Abstract
We describe a case of a 29-year-old man from Pakistan who presented with progressive neurologic symptoms over 1 week and was found to have a right parietal cerebral abscess. Neurosurgical drainage cultures showed growth of Actinomyces meyeri, Streptococcus intermedius, and Parvimonas micra. An abscessed molar was identified as the likely port of entry and was extracted. The patient was treated with metronidazole, vancomycin, and doxycycline because of prior anaphylaxis to penicillin. At 6-month follow-up, repeat magnetic resonance imaging showed no signs of residual abscess. Culture-independent identification techniques (e.g., ribosomal sequencing) increasingly identify Actinomyces meyeri as a causative agent and significant pathogen in spontaneous brain abscesses. As understanding about Actinomyces meyeri’s prevalence and pathogenesis improves, questions arise about optimal treatment strategy, which we discuss based on a literature review.
Keywords: Actinomyces, Actinomyces meyeri, Actinomycosis, Brain abscess
Introduction
Actinomyces meyeri is one of multiple Actinomyces species identified within the human microbiota and has a predilection for invasive and disseminated disease [1]. Culture-independent identification techniques (e.g., ribosomal sequencing) are increasingly revealing the role of A. meyeri and accompanying bacteria in the development of spontaneous brain abscesses [2].
We present here a case of a young man who was diagnosed with symptomatic polymicrobial right parietal abscess (A. meyeri, Streptococcus intermedius, and Parvimonas micra). In addition to describing this unusual case of A. meyeri cerebral abscess in further detail, we reviewed relevant literature to address current clinical challenges in diagnosis and management of this infection.
Case presentation
A 29-year-old man with history of unrepaired congenital heart disease was transferred to our hospital for the management of a symptomatic right parietal mass. He had been in his usual state of health until 6 days before presentation when he developed sensory loss in his left foot that persisted for 24 h. He presented to an outside emergency department where he had a normal computed tomography (CT) without contrast of the head and normal CT angiography of the chest, abdomen, and pelvis. Outpatient neurology follow-up was recommended, but his symptoms progressed rapidly during the following days to include complete left-sided body weakness, numbness, and focal convulsions of his left upper and lower extremities. On Day 5, he presented again to the outside hospital, where magnetic resonance imaging (MRI) of the head revealed a right parietal ring-enhancing lesion consistent with cerebral abscess. Antibiotics were held to increase potential yield of abscess drainage culture, and the patient was started on levetiracetam with subsequent reduction in focal convulsions. He was then transferred to our hospital for neurosurgical intervention.
The patient was originally from Pakistan and moved to the United States in 2006. At age 5, he was diagnosed with truncus arteriosus Type II, bicuspid truncal valve, and single ventricular septal defect. He was not a surgical candidate because of progression of disease and subsequently developed Eisenmenger’s syndrome with pulmonary hypertension, which was managed with nocturnal oxygen (4 L/min) and sildenafil. He had no recent travel and he last travelled to Pakistan in 2007. He worked as a part-time driver for taxi and fast-food companies and was not involved in any outdoor activities. He denied any sick contacts, personal history of or known exposure to tuberculosis, or incarceration. He had a significant remote history of recurrent dental problems requiring tooth extractions and outpatient oral antibiotic courses. His last visit to the dentist was for routine cleaning one year prior. The patient lived locally with his wife and denied any use of alcohol, tobacco, or other drugs.
On arrival to the emergency department, he had a temperature of 36.7 °C, heart rate of 88 beats/minute, respiratory rate of 27 breaths/minute, blood pressure of 120/69 mm Hg, and oxygen saturation of 92% on 4 L nasal cannula. Physical examination revealed a fatigued, thin, but well-nourished man in no acute distress. He was alert and oriented to time, space, and person. On neurologic examination, he had 4/5 strength and a sensory deficit to light touch of his left upper and lower extremities. Tone and reflexes were normal throughout. Head and neck exam did not reveal any sinus tenderness, signs of trauma, otorrhea, Roth spots, oropharyngeal exudate, or palpable cervical lymph nodes. He did have notably poor dentition with visible caries and gingivitis. Cardiovascular examination was remarkable for a known II/VI systolic ejection murmur at the left sternal border and a soft diastolic murmur and he did not have any jugular venous distention. Pulmonary and abdominal examinations were within normal limits. He had clubbing bilaterally but no peripheral stigmata of endocarditis. Extensive review of systems on admission was negative except for what has already been mentioned and frequent gingival bleeding with brushing.
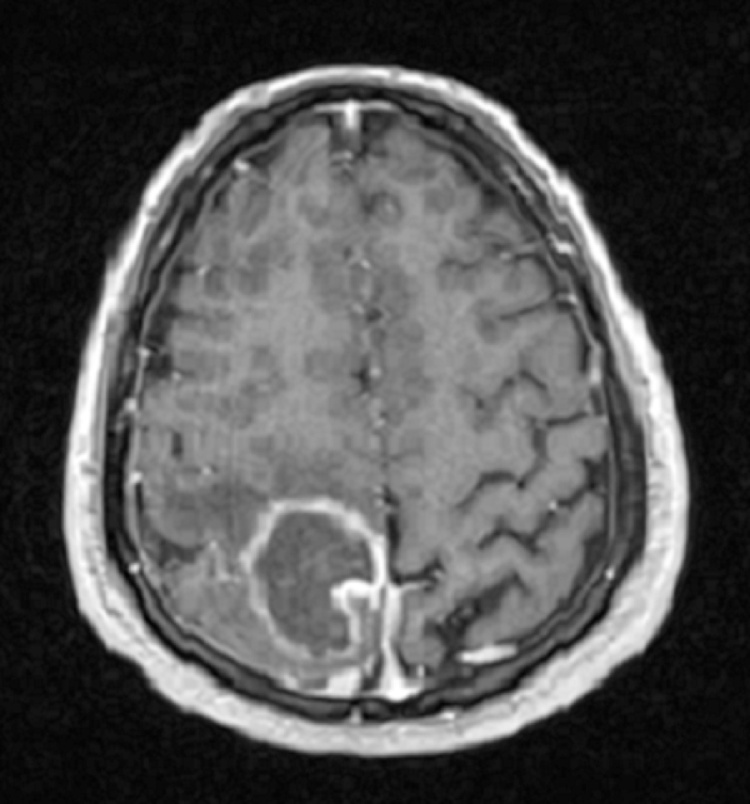
Complete biochemistry and hematology panel on presentation disclosed preserved renal and liver function, absence of leukocytosis, normal differential cell count, increased hemoglobin level of 20.2 mg/dL, and a normal platelet count of 145,000/μL. Other work-up included a negative HIV 4th- generation Ag/Ab test, non–detectable toxoplasma IgG and IgM serum antibodies, and negative blood cultures. Chest X-ray showed a dilated cardiac silhouette and mild pulmonary congestion. A repeat MRI of the head showed a ring-enhancing 3.7 × 3 × 3 cm mass within the right parietal lobe, with perilesional vasogenic edema (Fig. 1, Fig. 2). Lumbar puncture was not attempted due to lack of signs or symptoms of meningitis and potential risk of cerebral herniation. Transthoracic and transesophageal echocardiograms did not show any valvular vegetations.
Fig. 1.
Post-gadolinium T1 weighted Brain MRI. Ring-enhancing 3.7 × 3 ×3 cm mass centered within the right parietal lobe. Accompanying perilesional vasogenic edema contributes to the local mass effect. There is no midline shift.
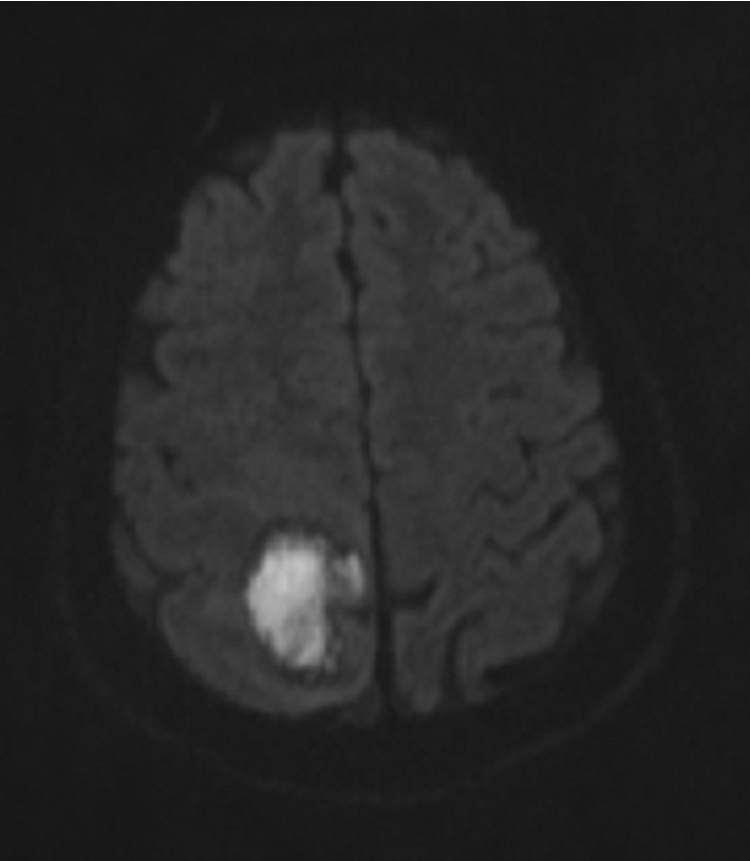
Fig. 2.
Diffusion Weighted Imaging Brain MRI. Pronounced high signal intensity in the center of the lesion corresponding to restricted diffusion of water molecules. Imaging findings are most compatible with cerebral abscess.
The patient underwent emergent stereotactic-guided neurosurgical drainage, which yielded 100 cc of purulent material. Postoperatively, he was started on oral trimethoprim-sulfamethoxazole 800–160 mg every 12 h, intravenous metronidazole 500 mg every 12 h, and intravenous vancomycin 1 g every 8 h. Antibiotic management was challenging because of the patient’s history of anaphylaxis to penicillin. Unfortunately, the patient was not desensitized to penicillin while under clinical observation, limiting choice of antibiotics. Cultures showed growth of Streptococcus intermedius, A. meyeri, and Parvimonas micra. The first two isolated organisms were susceptible to penicillin, ceftriaxone, vancomycin, clindamycin, and erythromycin. On subsequent complete dental evaluation, the patient was found to have a molar with an abscessed root, for which he underwent extraction. Once culture results were available, trimethoprim-sulfamethoxazole was discontinued and the patient was started on oral doxycycline 100 mg twice daily to cover A. meyeri. Doxycycline was chosen based on this organism’s reported antibiotic susceptibility and need for optimal central nervous system (CNS) penetration. He was discharged to an acute rehabilitation facility with oral doxycycline and intravenous vancomycin and metronidazole. Although systemic availability of metronidazole has been shown to be similar following oral and intravenous administration [3], intravenous administration is often recommended for the treatment of life-threatening infections [4], [5], [6], at least for initial therapy. We also opted for intravenous metronidazole over oral for ease of administration.
The patient stopped vancomycin prematurely due to reported rash in week 4 which was deemed later to be an infusion-related Red Man’s syndrome. An MRI 6 weeks into his antibiotic treatment showed interval signs of improvement with no evidence of abscess but with persistent hemorrhagic fluid, consistent with postoperative state. The patient was switched to oral doxycycline and oral metronidazole, and antiepileptics were stopped after electroencephalography was negative for seizure activity. Five months after his initial presentation, the patient underwent a second follow-up MRI brain examination that showed complete resolution of hemorrhagic fluid and no evidence of residual abscess. His clinical exam revealed stable residual left leg weakness. Metronidazole was stopped, and the patient was continued on doxycycline with a plan for 6 to 12 months based on published experience.
Discussion
The genus Actinomyces was identified by Bollinger and Harz in 1877 while studying “Lumpy Jaw” in cattle [5]. This group of gram-positive filamentous bacteria grows in anaerobic environments and is the etiologic agent of actinomycosis. Characteristic features include an indolent course, formation of abscesses with a wood-like consistency, presence of sulfur granules, and development of draining tracts. Actinomycosis is considered uncommon and appears to equally affect immune-competent and −compromised individuals, but there is limited epidemiologic data on prevalence because it is not a reportable disease [1], [5], [7].
Historically, diagnosis was based on clinical features, visualization of branching organisms on gram stain, and the presence of sulfur granules. The development of biochemical reactions and gas-liquid chromatography facilitated diagnosis, leading to identification of new species aside from Actinomyces israelii. More recently, the use of precise culture-independent identification techniques, such as 16S ribosomal RNA analysis, has revolutionized knowledge about this organism [4].
Actinomyces is part of the normal human microbiota of the oropharynx, distal esophagus, and genitourinary tract [1]. Depending on the location affected, actinomycosis is categorized as an oro-cervico-facial, thoracic, or abdomino-pelvic disease. Less frequently, actinomycosis can affect other body parts, including the musculoskeletal system, pericardium, and CNS [1]. Infections are highly associated with formation of dental plaque and caries [1] and can thereby penetrate deep tissues. Poor oral hygiene, dental procedures, aspiration of oral secretions, abdominal surgery, and acute intrabdominal processes have been identified as risk factors for actinomycosis [1]. Actinomyces is particularly known for its ability to cross tissue planes, a feature that has been used to explain involvement of contiguous tissues as well as spread to distant organs [5], [7]. Actinomyces occipital or parietal brain abscesses have been attributed to direct cranial extension from the oral cavity, however, little is known about the virulence factors that enable Actinomyces to invade tissues in this way [5].
A. meyeri was first described in 1911, when Kurt F. Meyer isolated the species from an empyema. Nomenclature changed over time, including Streptothrix meyeri, Actinobacterium meyeri, and eventually Actinomyces meyeri in 1977 [1], [4]. The colonization distribution of A. meyeri is similar to that of other species of Actinomyces. Organs infected by A. meyeri include brain, lung, muscle, skin, bone, liver, and gastrointestinal tract. The propensity of this bacteria to cause disseminated disease compared to other members of the genus is not well understood [1], [8]. We searched PubMed for case reports, case series, clinical trials, and reviews published in English in peer-reviewed journals, using the MeSH terms actinomyces, actinomycosis, actinomyces meyeri, brain abscess, cerebral, and/or central nervous system. Literature review yielded seven case reports of patients with A. meyeri brain abscess, which we summarize in Table 1.
Table 1.
Published Cases of A. meyeri Cerebral Abscesses.
| CR, publication year | Age/sex | Clinical presentation | Presumed Precipitant | Imaging findings | Other cultured organisms | Neurosurgical approach | Antibiotic treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| [17] 1984 | 28yo/F | HA, fever, AMS, meningismus, R-sided hemiparesis | Unknown | L parietal lobe brain abscess, ventriculitis | Streptobacillus moniliformis | Burr hole drainage, recurrent percutaneous punctures for external drainage | Benzathine penicillin 24 mill U x 1 m then 36 mill U + dexamethasone x 1 m | No recurrence at 1 year |
| [4] 2013 | 46yo/M | 3d HA and aphasia | Unknown | L lung mass, L fronto-parietal lobe brain abscess | Propionibacterium acnes, Fusobacterium nucleatum | Stereotactic brain biopsy | 4 mill U penicillin x 4 w, metronidazole x 4 w, amoxicillin x 11 m | Resolution of symptoms and significant reduction in mass size at 5m |
| [5] 2015 | 55yo/F | 2d HA, R hemisensory loss, unsteady gait | Dental extraction 7d prior | L parietal lobe brain abscess | Group B streptococcus, Staphilococcus capitis | Craniotomy and drainage | Vancomycin x 11 d, metronidazole IV x 1 m, Ceftriaxone x 4 m, amoxicillin x 6 m | No recurrence at 4m |
| [6] 2014 | 57yo/M | Hours AMS and new onset seizure | Dental procedure week prior | L parietal lobe abscess | Not reported | Stereotactic brain biopsy | Ceftriaxone and metronidazole IV x unknown duration, amoxicillin x 12 m | No recurrence at 1 year |
| [15] 1992 | 44yo/M | 1 m R-sided weakness and dysarthria | Unknown | L fronto-parietal lobe abscesses (x2), R occipital lobe abscess | Actinobacillus actinomycentemcomitans | Stereotactic brain biopsy and drainage | Amoxicillin 6 w, amoxicillin x 12 m | Clinical cure; follow-up period not specified |
| [19] 2016 | 55yo/M | Unknown | Pneumonitis | Brain abscess | Actinobacillus, mixed anaerobic flora | Brain biopsy | Ceftriaxone x 1 m, oral penicillin x 5 m | Lost to follow-up |
| [19] 2016 | 44yo/M | Unknown | Sinusitis | Bone and brain abscess | Microaerophilic streptococcus, Strep mitis | Brain biopsy and drainage | Ceftriaxone x 1 m, oral penicillin x 5 m | Lost to follow-up |
Abbreviations: CR = case report; yo = years-old; M = male; F = female; d = day(s); w = week(s); m = month(s); iv = intravenous; AMS = altered mental status; HA = headache; f/u = follow-up period.
Cerebral disease represents the most severe form of actinomycosis and usually presents as brain abscesses [1]. Patients who present with actinomycotic brain lesions often have concomitant lung disease. In some instances, this presentation has been misdiagnosed as lung cancer with brain metastasis [1]. In 1987, it was postulated that Actinomyces brain lesions had an average time of symptomatology of 2.1 months before presentation [9]. In our case, this presumption is challenged by the short duration of clinical symptoms and a normal head CT 1-week before hospital admission. Nevertheless, our patient likely had subtle changes of cerebritis not identifiable on CT scan.
The use of Multiple Parallel Sequencing (MPS) and 16S rRNA sequencing increasingly identify Actinomyces spp, and more specifically A. meyeri, as a significant pathogen in spontaneous brain abscesses. This was illustrated by the analysis of 37 samples from spontaneous intracerebral brain abscesses, with 16S rRNA gene analysis, MPS and traditional culture [2]. MPS detected 160 bacterial species, compared to 49 species identified by 16S rRNA analysis and only 4 species identified by culture. Out of the 37 spontaneous intracranial abscesses, MPS revealed the presence of Actinomyces spp in 14. Twelve of those samples contained A. meyeri and, like in our case, Parvimonas spp., Streptococcus intermedius and Fusobacterium nucleatum were also identified. The authors of the study hypothesized that spontaneous brain abscesses are the result of recurrent bacterial seeding, in which pioneer species able to survive in presence of oxygen arrive first (e.g., F. nucleatum), modifying the environment and making it suitable for subsequent colonization by strictly anaerobic bacteria [2], [4].
Because 16S rRNA sequencing is highly sensitive and specific at detecting bacterial genetic material, the role of each individual population in the formation of cerebral abscesses is unclear and many clinicians thus opt to treat them all. Actinomyces species are usually susceptible to vancomycin, penicillin, and other beta-lactam antibiotics but resistant to metronidazole. Susceptibility testing is possible but of uncertain utility due to poor correlation between in vivo and in vitro susceptibilities. Moreover, susceptibilities to antibiotics may vary between species, making clinical and radiographic follow-up a cornerstone of patient care [1], [10].
Conventional therapy for actinomyces infections involves intravenous penicillin, 18 to 24 million units daily for 2–6 weeks, followed by oral penicillin or amoxicillin for an extended period of 6 to 12 months. In cases of penicillin allergy, there is successful clinical experience described in treating actinomycosis with erythromycin, tetracycline, doxycycline, minocycline, and clindamycin, but CNS penetration of these antimicrobials varies widely [7]. Surgical debridement is critical in the treatment of actinomyces brain abscesses, as it increases the oxygen tension in the affected tissue and favors antibiotic penetration [10]. Anecdotal data suggests successful treatment of extensive actinomycosis with antibiotic therapy alone, mostly for pelvic disease [11], [12], [13], [14]. We found two case reports of patients with A. meyeri brain abscesses who required surgical drainage after failing antibiotic therapy alone [6], [15] and an exceptional case of a 2 cm Actinomyces odontolyticus brain abscess successfully treated with 6 months of penicillin alone [12].
A prolonged antibiotic course is supported only by data from the mid-twentieth century, when patients commonly presented with advanced disease, were subject to interrupted and suboptimal antibiotic therapy, and imaging modalities were limited [16]. Case series from the early antibiotic era reveal inconsistencies between penicillin dosage and treatment duration. Clinical recurrence was often treated with short antibiotic courses and associated with further relapses [16]. We found four articles describing patients with CNS actinomycosis who received short-course antibiotics following surgical drainage. One case reported successful treatment of A. meyeri brain abscess with one month of continuous benzathine penicillin infusion accompanied by dexamethasone; the patient remained disease-free at one year follow-up [17]. An additional three cases of Actinomyces israelii brain abscesses were successfully treated with 3 to 4 weeks of benzathine penicillin 2 million units daily; there was no reported recurrence during the follow-up period range of 6 to 36 months [10]. More recently, there were four reported cases of intracranial actinomycosis treated with intravenous antibiotics for 2 months, followed by another 2 months of oral erythromycin or ciprofloxacin; however, clinical outcomes were not reported [18]. Lastly, a prospective multisite study reported a median duration of antibiotic treatment of 9 weeks (range 6 to 16 weeks) for 14 patients with actinomycotic brain abscesses, with no recurrence of disease at one year follow-up [2]. More data are needed on the utility of prolonged courses of antibiotic therapy for cerebral actinomycosis to guide management.
In summary, we present a case of a 29-year-old man with polymicrobial and A. meyeri brain abscess that was likely odontogenic and without evidence of concomitant lung disease or endocarditis. Before the development of culture-independent identification techniques, Actinomyces spp. were rarely found in cerebral abscesses and were considered an unusual infection requiring a prolonged empirical course of therapy. Newer diagnostic techniques, including 16S rRNA sequencing, has revealed Actinomyces spp. as more frequently involved in spontaneous brain abscesses than previously recognized. More data are thus needed on optimal antibiotic management for A. meyeri and other Actinomyces brain abscesses, though shorter courses have anecdotally been associated with clinical recurrence of disease. In an era where physicians can provide close outpatient follow-up, duration of therapy should be tailored depending on the severity of disease, completion of debridement, clinical course, and radiographic re-imaging. Based on common source of infection (such as odontogenic), broad antibiotic coverage is often needed for polymicrobial infection.
Conflict of interest
The authors declare that they have no conflict of interest
Funding
Financial support for publication subsidized by the Office of Graduate Medical Education of Yale New Haven Health-Bridgeport Hospital. Research career development support provided by the National Institute on Drug Abuse (K23 DA033858 to JPM.) Funding source played no role in data collection, analysis, drafting of the manuscript, or decision to submit the manuscript for publication.
Author contributions
All authors participated in the clinical care of the patient described in this case report. Dr. Vazquez Guillamet conducted the literature review and drafted the primary version of the manuscript, with subsequent revisions and recommendations by Drs. Malinis and Meyer. All authors have read and approved the final version of the manuscript for submission.
Contributor Information
Laia Jimena Vazquez Guillamet, Email: LaiaJimena.VazquezGuillamet@bpthosp.org.
Maricar F. Malinis, Email: maricar.malinis@yale.edu.
Jaimie P. Meyer, Email: jaimie.meyer@yale.edu.
References
- 1.Kononen E., Wade W.G. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419–442. doi: 10.1128/CMR.00100-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kommedal O. Massive parallel sequencing provides new perspectives on bacterial brain abscesses. J Clin Microbiol. 2014;52(6):1990–1997. doi: 10.1128/JCM.00346-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houghton G.W. Comparison of the pharmacokinetics of metronidazole in healthy female volunteers following either a single oral or intravenous dose. Br J Clin Pharmacol. 1979;8(4):337–341. doi: 10.1111/j.1365-2125.1979.tb04715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park H.J. A case of disseminated infection due to actinomyces meyeri involving lung and brain. Infect Chemother. 2014;46(4):269–273. doi: 10.3947/ic.2014.46.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy U. Actinomyces meyeri brain abscess following dental extraction. BMJ Case Rep. 2015:2015. doi: 10.1136/bcr-2014-207548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Valle T. Actinomyces meyeri brain abscess. Med Clin (Barc) 2014;143(7):331–332. doi: 10.1016/j.medcli.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Mandell G.L., Bennett J.E., Dolin R. 7th ed. Churchill Livingstone/Elsevier; Philadelphia: 2010. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. xl, 4028 p. [Google Scholar]
- 8.Apotheloz C., Regamey C. Disseminated infection due to Actinomyces meyeri: case report and review. Clin Infect Dis. 1996;22(4):621–625. doi: 10.1093/clinids/22.4.621. [DOI] [PubMed] [Google Scholar]
- 9.Smego R.A., Jr. Actinomycosis of the central nervous system. Rev Infect Dis. 1987;9(5):855–865. doi: 10.1093/clinids/9.5.855. [DOI] [PubMed] [Google Scholar]
- 10.Jamjoom A.B., Jamjoom Z.A., al-Hedaithy S.S. Actinomycotic brain abscess successfully treated by burr hole aspiration and short course antimicrobial therapy. Br J Neurosurg. 1994;8(5):545–550. doi: 10.3109/02688699409002946. [DOI] [PubMed] [Google Scholar]
- 11.Fu P.K., Tsai C.A. Management of patients with huge pelvic actinomycosis complicated with hydronephrosis: a case report. J Microbiol Immunol Infect. 2010;43(5):442–446. doi: 10.1016/S1684-1182(10)60068-0. [DOI] [PubMed] [Google Scholar]
- 12.Pauker S.G., Kopelman R.I. Clinical problem-solving. A rewarding pursuit of certainty. N Engl J Med. 1993;329(15):1103–1107. doi: 10.1056/NEJM199310073291508. [DOI] [PubMed] [Google Scholar]
- 13.Nozawa H. Pelvic actinomycosis presenting with a large abscess and bowel stenosis with marked response to conservative treatment: a case report. J Med Case Rep. 2007;1:141. doi: 10.1186/1752-1947-1-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P.T. Huge pelvic mass, cutaneous and vaginal fistulas, and bilateral hydronephrosis: a rare presentation of actinomycosis with a good response to conservative treatment and with long-term sequelae of renal atrophy and hydronephrosis. Taiwan J Obstet Gynecol. 2008;47(2):206–211. doi: 10.1016/S1028-4559(08)60082-0. [DOI] [PubMed] [Google Scholar]
- 15.Kuijper E.J. Disseminated actinomycosis due to Actinomyces meyeri and Actinobacillus actinomycetemcomitans. Scand J Infect Dis. 1992;24(5):667–672. doi: 10.3109/00365549209054655. [DOI] [PubMed] [Google Scholar]
- 16.Sudhakar S.S., Ross J.J. Short-term treatment of actinomycosis: two cases and a review. Clin Infect Dis. 2004;38(3):444–447. doi: 10.1086/381099. [DOI] [PubMed] [Google Scholar]
- 17.Dijkmans B.A. Brain abscess due to Streptobacillus moniliformis and Actinobacterium meyerii. Infection. 1984;12(4):262–264. doi: 10.1007/BF01645956. [DOI] [PubMed] [Google Scholar]
- 18.Akhaddar A. Focal intracranial infections due to Actinomyces species in immunocompetent patients: diagnostic and therapeutic challenges. World Neurosurg. 2010;74(2-3):346–350. doi: 10.1016/j.wneu.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Rolfe R. Actinomyces meyeri: a common agent of actinomycosis. Am J Med Sci. 2016;352(1):53–62. doi: 10.1016/j.amjms.2016.03.003. [DOI] [PubMed] [Google Scholar]