Abstract
In the present study, virus-like particles (VLPs) were evaluated as a candidate poultry vaccine against avian influenza virus (AIV) subtype H9N2. Specific pathogen-free chickens received a single injection of the VLP vaccine expressing HA and M1 protein of AIV H9N2 (H9 HA VLP) at escalating doses in the presence or absence of ISA70 water-in-oil adjuvant. At 3 weeks post vaccination, we performed hemagglutination inhibition (HI) test and enzyme-linked immunosorbent assay (ELISA) to determine serological immune responses, and challenge studies using SPF chickens. A single dose of H9 HA VLP vaccine induced high levels of HI antibodies and lowered frequencies of virus isolation after the wild-type virus challenge. The addition of ISA70 adjuvant significantly increased the immunogenicity of H9 HA VLP vaccines. Furthermore, it allows differentiation of AIV-infected chickens from vaccinated chickens with an ELISA using nucleocapsid antigen, which offers a promising strategy to differentiate infected from vaccinated animals (DIVA). These results provide support for continued development of the VLP as an animal vaccine against influenza virus.
Keywords: Virus-like particle, Avian influenza, Vaccine, DIVA
1. Introduction
Low pathogenic avian influenza (LPAI) H9N2 viruses have been circulating in multiple avian species in Eurasia resulting in great economic losses due to declined egg production or moderate to high mortality [1–6]. Furthermore, the H9N2 LPAI virus is known to possess human-like receptor specificity [7] and transmissibility to mammalian species including humans, raising public concerns about increasing pandemic potential [8,9].
H9N2 LPAI viruses have been endemic in domestic poultry farms in Korea since 1996 and have often cause slight to moderate mortality (5–30%) with apparent clinical signs that is characterized by depression, edema of head, cyanosis of comb and legs, drop in egg production [10]. Since then, the H9N2 viruses that belong to the Korea group have been prevalent in chickens and have continuously evolved through reassortment with isolates circulating in the wild bird and live bird market [5,11,12].
In order to control the LPAI outbreaks, since 2007, the Korean veterinary authority has permitted the use of inactivated oil-adjuvant H9N2 LPAI vaccine [13,14], but only a few inactivated commercial vaccines have been assessed in chicken. In addition, one limitation of the inactivated vaccines is that vaccinated birds cannot be differentiated serologically from naturally infected birds using the commonly available diagnostic tests. Therefore, several different strategies to differentiate infected from vaccinated animals (DIVA) have been proposed for avian influenza to overcome this limitation [15,16].
Influenza virus-like particles (VLPs) have been suggested and developed as a new generation of non-egg-based cell culture-derived vaccine candidates against influenza infection [17]. VLP vaccines containing influenza hemagglutinin (HA) and neuraminidase (NA) antigens are produced easily in insect or mammalian cells via the simultaneous expression of HA and NA along with a viral core protein, such as influenza matrix (M1) [18]. The protective mechanism of influenza VLP vaccine is known to be similar to that of the commercial influenza vaccines inducing neutralizing antibodies and hemagglutination inhibition (HI) activities [17]. Furthermore, VLP vaccine has strong immunogenicity and protective efficacy in pandemic and highly pathogenic influenza challenge models [19–22]. In previous studies, H5N3 VLP vaccine was applied to duck species and provided the possibility of reliable use of VLP vaccine in poultry species [23,24].
In the present study, we developed H9 influenza VLP vaccine and evaluated its immunogenicity and protective efficacy in specific-pathogen-free (SPF) chickens. Furthermore, we assessed VLP vaccines by using a companion nucleocapsid protein (NP) coated enzyme linked immunosorbent assay (ELISA) test, which may provide a useful method to discriminate between chickens infected naturally and chickens vaccinated only.
2. Materials and methods
2.1. Preparation of avian influenza H9 HA VLPs
Influenza H9 VLPs containing HA and M1 proteins were produced by following a procedure previously described [25]. Briefly, to generate the recombinant baculoviruses (rBVs) expressing influenza proteins, full length HA cDNA derived from influenza A/Chicken/Korea/01310/2001 (H9N2) virus and M1 were cloned into pFastBac (Invitrogen, Carlsbad, CA) and transferred into Bacmid BV DNA (rAcNPV) followed by transformation with DH10Bac cells (Invitrogen, Carlsbad, CA). The rBVs expressing HA and M1 proteins were generated by transfecting sf9 insect cells with Bacmid recombinant BV DNA, and plaque isolation from culture supernatants of transfected sf9 insect cells. To generate influenza H9 VLPs, sf9 insect cells (ATCC, CRL-1711) were co-infected with rBVs expressing H9 HA and M1 at a multiplication of infection of 3 and 1 respectively. Culture media were collected and clarified by low speed centrifugation (2000 × g, 30 min, 4 °C) at 2 days post infection. Culture supernatants were concentrated and filtrated by Quixstand bench-top system (GE Healthcare, Waukesha, WI) using hollow fiber cartridge (300,000 Da of nominal molecular weight cut-off). Further purification was performed by 30 and 60% sucrose layer gradient ultracentrifugation (28,000 × g, for 60 min) following dialysis with HEPES buffered saline solution (HBS) and then H9 VLP solution was concentrated by Viva spin (Sartorius, Bohemia, NY) protein concentrator. The final protein concentration of H9 VLPs was quantified by a protein assay kit (Bio-rad, Irvine, CA) and biological activity was determined by a hemagglutination assay as previously described [26]. Units of hemagglutination activity are presented as a factor of dilution that prevents the precipitation of red blood cells.
2.2. Vaccine and viruses
Vaccines, with or without adjuvant, were prepared by emulsifying the escalating concentration (2 μg, 5 μg, 10 μg, and 20 μg) of VLP antigen solution with Montanide ISA70 (SEPPIC, France) in the ratio 30:70 (v/v). To evaluate vaccine efficacy, chickens were challenged intranasally with 106 EID50 of homologous H9N2 influenza A virus (A/Chicken/Korea/01310/2001).
2.3. Animals and experimental design
Sixty 6-week-old SPF chickens (10 chickens per group) were divided into six groups. Chickens were immunized with escalating dose of (2 μg, 5 μg, 10 μg, or 20 μg) VLP vaccines with adjuvant or 20 μg of VLP without adjuvant, via an intra-muscular route. Ten SPF chickens were vaccinated with commercial H9N2 whole-virus inactivated vaccine “Chicken-Vac” (DaeSung, Korea), containing the 212HAU concentration of inactivated allantoic fluid with mineral-oil adjuvant in the ratio 30:70 (v/v), according to the manufacturer’s instruction (0.5 ml injection via intra-muscular route). As a non-vaccinated control group, another 10 SPF chickens were injected with emulsified solution of distilled water with ISA 70 in the same ratio with VLP vaccines.
At 3 weeks after a single dose of vaccination, under Animal Biosafety Level 2 enhanced conditions, chickens were challenged intranasally with the H9N2 homologous virus. To determine the replication of challenge influenza virus at day 5 post-challenge as previously described [14], trachea and cecal tonsil tissues were collected from each chicken and homogenized. 10% (w/v) of tissue homogenates were prepared in PBS containing 400 μg/ml gentamycin, then the suspensions were centrifuged and a clear liquid supernatant at the topper layer was collected. All specimens were inoculated into the allantoic cavity of 9–11-day old SPF chicken embryonated eggs. After 72 h of incubation, the eggs were chilled and allantoic fluids were harvested and tested for hemagglutination activity.
For AIV-infected sera, fifteen 6-week-old SPF chickens were inoculated intranasally with the same challenge virus, and serum samples were collected from AIV infected chickens at 2 weeks after challenge and used to determine antibodies in comparison with vaccine derived antibodies.
2.4. Serology
In order to determine the immunogenicity of VLP vaccines, serum samples were collected prior to vaccination and 3 weeks after the vaccination for HI test and ELISA. HI tests were performed as described in the OIE standard HI method using formalin-inactivated homologous antigen. AIV NP-specific antibody levels were measured using a commercially available multispecies competitive NP-ELISA kit (Bionote, Korea), which have been implemented to detect antibodies against AIV NP in different avian species, according to manufacturer‘s instruction.
2.5. Statistical analysis
Statistical analysis of differences between vaccinated groups and unvaccinated control group was performed using a Fisher’s exact test. ANOVA with Tukey–Kramer post-test was performed for statistical test of serum antibody titers. Statistical significance was designated for differences with p-values less than or equal to 0.05.
3. Results
3.1. Immune responses to vaccination with influenza H9 HA VLPs
Influenza H9 VLPs containing the HA protein derived from A/Chicken/Korea/01310/2001 (H9N2) virus were produced in insect cells by the rBV expression system. Purification procedures were performed by ultra-filtration and sucrose gradient ultra-centrifugation. Purified H9 HA VLPs were found to have approximately 8000 units of hemagglutination activity (HAU) at the protein concentration of 1.2 mg per milliliter. This result indicates that the HA protein incorporated into VLPs maintains a biologically active conformation in the receptor binding site. The native conformation in the receptor binding site in the influenza VLPs was recently shown to be important for maintaining the immunogenicity of HA VLPs and inducing protective immune responses [27].
To evaluate the immunogenicity of H9 HA VLP vaccines, groups of chickens were intramuscularly immunized and immune responses in sera were determined 3 weeks after a single dose of vaccination. As shown in Fig. 1, as low as 2 μg VLP vaccine induced significant levels of virus-specific antibodies that are equivalent to 27.1 HI units. However, any detectable levels of antibody responses were not observed in the control group that received mock vaccination with ISA70 adjuvant. Levels of virus-specific antibody responses showed an increase up to 10 μg VLPs in a dose-dependent manner. Importantly, H9 HA VLP vaccines (5 or 10 μg dose) showed antibody responses that are comparable to the whole-viral vaccine control. Without ISA70 adjuvant, low antibody responses equivalent to 24.3 HI units were induced even with 20 μg VLPs. Therefore, the inclusion of ISA70 adjuvant in the H9 VLP vaccination showed a significant immune enhancing effect on VLP vaccines by over 10 fold. These results suggest that influenza H9 HA VLP vaccines can induce virus-specific functional antibody responses.
Fig. 1.
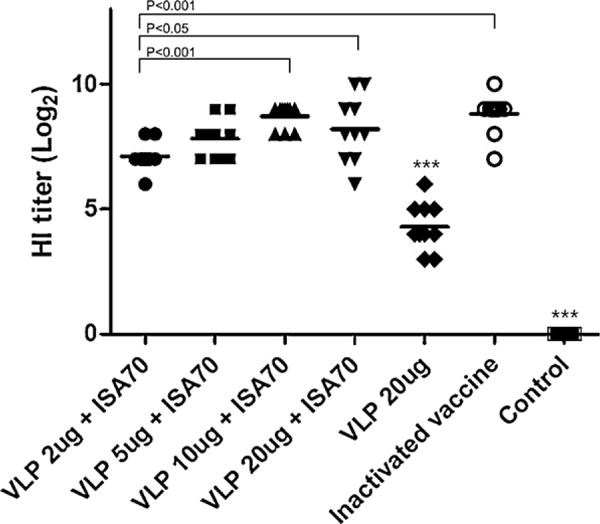
Mean serum HI titers (log 2) induced in SPF chickens after a single dose of vaccination. Sixty 6-week-old SPF chickens (10 chickens per group) were intramuscularly immunized with influenza H9 HA VLPs (2, 5, 10, or 20 μg total protein) or inactivated whole viral vaccine (0.5 ml dose) commercially manufactured HI titers against the homologous antigen [A/chicken/Korea/310/2001(H9N2)] were determined at 3 weeks after vaccination. ISA70: an adjuvant; control: ISA70 adjuvant only without vaccine. ***p < 0.001 by ANOVA with Tukey–Kramer post-test compared to other groups.
3.2. Reduced viral shedding in chickens vaccinated with influenza H9 HA VLPs
The mock control group showed the highest frequency of virus detection in the trachea and cecal tonsil as 9 out of 10 chickens’ trachea and 8 out of 10 chickens’ tonsil were positive for viral replication (Table 1). However, chickens vaccinated with 2 μg of VLPs with ISA70 adjuvant showed the virus detection in the trachea and cecal tonsil as 1 out of 10 chickens’ trachea and 2 out of 10 chickens’ tonsil were positive for viral replication. Chickens vaccinated with 5 μg of VLPs with ISA70 adjuvant showed the virus detection in the trachea and cecal tonsil as 2 out of 10 chickens’ trachea and 2 out of 10 chickens’ tonsil were positive for viral replication. Additionally, no virus was isolated from the tracheal or cecal tonsil tissues of chickens vaccinated with over 10 μg of VLP with ISA70 adjuvant or whole-virus inactivated vaccine. Moreover, vaccination with 20 μg VLP without adjuvant also significantly reduced viral shedding from cecal tonsil compared to the unvaccinated control group.
Table 1.
Vaccination with H9 HA VLP vaccine lowers frequencies of virus isolation from SPF chickens challenged with wild type H9N2 AIV.a
| Group | Virus isolationb/total
|
|
|---|---|---|
| Trachea | Cecal tonsil | |
| VLP 2 μg + ISA70 | 1/10** | 2/10* |
| VLP 5 μg + ISA70 | 2/10** | 2/10* |
| VLP 10 μg + ISA70 | 0/10*** | 0/10*** |
| VLP 20 μg + ISA70 | 0/10*** | 0/10*** |
| VLP 20 μg only | 7/10 | 3/10* |
| Commercial vaccinec | 0/10*** | 0/10*** |
| Challenge control | 9/10 | 8/10 |
Groups of SPF chickens were challenged intranasally with 106 EID50 of homologous H9N2 virus at 3 weeks post vaccination.
Virus re-isolation was done by chicken embryo inoculation at 5 days post challenge.
Commercial vaccine contains identical AIV strains and mineral-oil adjuvant.
p < 0.05 by Fisher’s exact test, compared to challenge control group.
p < 0.01 by Fisher’s exact test, compared to challenge control group.
p < 0.001 by Fisher’s exact test, compared to challenge control group.
3.3. Differentiation of infected chickens from the vaccinated with a H9 HA VLP vaccine
As expected, all serum samples of chickens vaccinated with VLP vaccine (n = 10) were negative by the NP-cELISA [1 − (S/N) ≤0.5] (Fig. 2). In contrast, the sera of chickens vaccinated with whole virus inactivated vaccine (n = 10) were all positive for NP immune responses [1 − (S/N) ≥0.5]. We tested AIV-infected sera (n = 15) using the NP-cELISA and were all positive for NP immune responses [1 − (S/N) ≥0.5]. Therefore, VLP vaccine and the companion DIVA test, NP-ELISA, could allow the utilization of the DIVA strategy.
Fig. 2.
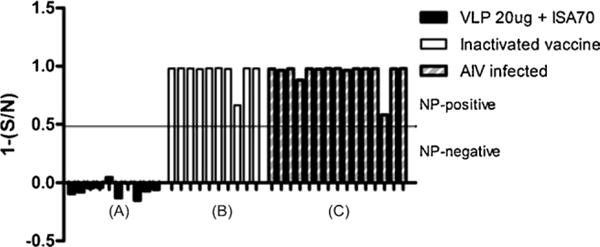
NP serum antibody levels induced by VLP vaccine, inactivated whole virus vaccine, or AIV infection by NP ELISA. SPF chickens were immunized with 20 μg of VLP antigen with ISA70 adjuvant (A) or whole virus inactivated vaccine (B). Antibody levels were determined by NP-cELISA at 3 weeks post vaccination. AIV infected (C) sera were collected at 2 weeks post H9N2 virus challenge. Each bar represents the NP specific antibody value of each chicken.
4. Discussion
An advantage of a VLP vaccine approach is a virus mimicking particle with multiple viral antigens and epitopes that stimulate a diverse set of immune responses [28,29]. Influenza VLP vaccines have been shown to be very immunogenic and safe in various animal models [17,21,23,28,29]. In our previous studies, antibody responses to M1 were found to be minimal even after prime-boost of two immunizations with influenza M1 core VLPs [30]. Also, mice immunized with M1 VLPs alone were not protected [31], indicating that immune responses to M1 do not play a significant role in inducing protective immunity. In the present study, we demonstrated that H9 HA VLP vaccine elicited high levels of antibody responses to the virus as shown by hemagglutination inhibition activity and also lessened the number of chickens with viral shedding from respiratory and gastrointestinal tract. To our knowledge, this study is the first work intended to generate and evaluate poultry H9 HA VLP vaccines in chickens, and to provide a promising DIVA strategy. Recently, Prel et al. introduced VLP vaccine by using a triple baculovirus recombinant coding for HA, NA, and M1 proteins from H5N3 AIV and showed protection conferred by these VLPs in Muscovy duck [23]. Here, we evaluated H9 HA VLP vaccine in chickens which are known to be more susceptible species to AIV than duck species. Our results should further support for the possibility of reliably immunizing VLP vaccines in poultry species.
The use of VLP vaccines would help in limiting the safety concerns compared to live-attenuated and inactivated whole-virus vaccines. For example, a strong possibility was suggested that the introduction of highly pathogenic H5N2 of American lineage into Japan was due to a transfer of live virus from improperly inactivated vaccine or from contaminated vaccine [32]. Because of the noninfectious nature of VLPs and their lack of viral genomic material, the VLP vaccine represents a desirable safety feature as a vaccine candidate [17]. In addition, during AIV circulation in poultry population, HA and NA surface antigens undergo progressive amino acid substitutions which could cause evasion of the previously acquired immunity by vaccination. Because VLP vaccine approach allows rapid vaccine production, it could provide easy updates of the mutated or newly emerged strains.
ISA 70 has already been demonstrated as a safe and effective adjuvant in numerous poultry disease models with enhancing antibody and/or cell-mediated immune responses [33]. Enhanced immune reactions induced by ISA70 adjuvant were characterized by proliferation of macrophages, epithelial cells, and fibroblasts around the small cysts in the muscle [34]. Based on our results, 10 μg of VLP with the ISA70 adjuvant provide significantly higher mean antibody titers than the same or high dose of VLP vaccine without adjuvant. Furthermore, a lower dose of VLP vaccines (2 μg) with an ISA70 adjuvant provided significantly enhanced antibody titers than a high dose of VLPs (20 μg) in the absence of adjuvant. The combined effects of vaccine doses and increased HI titers observed suggest that the immune enhancing effects on VLP vaccine antigens by the addition of ISA70 adjuvant are significant. These results suggest that influenza VLPs can confer effective protection in chickens and that inclusion of ISA70 adjuvant significantly enhances the protective immunity by influenza VLPs. Therefore, the addition of ISA70 adjuvant to the VLP vaccination could provide enhanced protective immune response against AIV even with vaccination with low doses of VLP vaccines.
Vaccination against AIV, an effective control measure for the eradication of LPAI or HPAI, may result in issues related to surveillance programs and international trades of poultry and poultry products [15,35]. Therefore, several different DIVA strategies have been suggested using appropriate vaccines and companion serologic tests for discriminating between naturally infected and vaccinated-only animals [16,35,36]. Particularly, many different types of subunit vaccines, including virus vectored vaccines and vaccines using proteins expressed in culture systems, have been shown to provide protection against AIV and allow DIVA strategy [15,23,37]. The present study provides evidence that H9 HA VLP vaccination can be a promising DIVA strategy by companion NP-coated ELISA tests.
Vaccine cost has been thought to be an important issue that influences the feasibility of recombinant subunit vaccines including VLP for poultry. Based on our results, VLP vaccine has potential advantages over egg-based vaccine, particularly for providing significant protection even with low doses of VLP vaccines compared to unvaccinated animals. In addition, purified influenza VLP vaccines have very high-yield and hemagglutination activity (213 HAU), which are expected to be advantageous for manufacturing not only cost-effective vaccines but also diagnostic antigens of HPAI. Based on our analysis, the cost of VLP production would be equal to or less than the conventional inactivated whole virus vaccine. Further, the cost may be reduced if the purification procedures are improved and minimized in the future. In addition, during HPAI outbreaks, vaccine manufacturers using VLP vaccine technology could produce vaccines without the need for expensive high-bio-safety-requiring biocontainment facilities.
In conclusion, H9 HA VLP vaccine developed in this study was safe, immunogenic, and vaccination with H9 VLPs lessened the number of chickens with viral shedding from respiratory and gastrointestinal tract. Furthermore, we could differentiate VLP vaccinated chickens from AIV infected chickens, supporting that VLP vaccine allows an effective DIVA strategy. These results provide support for continued development of the VLP as a poultry vaccine against AIV.
Acknowledgments
This work was supported by grant no. 610001-03-1-SU000 from Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea. We are grateful to Tae-Hyun Lim, Myung-Seob Kim, Ha-Na Youn, Hyo-Seon Joo, Kyung-Min Kim, Byung-Yoon Kim, and Soo-Won Choi for their technical support.
References
- 1.Guo YJ, Krauss S, Senne DA, Mo IP, Lo KS, Xiong XP, et al. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267(2):279–88. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- 2.Nagarajan S, Rajukumar K, Tosh C, Ramaswamy V, Purohit K, Saxena G, et al. Isolation and pathotyping of H9N2 avian influenza viruses in Indian poultry. Vet Microbiol. 2009;133(1–2):154–63. doi: 10.1016/j.vetmic.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Wu R, Sui ZW, Zhang HB, Chen QJ, Liang WW, Yang KL, et al. Characterization of a pathogenic H9N2 influenza A virus isolated from central China in 2007. Arch Virol. 2008;153(8):1549–55. doi: 10.1007/s00705-008-0139-1. [DOI] [PubMed] [Google Scholar]
- 4.Monne I, Cattoli G, Mazzacan E, Amarin NM, Al Maaitah HM, Al-Natour MQ, et al. Genetic comparison of H9N2 AI viruses isolated in Jordan in 2003. Avian Dis. 2007;51(Suppl. 1):451–4. doi: 10.1637/7563-033106R.1. [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Shin JY, Song MS, Lee YM, Choi JG, Lee EK, et al. Continuing evolution of H9 influenza viruses in Korean poultry. Virology. 2007;359(2):313–23. doi: 10.1016/j.virol.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Toroghi R, Momayez R. Biological and molecular characterization of avian influenza virus (H9N2) isolates from Iran. Acta Virol. 2006;50(3):163–8. [PubMed] [Google Scholar]
- 7.Matrosovich MN, Krauss S, Webster RG. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology. 2001;281(2):156–62. doi: 10.1006/viro.2000.0799. [DOI] [PubMed] [Google Scholar]
- 8.Butt KM, Smith GJ, Chen H, Zhang LJ, Leung YH, Xu KM, et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol. 2005;43(11):5760–7. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YP, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, et al. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc Natl Acad Sci USA. 2000;97(17):9654–8. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CW, Song CS, Lee YJ, Mo IP, Garcia M, Suarez DL, et al. Sequence analysis of the hemagglutinin gene of H9N2 Korean avian influenza viruses and assessment of the pathogenic potential of isolate MS96. Avian Dis. 2000;44(3):527–35. [PubMed] [Google Scholar]
- 11.Lee HJ, Kwon JS, Lee DH, Lee YN, Youn HN, Lee YJ, et al. Continuing evolution and interspecies transmission of influenza viruses in live bird markets in Korea. Avian Dis. 2010;54(Suppl. 1):738–48. doi: 10.1637/8785-040109-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 12.Moon HJ, Song MS, Cruz DJ, Park KJ, Pascua PN, Lee JH, et al. Active reassortment of H9 influenza viruses between wild birds and live-poultry markets in Korea. Arch Virol. 2010;155(2):229–41. doi: 10.1007/s00705-009-0577-4. [DOI] [PubMed] [Google Scholar]
- 13.Choi JG, Lee YJ, Kim JY, Kim YH, Paek MR, Yang DK, et al. Molecular identification of the vaccine strain from the inactivated oil emulsion H9N2 low pathogenic avian influenza vaccine. J Vet Sci. 2010;11(2):161–3. doi: 10.4142/jvs.2010.11.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JG, Lee YJ, Kim YJ, Lee EK, Jeong OM, Sung HW, et al. An inactivated vaccine to control the current H9N2 low pathogenic avian influenza in Korea. J Vet Sci. 2008;9(1):67–74. doi: 10.4142/jvs.2008.9.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suarez DL. Overview of avian influenza DIVA test strategies. Biologicals. 2005;33(4):221–6. doi: 10.1016/j.biologicals.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Capua I, Cattoli G. Diagnosing avian influenza infection in vaccinated populations by systems for differentiating infected from vaccinated animals (DIVA) Dev Biol (Basel) 2007;130:137–43. [PubMed] [Google Scholar]
- 17.Kang SM, Pushko P, Bright RA, Smith G, Compans RW. Influenza virus-like particles as pandemic vaccines. Curr Top Microbiol Immunol. 2009;333:269–89. doi: 10.1007/978-3-540-92165-3_14. [DOI] [PubMed] [Google Scholar]
- 18.Haynes JR. Influenza virus-like particle vaccines. Expert Rev Vaccines. 2009;8(4):435–45. doi: 10.1586/erv.09.8. [DOI] [PubMed] [Google Scholar]
- 19.Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009;83(11):5726–34. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pushko P, Kort T, Nathan M, Pearce MB, Smith G, Tumpey TM. Recombinant H1N1 virus-like particle vaccine elicits protective immunity in ferrets against the 2009 pandemic H1N1 influenza virus. Vaccine. 2010;28(30):4771–6. doi: 10.1016/j.vaccine.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 21.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405(1):165–75. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao P, Luo M, Zhu D, Qu S, Yang Z, Gao M, et al. Virus-like particle vaccine comprised of the HA, NA, and M1 proteins of an avian isolated H5N1 influenza virus induces protective immunity against homologous and heterologous strains in mice. Viral Immunol. 2009;22(4):273–81. doi: 10.1089/vim.2009.0017. [DOI] [PubMed] [Google Scholar]
- 23.Prel A, Le Gall-Recule G, Cherbonnel M, Grasland B, Amelot M, Jestin V. Assessment of the protection afforded by triple baculovirus recombinant coexpressing H5, N3, M1 proteins against a homologous H5N3 low-pathogenicity avian influenza virus challenge in Muscovy ducks. Avian Dis. 2007;51(Suppl. 1):484–9. doi: 10.1637/7683-072106R.1. [DOI] [PubMed] [Google Scholar]
- 24.Prel A, Le Gall-Recule G, Jestin V. Achievement of avian influenza virus-like particles that could be used as a subunit vaccine against low-pathogenic avian influenza strains in ducks. Avian Pathol. 2008;37(5):513–20. doi: 10.1080/03079450802357001. [DOI] [PubMed] [Google Scholar]
- 25.Kang SM, Yoo DG, Lipatov AS, Song JM, Davis CT, Quan FS, et al. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS One. 2009;4(3):e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song JM, Kim YC, Lipatov AS, Pearton M, Davis CT, Yoo DG, et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol. 2010;17(9):1381–9. doi: 10.1128/CVI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84(15):7760–9. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25(19):3871–8. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 29.Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, Bright RA. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One. 2009;4(6):e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, et al. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011;6(1):e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81(7):3514–24. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krauss S, Obert CA, Franks J, Walker D, Jones K, Seiler P, et al. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog. 2007;3(11):e167. doi: 10.1371/journal.ppat.0030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang SI, Lillehoj HS, Lee SH, Lee KW, Park MS, Bauchan GR, et al. Immunoenhancing effects of Montanide ISA oil-based adjuvants on recombinant coccidia antigen vaccination against Eimeria acervulina infection. Vet Parasitol. 2010;172(3–4):221–8. doi: 10.1016/j.vetpar.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 34.Yamanaka M, Okabe T, Nakai M, Goto N. Local pathological reactions and immune response of chickens to ISA-70 and other adjuvants containing Newcastle disease virus antigen. Avian Dis. 1993;37(2):459–66. [PubMed] [Google Scholar]
- 35.Avellaneda G, Mundt E, Lee CW, Jadhao S, Suarez DL. Differentiation of infected and vaccinated animals (DIVA) using the NS1 protein of avian influenza virus. Avian Dis. 2010;54(Suppl. 1):278–86. doi: 10.1637/8644-020409-Reg.1. [DOI] [PubMed] [Google Scholar]
- 36.Kwon JS, Kim MC, Jeong OM, Kang HM, Song CS, Kwon JH, et al. Novel use of a N2-specific enzyme-linked immunosorbent assay for differentiation of infected from vaccinated animals (DIVA)-based identification of avian influenza. Vaccine. 2009;27(24):3189–94. doi: 10.1016/j.vaccine.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 37.Swayne DE, Avellaneda G, Mickle TR, Pritchard N, Cruz J, Bublot M. Improvements to the hemagglutination inhibition test for serological assessment of recombinant fowlpox-H5-avian-influenza vaccination in chickens and its use along with an agar gel immunodiffusion test for differentiating infected from noninfected vaccinated animals. Avian Dis. 2007;51(3):697–704. doi: 10.1637/0005-2086(2007)51[697:ITTHIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]


