Abstract
Objectives
Mutations in Cbl or Cbl-b gene occur in 10% of MPD patients and are associated with poor prognosis. Hematopoietic Cbl/Cbl-b double knockout (DKO) leads to a disease in mice phenotypically similar to human MPDs. The aim of this study was to evaluate the anti-MPD activity of a clinical safe drug, Fasudil identified in an in vitro kinase inhibitor as an inhibitor of proliferation of DKO mouse hematopoietic stem/progenitor cells (HSPCs).
Methods
Fasudil exhibited relatively selective anti-proliferative activity against Cbl/Cbl-b DKO vs. control murine bone marrow HSPCs. We established a mouse model with uniform time of MPD onset by transplanting Cbl/Cbl-b DKO HSPCs into busulfan-conditioned NOD/SCID/gamma chain-deficient mice. Four weeks post-transplant, mice were treated with 100 mg/kg fasudil (13 mice) or water (control, 8 mice) daily by oral gavage, followed by blood cell count every two weeks.
Results
By two weeks of treatment, total white cell and monocyte counts were significantly lower in mice treated with fasudil. We observed a trend towards improved survival in fasudil-treated mice that didn’t reach statistical significance. Notably, prolonged survival beyond 27 weeks was observed in 2 fasudil-treated mice, nearly twice the 16-week average life-span in the Cbl/Cbl-b DKO MPD model.
Conclusions
Our results suggest a therapeutic potential for fasudil, a clinically-safe drug with promising results in vascular diseases, in the treatment of MPDs or other mutant Cbl-driven myeloid disorders.
Keywords: Myeloproliferative diseases, Fausdil, Rho kinase (ROCK), Cbl, Myosin light chain (MLC), Mouse model
Introduction
Myeloproliferative neoplasms/disorders (MPNs/MPDs) are clonal hematopoietic stem cell disorders characterized by over-proliferation of one or more myeloid cell lineages in the bone marrow and increased numbers of mature and immature myeloid cells in the peripheral blood. Excess proliferation is frequently associated with splenomegaly and cardiovascular complications as well as increased risk of transformation to acute leukemia. MPNs/MPDs are heterogeneous disorders with variable clinical courses1. Patients with longstanding disease frequently develop myelofibrosis with extra-medullary hematopoiesis, and therapeutic options are extremely limited in that situation. Allogeneic hematopoietic stem cell transplantation is currently the only curative option for advanced MPDs, however many patients are ineligible for transplantation because of advanced age, medical co-morbidities, and multiple organ dysfunctions secondary to systemic fibrosis2.
Recently, mutations in the Cbl gene family member Cbl, and less commonly Cbl-b, were reported by multiple independent investigators to be present in about 10% of patients with MPNs/MPDs, and these patients tend of have poorer prognosis3, 4. Cbl and Cbl-b are highly-related E3 ubiquitin ligases that function in hematopoietic cells to negatively regulate signaling of tyrosine kinase-coupled cell surface receptors activated by growth factors, antigenic signals, cytokines and other stimuli5. Mutations in the Cbl gene lead to loss of negative regulatory control mediated by Cbl proteins, uncontrolled signaling from surface growth factor/cytokine receptors, and uncontrolled cellular proliferation that may evolve into malignancy6.
We have previously demonstrated that the Cbl/Cbl-b double knockout (DKO) mice, in which a floxed Cbl gene is deleted by MMTV Cre and Cbl-b gene is constitutively deleted, develop a hematological disease that is phenotypically similar to human MPNs/MPDs7. We undertook an in vitro tyrosine kinase screen that demonstrated a the differential anti-proliferative activity of fasudil on bone marrow cells isolated from Cbl/Cbl-b double knockout mice compared to control bone marrow cells from mice from same genetic background (data not shown). Fasudil is already clinically approved for use in Japan for the prevention of vasospasm that follows subarachnoid hemorrhage8, 9, and has shown clinically-beneficial activity in patients with pulmonary hypertension10 and clinical trials are on ongoing to confirm such observations11. If fasudil activity can be shown in a disease model for MPNs/MPDs, it would be a highly attractive agent to be taken to early phase clinical trials in patients with MPNs/MPDs given the fact that fasudil is a clinically approved drug and has a demonstrated safety profile in hundreds of patients12.
Materials and Methods
Reagents and antibodies
Fasudil was purchased from LC Laboratories (Woburn, MA). Busulfan, Cremaphor were purchased from Sigma-Aldrich (St. Louis, MO). Flourochrome-labeled monoclonal antibodies to CD45.1 and CD45.2 were purchased from BD Bioscience (San Jose, CA). The murine recombinant cytokines stem cell factor (SCF), thromobopoieitn (TPO) and FMS-like tyrosine kinase 3 ligand (FLT3L) were obtained from PeproTech (Rocky Hill, NJ). X-VIVO medium was from Lonza (Basel, Swiss). Antibodies to myosin light chain 2 (MLC) were purchased from Cell Signaling Technology (Danvers, MA).
In vitro bone marrow cell proliferation assay
Cell proliferation was assayed using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Promega, Madison, WI) according to the manufacturer’s protocol. In brief, lineage negative cells were purified from the whole bone marrow (BM) cells using lineage-depletion kit (Miltenyi Biotec, San Diego, CA) to remove mature cell populations, thus yielding enriched hematopoietic stem and progenitor cell fraction. 3 × 103 purified cells were plated per well in 96-well plates and cultured in the presence of 50ng/ml SCF, 10ng/ml TPO and 10ng/ml FLT3L for 3 days. Luminescent signal after adding the Cell Titer-Glo reagent was read using LUMIstar OPTIMA (BMG LABTECH, Cary, NC).
Animals
Cblflox/flox; Cblb−/− and MMTV-Cre transgenic mice were backcrossed to C57BL/6 for at least five generations before being intercrossed to generate the MMTV-Cre; Cblflox/flox; Cblb−/− mice as described previously7. For ease, these mice will be referred to as the Cbl/Cbl-b DKO mice in the remainder of the text. NOD/SCID/gamma chain-deficient (NSG) mice (6–8 weeks old) used for bone marrow (BM) transplantation-based MPD model were purchased from the Jackson Laboratory (Bar Harbor, ME). All animals were housed under specific pathogen-free conditions in the animal care facility at the Center for Comparative Medicine of the University of Nebraska Medical Center (UNMC). All mouse experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the UNMC.
Bone Marrow Transplantation
The BM cell donor Cbl/Cbl-b DKO mice were sacrificed at 2 months after birth, when they had started to show signs of MPD. BM was collected from the femurs and leg bones, and marrow cells were suspended in phosphate-buffered saline (PBS). Busulfan was dissolved in DMSO at 100 mg/mL and mixed with nine volumes of 50% Cremophor formulation (10% Cremophor EL, 15% propylene glycol, 25% ethanol, 50% PBS). Immediately before injection, the above solution was diluted with 5% glucose to adjust the final concentration of busulfan to 4 mg/mL. Recipient NSG mice were conditioned by intraperitoneal injection of two doses of busulfan at 20 mg/kg body weight twnety-four hours apart, as described13. Twenty-four hours after the second busulfan injection, 4 × 106 total BM cells isolated from Cbl/Cbl-b DKO donors were injected intravenously in recipient mice. Peripheral blood was obtained by submandibular vein bleeding [14] every 2 weeks, starting at 4 weeks after transplant, and total white blood cell, granulocyte and monocyte counts were performed on a Scil Vet abc Animal Blood Counter (Scil Animal Care, Gurnee, IL). Mice were euthanized when they exhibited severe distress from disease.
Flow cytometry
All transplant recipient NSG mice were monitored by peripheral blood analysis every two weeks after transplantation. Peripheral blood was obtained by submandibular vein bleeding14. Red blood cells were lysed by ACK lysis buffer (Quality Biological, Gaithersburg, MD) and mononuclear cells were labeled with antibodies against CD45.1 (recipient cells) and CD45.2 (donor cells). Flow cytometry was performed on a BD LSRII or Aria II at the UNMC Flow Cytometry Core Facility. Data were analyzed using the FlowJo software (Tree Star, Ashland, OR).
Fasudil treatment
Starting four weeks after transplantation, 13 mice received 100 mg/kg fasudil dissolved in water by gavage once daily (treatment group) and 8 mice received the same volume of water (control group). Treatments were continued until the end of the experiment. Total white blood cell, granulocyte and monocyte counts were performed as above, every 2 weeks, until the mice were euthanized due to severe distress from disease. The onset and persistence of disease over time, and survival times were recorded.
Western blotting
Peripheral blood samples were obtained prior to euthanasia of mice. Protein lysates were prepared by lysing cells in a RIPA buffer and western blot was carried out as described previously15.
Statistical analysis
The statistical significance of differences in total white blood cell (WBC), granulocyte and monocyte counts between control and fasudil-treated groups were determined using Student’s T test. Log-rank (Mantel-Cox) test was used to compare survival between treated and control groups. GraphPad Prism was used for statistical analysis. P values <0.05 were considered statistically significant.
Results
Fasudil selectively inhibit Cbl/Cbl-b DKO cell expansion in vitro
To validate the potential anti-proliferative activity of fasudil observed in a larger screen using a kinase inhibitor library (data not shown), we first performed a proliferation assay, in which lineage-negative (Lin−) BM cells representing hematopoietic stem and progenitor cell populations were isolated from the wild-type (WT) control or the Cbl/Cbl-b DKO mice and cultured in vitro in the presence of cytokines for three days with water alone or with various concentrations of fasudil (Figure-1). Fasudil, at 0.01 to 1 uM concentrations induced a dose-dependent inhibition of proliferation of both the control and the DKO BM cells. However, the inhibitory effect was more pronounced on the Cbl/Cbl-b DKO cells, especially at 0.1 and 1 uM concentrations, with the differences at 1 uM being statistically significant. These results not only confirmed the anti-proliferative activity of fasudil against hematopoietic cells but also suggested the relative selectivity of fasudil against Cbl/Cbl-b DKO cells compared to control BM cells.
Figure-1. Fasudil inhibited Cbl/Cbl-b DKO Lin- bone marrow (BM) cell proliferation in vitro.
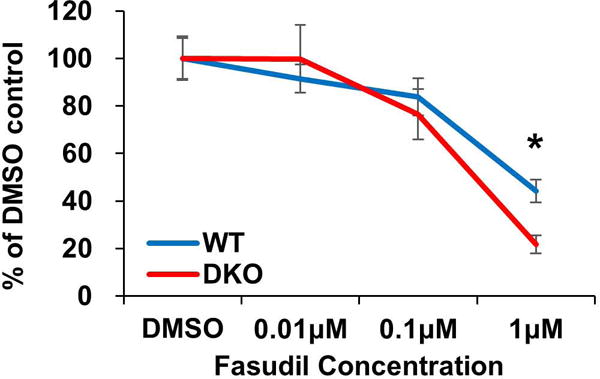
BM cells were collected from WT control or Cbl/Cbl-b DKO mice and Lin- cells were purified using magnetic beads and 3 × 103 lin- cells were plated in each well in 96-well plate and cultured in X-VIVO medium with SCF 50 ng/ml, TPO 10 ng/ml, FLT3L 10 ng/ml. Cell proliferation was assayed using CellTiter-Glo® Luminescent Cell Viability Assay. Data shown is one representative experiment of three independent repeats with similar results. Data is shown as mean ± SD (* p<0.05).
All recipient NSG mice developed MPD
Because leukocytes derived from the recipient NSG and Cbl/Cbl-b DKO donor mice express CD45.1 and CD45.2 allotypes, respectively16, cell origins could be readily distinguished by cell-surface staining for CD45 allotypes followed by flow cytometry. Analysis of the transplanted NSG recipient mice demonstrated that only those receiving the Cbl/Cbl-b DKO BM donor cells, but not those receiving the control BM donor cells, exhibited severe MPD, as demonstrated by the leukocytosis, hepatosplenomegaly and rapid lethality (Figure-2 A–D). Using this transplantation system, we next established a cohort of recipients NSG mice that were transplanted with the DKO BM cells. All recipient NSG mice survived transplantation and demonstrated engraftment with donor cells, with a mean donor chimerism of 88% (Figure-3A) All mice developed signs of MPD, based on elevated total WBC, granulocyte (GRA), and monocyte (MON) counts as early as 4 weeks after transplantation (Figure-3B–D).
Figure-2. DKO donor cell induced MPD in NSG recipient mice.
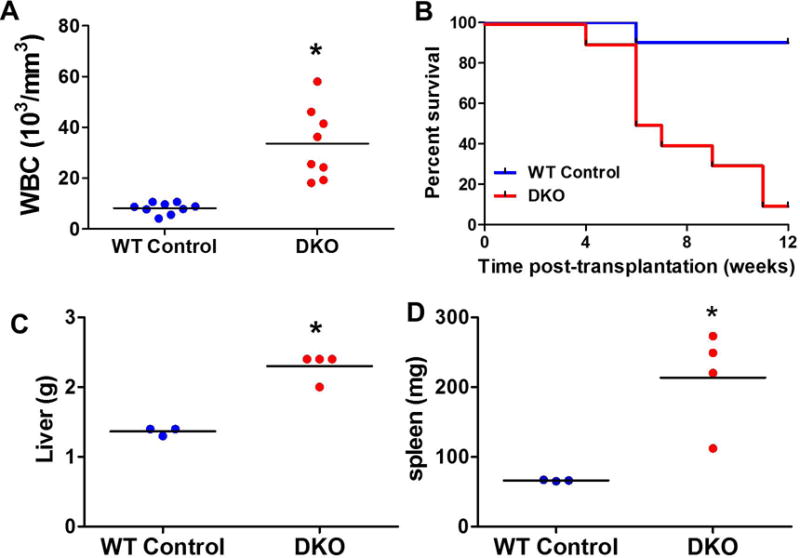
(A–B) 4 × 106 whole BM cells from Control or DKO mice were transplanted into pre-conditioned NSG recipients. 6 weeks after transplantation, whole blood cell count was performed (A) and overall survival was recorded (B). (C–D) 2 × 106 whole BM cells from Control or DKO mice were transplanted into pre-conditioned NSG recipients. Recipients were sacrificed at 4 weeks after transplantation and liver (C) and spleen (D) was weighted to evaluate the hepatosplenomegaly. Each dot represents one individual recipients (*:p<0.05).
Figure-3. Fasudil decreased MPD disease burden.
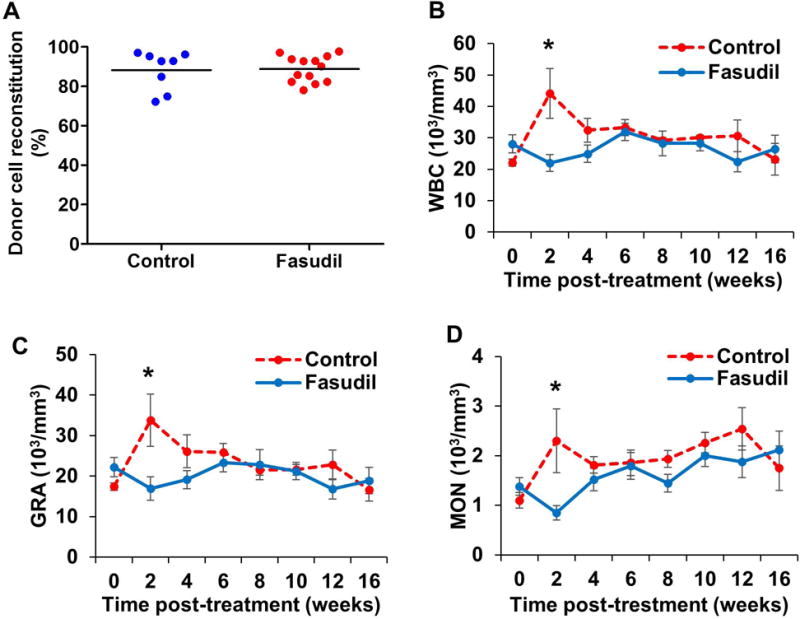
Four weeks after bone marrow transplantation (week 0), engraftment of donor cells was analyzed by flow cytometry (A). Recipient mice were then treated with water (control) or fasudil. Peripheral blood were collected and analyzed every two weeks for total white blood cell (WBC) (B), granulocyte (GRA) (C), and monocyte (MON) (D) counts. Data is shown as mean ± SD (* p<0.05).
Fasudil decreases MPD disease burden in mice
After 2 weeks of treatment with fasudil, total WBC, GRA, and MON counts were significantly lower (Figure-3) in mice treated with fasudil (p=0.004, 0.012, and 0.009, respectively). For the entire fasudil-treated mouse group (Figure-4), we observed a trend towards improved survival compared to that in the control group, although this did not reach statistical significance (p=0.07). However, an analysis of the male recipients only (n=6 for control and n=7 for fasudil treatment) revealed a significant survival advantage in the fasudil-treated group compared to the control group (p=0.04).
Figure-4. Survival of Cbl/Cbl-b DKO mice treated with fasudil (n=13) compared to controls (n=8).
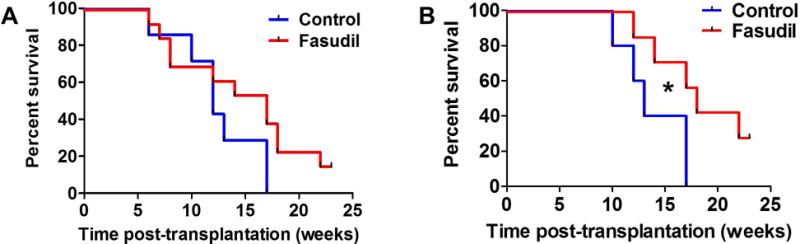
(A) All mice (no significant difference seen; p=0.07), (B) male mice (n=12) showing a statistically significant difference in survival with fasudil treatment (p=0.04).
Fasudil treatment imparted prolonged survival in a subset of mice with Cbl/Cbl-b DKO BM transplant-induced MPD
While all untreated mice succumbed to MPD between 12 and 16 weeks after transplant, survival beyond 27 weeks was observed in 2 fasudil-treated mice, which is nearly twice the mean life-span of the untreated mice with the Cbl/Cbl-b DKO BM transplant-induced MPD (16 weeks). The 2 long-term survivors had undetectable levels of myosin light chain (MLC), a downstream target of ROCK phosphorylation (Figure-5).
Figure-5. Fasudil reduced myosin light chain (MLC) level in recipient mice.
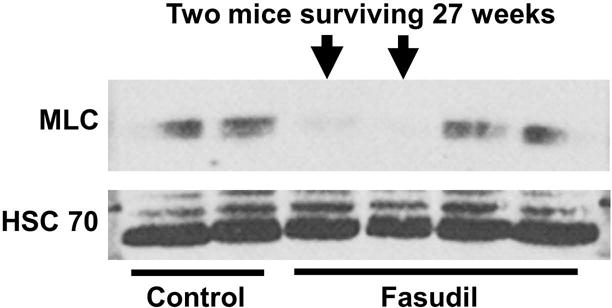
Whole bone marrow cells were collected from recipient mice and analyzed for the expression MLC; a downstream target of fasudil. Undetectable MLC, by Western blot, in the 2 mice that had extended survival (beyond 27 weeks).
Discussion
In this study, we assessed the potential therapeutic activity of fasudil, a Rho kinase inhibitor, in a model of MPD arising from the transplant of Cbl/Cbl-b DKO BM hematopoietic stem and progenitor cells into NSG recipient mice. This effort was based on an in vitro screen that identified fasudil with preferential a relatively selective anti-proliferative activity against the highly proliferative Cbl/Cbl-b DKO mouse BM cells compared to the control cells. Given its safety profile in clinical use in hundreds of patients with vascular conditions, our results support a potential therapeutic role of fasudil in MPDs.
Rho kinases (ROCKs), which are targeted by fasudil, are involved in regulating a myriad of physiologic functions including cytoskeletal reorganization, cell migration, adhesion, survival and proliferation. They do so via activating several different downstream pathways such as myosin light chain (MLC) phosphatase. To date, most of the conclusions with regard to the function of ROCKs have involved the use of cell lines, pharmacologic inhibitors and dominant negative approaches. Importantly, the role of ROCK in hematopoiesis, myeloid biology, or leukemogenesis in vivo remains poorly understood. Mali et al.17 demonstrated constitutive activation of ROCK in leukemic cells expressing activating forms of KIT, Flt-3 and breakpoint cluster region–Abelson kinase (BCR-ABL), which are oncogenes commonly found in patients with systemic mastocytosis, acute myeloid leukemia (AML) and chronic myeloid leukemia, respectively. Mali et al. showed that pharmacologic inhibition of ROCK in leukemic cells inhibited the growth and survival of leukemic cells in vitro and, importantly, prolonged survival of leukemic mice17.
Our results suggest a promising role of fasudil in decreasing the morbidity of MPD through reduction in WBC, granulocyte and monocyte counts. There was a trend towards survival advantage as well, which didn’t reach statistical significance in the overall cohort analyzed. Given the fact that fasudil significantly impaired the Cbl/Cbl-b DKO BM cell expansion in vitro (Figure-1), the lack of a statistically significant difference between the groups could be explained by: (1) the small sample size, or (2) superior metabolism of fasudil in rodents with reduced systemic exposure with single daily dosing, as has been shown8, 18–20. The fasudil effect however was statistically significant when the males alone were compared. The differential effect of sex on the survival of transplanted NSG mice is of interest. We observed a more aggressive disease phenotype in female mice in our Cbl/Cbl-b DKO murine model, which likely explains the poorer survival and lesser benefit of fasudil treatment in this subset (Figure-5). Sánchez-Aguilera et al.21 have recently shown that estrogen receptor (ER) differentially regulates the survival and proliferation of hematopoietic stem/progenitor cells (HSPCs) and that tamoxifen treatment blocked the development of JAK2V617F-induced myeloproliferative neoplasm in vivo, induced the apoptosis of human JAK2V617F+ HSPCs in a xenograft model, and sensitized MLL-AF9+ leukemias to chemotherapy. Apoptosis was selectively observed in mutant cells, and tamoxifen treatment only had a minor impact on steady-state hematopoiesis in disease-free animals21. JAK2V617F is the most common genetic abnormality reported in MPDs22, 23 and is associated with constitutive activation of downstream STAT5 signaling and heightened sensitivity to erythropoietin and other hematopoietic growth factors leading to myeloid lineage expansion and subsequently MPD23. A differential effect of ER signaling akin to that demonstrated on JAK2V617F–driven clonal hematopoiesis may explain the more aggressive disease phenotype we observed in female recipient mice in our Cbl/Cbl-b DKO murine model.
Lastly, the association between the prolonged survival observed in 2 mice treated with fasudil (>27 weeks), which is almost double the mean survival time in our Cbl/Cbl-b DKO model, and the total depletion of MLC in the peripheral blood of these survivors (Figure-5) is intriguing. Mali et al. found MLC to be constitutively hyperphosphorylated on Ser19 in leukemic cells, whose activation could be rapidly inhibited upon treating the leukemic cells with ROCK inhibitors, suggesting that the antileukemic activity of ROCK inhibition is mediated through inhibition of the downstream MLC signaling17. Inhibition of MLC leads to the destabilization of actin filaments and subsequently cell death24. It remains unclear why such a dramatic benefit was seen in these 2 mice compared to the others, despite an identical genetic background.
Our data suggests that fausdil has the potential to ameliorate the disease burden in MPD and, given its demonstrated safety in large cohorts of patients with subarachnoid hemorrhage, testing its safety and efficacy in early phase clinical trials in patients with MPD in warranted. There is an oral formulation of fasudil that is currently in clinical trials in patients with primary pulmonary hypertension (PH). PH is an under-recognized complication of long-standing MPD (in the “spent phase”) and leads to significant morbidity. Ruxolitinib, a JAK2 inhibitor currently in clinical use to ameliorate systemic symptoms of MPD, was shown to improve echocardiographic findings in 66% of patients with MPD-associated PH in a small trial of 12 patients. That improvement was associated with reduction in nitric oxide (NO) plasma levels25. Given the lack of overlapping toxicities between fasudil and JAK2 inhibitors, a clinical trial combining both of these agents in patients with MPDs would be of considerable interest given the potential for their additive, and possibly synergistic, effects.
Acknowledgments
We thank the Band Laboratory members for discussion, the UNMC Flow Cytometry, Histology and Comparative Medicine Core staff for assistance and Dr. Kay-Uwe Wagner for the use of cell analyzer. This work was supported by: the NIH grants CA87986, CA105489, CA99163 and CA116552 to HB and CA96844 and CA144027 to VB; the Department of Defense grants W81XWH-07-1-0351 and W81XWH-11-1-0171 (VB); and the NCI CCSG to the Buffett Cancer Center. WA and BM received the UNMC graduate assistantships.
Footnotes
Disclosure of Conflict of Interest:
All authors disclose no conflict of interest.
References
- 1.Spivak JL. The chronic myeloproliferative disorders: clonality and clinical heterogeneity. Semin Hematol. 2004;41(2 Suppl 3):1–5. doi: 10.1053/j.seminhematol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: when, which agent, and how? Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2014;2014(1):277–86. doi: 10.1182/asheducation-2014.1.277. [DOI] [PubMed] [Google Scholar]
- 3.Grand FH, Hidalgo-Curtis CE, Ernst T, Zoi K, Zoi C, McGuire C, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009;113(24):6182–92. doi: 10.1182/blood-2008-12-194548. [DOI] [PubMed] [Google Scholar]
- 4.Makishima H, Cazzolli H, Szpurka H, Dunbar A, Tiu R, Huh J, et al. Mutations of e3 ubiquitin ligase cbl family members constitute a novel common pathogenic lesion in myeloid malignancies. J Clin Oncol. 2009;27(36):6109–16. doi: 10.1200/JCO.2009.23.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohapatra B, Ahmad G, Nadeau S, Zutshi N, An W, Scheffe S, et al. Protein tyrosine kinase regulation by ubiquitination: critical roles of Cbl-family ubiquitin ligases. Biochimica et biophysica acta. 2013;1833(1):122–39. doi: 10.1016/j.bbamcr.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kales SC, Ryan PE, Nau MM, Lipkowitz S. Cbl and human myeloid neoplasms: the Cbl oncogene comes of age. Cancer Res. 70(12):4789–94. doi: 10.1158/0008-5472.CAN-10-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naramura M, Nandwani N, Gu H, Band V, Band H. Rapidly fatal myeloproliferative disorders in mice with deletion of Casitas B-cell lymphoma (Cbl) and Cbl-b in hematopoietic stem cells. Proc Natl Acad Sci U S A. 107(37):16274–9. doi: 10.1073/pnas.1007575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu GJ, Wang ZJ, Wang YF, Xu LL, Wang XL, Liu Y, et al. Systematic assessment and meta-analysis of the efficacy and safety of fasudil in the treatment of cerebral vasospasm in patients with subarachnoid hemorrhage. Eur J Clin Pharmacol. 68(2):131–9. doi: 10.1007/s00228-011-1100-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Zhou D, Guo J, Ren Z, Zhou L, Wang S, et al. Efficacy and safety of fasudil in patients with subarachnoid hemorrhage: final results of a randomized trial of fasudil versus nimodipine. Neurol Med Chir (Tokyo) 51(10):679–83. doi: 10.2176/nmc.51.679. [DOI] [PubMed] [Google Scholar]
- 10.Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91(3):391–2. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raja SG. Evaluation of clinical efficacy of fasudil for the treatment of pulmonary arterial hypertension. Recent patents on cardiovascular drug discovery. 2012;7(2):100–4. doi: 10.2174/157489012801227238. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki Y, Shibuya M, Satoh S, Sugimoto Y, Takakura K. A postmarketing surveillance study of fasudil treatment after aneurysmal subarachnoid hemorrhage. Surg Neurol. 2007;68(2):126–31. doi: 10.1016/j.surneu.2006.10.037. discussion 31–2. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa J, Hsieh MM, Uchida N, Phang O, Tisdale JF. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rgamma(null) mice. Stem cells. 2009;27(1):175–82. doi: 10.1634/stemcells.2008-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab animal. 2005;34(9):39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 15.Burnette WN. Western blotting. Clinical chemistry. 2011;57(1):132–3. doi: 10.1373/clinchem.2010.149765. [DOI] [PubMed] [Google Scholar]
- 16.An W, Nadeau SA, Mohapatra BC, Feng D, Zutshi N, Storck MD, et al. Loss of Cbl and Cbl-b ubiquitin ligases abrogates hematopoietic stem cell quiescence and sensitizes leukemic disease to chemotherapy. Oncotarget. 2015 doi: 10.18632/oncotarget.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mali RS, Ramdas B, Ma P, Shi J, Munugalavadla V, Sims E, et al. Rho kinase regulates the survival and transformation of cells bearing oncogenic forms of KIT, FLT3, and BCR-ABL. Cancer cell. 2011;20(3):357–69. doi: 10.1016/j.ccr.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowerman M, Murray LM, Boyer JG, Anderson CL, Kothary R. Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy. BMC Med. 10:24. doi: 10.1186/1741-7015-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald DA, Shi C, Shenkar R, Stockton RA, Liu F, Ginsberg MH, et al. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke. 43(2):571–4. doi: 10.1161/STROKEAHA.111.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying H, Biroc SL, Li WW, Alicke B, Xuan JA, Pagila R, et al. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol Cancer Ther. 2006;5(9):2158–64. doi: 10.1158/1535-7163.MCT-05-0440. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Aguilera A, Arranz L, Martin-Perez D, Garcia-Garcia A, Stavropoulou V, Kubovcakova L, et al. Estrogen signaling selectively induces apoptosis of hematopoietic progenitors and myeloid neoplasms without harming steady-state hematopoiesis. Cell stem cell. 2014;15(6):791–804. doi: 10.1016/j.stem.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106(6):2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 23.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England journal of medicine. 2005;352(17):1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 24.Street CA, Bryan BA. Rho kinase proteins–pleiotropic modulators of cell survival and apoptosis. Anticancer research. 2011;31(11):3645–57. [PMC free article] [PubMed] [Google Scholar]
- 25.Tabarroki A, Lindner DJ, Visconte V, Zhang L, Rogers HJ, Parker Y, et al. Ruxolitinib leads to improvement of pulmonary hypertension in patients with myelofibrosis. Leukemia. 2014;28(7):1486–93. doi: 10.1038/leu.2014.5. [DOI] [PubMed] [Google Scholar]


