;279(18):18600–7. doi: 10.1074/jbc.M311643200. [DOI] [PubMed] [Google Scholar]
- 88.Noels H, Zhou B, Tilstam PV, Theelen W, Li X, Pawig L, et al. Deficiency of endothelial CXCR4 reduces reendothelialization and enhances neointimal hyperplasia after vascular injury in atherosclerosis-prone mice. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(6):1209–20. doi: 10.1161/ATVBAHA.113.302878. [DOI] [PubMed] [Google Scholar]
- *89.Zhou BO, Ding L, Morrison SJ. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. eLife. 2015;4:e05521. doi: 10.7554/eLife.05521. Following radiation-mediated injury, Zhou et. al. report that ANGPT1 from LEPR+ stroma and HSCs are necessary to promote BM endothelial cell niche integrity, at the expense of pan-hematopoietic recovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knospe WH, Gregory SA, Husseini SG, Fried W, Trobaugh FE., Jr Origin and recovery of colony-forming units in locally curetted bone marrow of mice. Blood. 1972;39(3):331–40. [PubMed] [Google Scholar]
- 91.Jacobson LO. Modification of radiation injury. Bull N Y Acad Med. 1954;30(9):675–92. [PMC free article] [PubMed] [Google Scholar]
- 92.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 94.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nature medicine. 2004;10(1):64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 95.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(50):20012–7. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nature reviews Immunology. 2013;13(5):376–89. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 99.Kowalczyk MS, Tirosh I, Heckl D, Rao TN, Dixit A, Haas BJ, et al. Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Res. 2015;25(12):1860–72. doi: 10.1101/gr.192237.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111(12):5553–61. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dykstra B, de Haan G. Hematopoietic stem cell aging and self-renewal. Cell Tissue Res. 2008;331(1):91–101. doi: 10.1007/s00441-007-0529-9. [DOI] [PubMed] [Google Scholar]
- 102.Van Zant G, Liang Y. The role of stem cells in aging. Experimental hematology. 2003;31(8):659–72. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 103.Vas V, Senger K, Dorr K, Niebel A, Geiger H. Aging of the microenvironment influences clonality in hematopoiesis. PloS one. 2012;7(8):e42080. doi: 10.1371/journal.pone.0042080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119(11):2500–9. doi: 10.1182/blood-2011-11-391730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **105.Guidi N, Sacma M, Standker L, Soller K, Marka G, Eiwen K, et al. Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. The EMBO journal. 2017 doi: 10.15252/embj.201796968. In this study, Guidi et. al. demonstrate that an aged BM microenvironment can promote aging phenotypes in young hematopoietic cells through a decrease in stromal-derived osteopontin. Infusion of osteopontin can help restore the functional output of an aged hematopioetic system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *106.Kusumbe AP, Ramasamy SK, Itkin T, Mae MA, Langen UH, Betsholtz C, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532(7599):380–4. doi: 10.1038/nature17638. In this report, Kusumbe et. al. demonstrate that BM microenvironmental vessel subtypes and perivascular cells are altered during normal aging and can be functionally enhanced through the restoration of endothelial NOTCH signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA, et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86(9):3353–63. [PubMed] [Google Scholar]
- 108.Rafii S, Shapiro F, Rimarachin J, Nachman RL, Ferris B, Weksler B, et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84(1):10–9. [PubMed] [Google Scholar]
- 109.Chute JP, Saini AA, Chute DJ, Wells MR, Clark WB, Harlan DM, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100(13):4433–9. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 110.Chute JP, Saini AA, Kampen RL, Wells MR, Davis TA. A comparative study of the cell cycle status and primitive cell adhesion molecule profile of human CD34+ cells cultured in stroma-free versus porcine microvascular endothelial cell cultures. Experimental hematology. 1999;27(2):370–9. doi: 10.1016/s0301-472x(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 111.Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Experimental hematology. 2004;32(12):1226–37. doi: 10.1016/j.exphem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Li W, Johnson SA, Shelley WC, Ferkowicz M, Morrison P, Li Y, et al. Primary endothelial cells isolated from the yolk sac and para-aortic splanchnopleura support the expansion of adult marrow stem cells in vitro. Blood. 2003;102(13):4345–53. doi: 10.1182/blood-2003-03-0729. [DOI] [PubMed] [Google Scholar]
- 113.Brandt JE, Bartholomew AM, Fortman JD, Nelson MC, Bruno E, Chen LM, et al. Ex vivo expansion of autologous bone marrow CD34(+) cells with porcine microvascular endothelial cells results in a graft capable of rescuing lethally irradiated baboons. Blood. 1999;94(1):106–13. [PubMed] [Google Scholar]
- 114.Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117(23):6083–90. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chaurasia P, Gajzer DC, Schaniel C, D’Souza S, Hoffman R. Epigenetic reprogramming induces the expansion of cord blood stem cells. The Journal of clinical investigation. 2014;124(6):2378–95. doi: 10.1172/JCI70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19288–93. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, Rafii S. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120(6):1344–7. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *118.Gori JL, Butler JM, Chan YY, Chandrasekaran D, Poulos MG, Ginsberg M, et al. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. The Journal of clinical investigation. 2015;125(3):1243–54. doi: 10.1172/JCI79328. In this article, Gori et. al. utilize an ex vivo endothleial cell niche to support the generation and expansion of engraftable multipotent hematopoietic progenitors from human and non-human primate-derived pluripotent stem cells in a NOTCH ligand-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *119.Gori JL, Butler JM, Kunar B, Poulos MG, Ginsberg M, Nolan DJ, et al. Endothelial Cells Promote Expansion of Long-Term Engrafting Marrow Hematopoietic Stem and Progenitor Cells in Primates. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2016-0240. Gori et. al. describe the ex vivo expansion of BM-derived HSPCs from non-human primates on endothelial cells, demontrating the potential for the improvement of autologous gene-corrected HSC tranplantations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gartner S, Kaplan HS. Long-term culture of human bone marrow cells. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(8):4756–9. doi: 10.1073/pnas.77.8.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Al-Lebban ZS, Lange RD, Jones JB, Lothrop CD. Long-term bone marrow culture systems: normal and cyclic hematopoietic dogs. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire. 1987;51(2):162–8. [PMC free article] [PubMed] [Google Scholar]
- 122.Bagby GC, Jr, McCall E, Bergstrom KA, Burger D. A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood. 1983;62(3):663–8. [PubMed] [Google Scholar]
- 123.Quesenberry PJ, Gimbrone MA., Jr Vascular endothelium as a regulator of granulopoiesis: production of colony-stimulating activity by cultured human endothelial cells. Blood. 1980;56(6):1060–7. [PubMed] [Google Scholar]
- 124.Gerson SL, Friedman HM, Cines DB. Viral infection of vascular endothelial cells alters production of colony-stimulating activity. The Journal of clinical investigation. 1985;76(4):1382–90. doi: 10.1172/JCI112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salter AB, Meadows SK, Muramoto GG, Himburg H, Doan P, Daher P, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113(9):2104–7. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chute JP, Muramoto GG, Salter AB, Meadows SK, Rickman DW, Chen B, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109(6):2365–72. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Montfort MJ, Olivares CR, Mulcahy JM, Fleming WH. Adult blood vessels restore host hematopoiesis following lethal irradiation. Experimental hematology. 2002;30(8):950–6. doi: 10.1016/s0301-472x(02)00813-5. [DOI] [PubMed] [Google Scholar]
- 128.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Developmental cell. 2013;26(2):204–19. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468(7321):310–5. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539–53. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]