Abstract
A novel family of small molecule inhibitors of voltage-gated sodium channels (Navs) based on the structure of batrachotoxin (BTX) – a well-known channel agonist – is described. Protein mutagenesis and electrophysiology experiments reveal the binding site as the inner pore region of the channel, analogous to BTX, alkaloid toxins, and local anesthetics. Homology modeling of the eukaryotic channel based on recent crystallographic analyses of bacterial NaVs suggests a mechanism of action for ion conduction block.
Graphical Abstract
Voltage-gated sodium ion channels (NaVs) are responsible for the initiation and propagation of action potentials in excitable membranes.[1] Eukaryotic NaVs are comprised of an α-subunit, which assembles as four homologous domains (D1–D4) to form the central ion conduction pore, and two auxiliary β-subunits. Transient changes in membrane voltage potential effect rapid alterations in channel conformation, which are critical for proper ion gating. Biophysical and computational studies to characterize NaV dynamics have been aided through structural investigations that include protein X-ray crystallography,[2] mutagenesis experiments,[3] and ligand binding studies.[4] The value of small molecules for interrogating NaV structure-function is considerable, as SAR data in combination with site-directed mutagenesis have shaped current views of the molecular form of the eukaryotic channel structure.[5] These insights notwithstanding, the ability of small molecule NaV modulators to alter ion gating kinetics by influencing conformational states of the channel is poorly understood.[6] Given the intensive interest in NaVs as targets for drug design,[7] deeper insight into the complex molecular interactions between ligand and channel is sought.
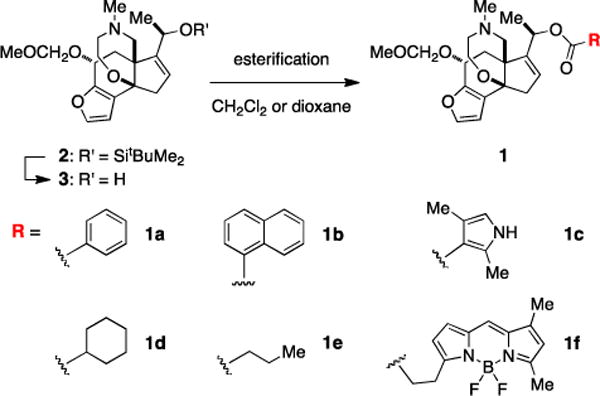
Batrachotoxin (BTX), a natural product isolated from poison dart frogs of the genus Phyllobates,[8] is a potent modulator of NaVs.[9] Binding of this molecule to the inner pore of the channel (Site II) elicits a multitude of functional responses, including a hyperpolarized shift on the voltage-dependence of activation, inhibition of both fast and slow inactivation, and reduction in ion selectivity.[10] The uniqueness of BTX as a NaV agonist has motivated our efforts to understand the molecular details of its binding interactions with the channel and its structure-function properties. As part of this program, we have prepared simplified BTX-like structures 1 (Figure 1).[11] Electrophysiology recordings with wild-type and mutant NaV isoforms demonstrate that BTX analogues comprising the C, D, and E rings of the natural product block NaV current – a stark contrast to the behavior of BTX itself. Protein mutagenesis data suggest that BTX analogues lodge in the inner pore lining of the channel, thus sharing a common receptor site with the parent compound. Surprisingly, both enantiomers of 1 display nearly identical potency as NaV inhibitors.[12] A homology model of the binding interactions of 1 with the channel pore is presented in an attempt to rationalize the lack of stereorecognition in the receptor site. Collectively, these results establish 1 as a novel chemotype for NaV inhibition and as a chemical probe for understanding the molecular mechanism of NaV function.
Figure 1.
C/D/E ring analogues of batrachotoxin (1) act as potent antagonists of voltage-gated sodium ion channels (NaVs).
Early structure-activity relationship studies on the effects of semi-synthetic BTX derivatives against NaVs highlight the importance of the ester and amine groups in addition to oxygen functional groups at C3, C9, and C11 for toxin function.[13] Protein mutagenesis experiments,[3] ligand binding studies,[4] and competitive ligand displacement assays[14] give substantive evidence that toxin interaction with NaV is localized to Site II. Homology modeling and single-point mutagenesis data suggest that the C/D/E ring portion of BTX makes primary contacts with the inner pore lining.[3f,15] The lack of availability of BTX from natural sources restricts access to new, modified BTX compounds and further examination of this model.[9c] Investigations of structure-function with BTX analogues and protein mutants can give insight into the unique ability of BTX to influence voltage activation and inactivation through binding the central cavity of the channel. Accordingly, de novo chemical synthesis of the toxin and related structures is the only current available strategy for accessing such probes.[16]
Truncated forms of BTX comprised of the C/D/E ring units and C20 ester moiety are available in two steps from silyl ether 2 (Scheme 1). Derivatives that contain either the acylpyrrole group found in BTX or those bearing benzoate, naphthoate, cyclohexylcarboxylate, and butyrate are generated in good yields (33–95%) using either acid chloride or mixed-anhydride reaction partners. Coupling of a mixed-anhydride form[17] of BODIPY® FL to 3 gives a fluorescent conjugate 1f, which was prepared to examine the steric dimensions of the receptor site and for its potential as an imaging tool for ligand displacement assays.[14] Each of these compounds has been synthesized from 2 in multi-milligram quantities.
Scheme 1. Synthesis of BTX analogue compounds.a.
a(a) benzoyl chloride, DMAP, CH2Cl2, 95%; (b) 1-naphthoyl chloride, DMAP, CH2Cl2, 89%; (c) (ethyl carbonic) 2,4-dimethyl-1H-pyrrole-3-carboxylic anhydride, i-Pr2NEt, 1,4-dioxane, 90 °C, 72%; (d) cyclohexanecarboxylic(ethyl carbonic) anhydride, 1,4-dioxane, 90 °C, 65%; (e) butyric(ethyl carbonic) anhydride, 1,4-dioxane, 90 °C, 75%; (f) BODIPY® FL propionic acid, 2-methyl-6-nitrobenzoic anhydride, Et3N, DMAP, CH2Cl2, 33%.
Electrophysiology measurements were performed in a whole-cell voltage-clamp format against the α-subunit of the rat skeletal muscle NaV (rNaV1.4), heterologously expressed in Chinese hamster ovarian (CHO) cells. Currents were elicited by 10-ms step depolarizations from a holding potential of −100 to 0 mV and were recorded at steady state. In this assay, a channel modified by BTX, which binds preferentially to the open-state of the channel (use-dependence) exhibits a sustained current.[3,10] By contrast, application of benzoate 1a or naphthoate 1b (100 μM) shows partial block of Na+ current in a use-dependent manner (Figure 2A, data shown for 1b). Measured IC50 values for these two compounds are 81.4 ± 4.8 and 17.4 ± 0.4 μM, respectively (Figures 2B, S1). More surprisingly, experiments performed with either antipode of 1b (i.e., 1b*) demonstrate that inhibition of the channel by these agents is not stereoselective.
Figure 2.
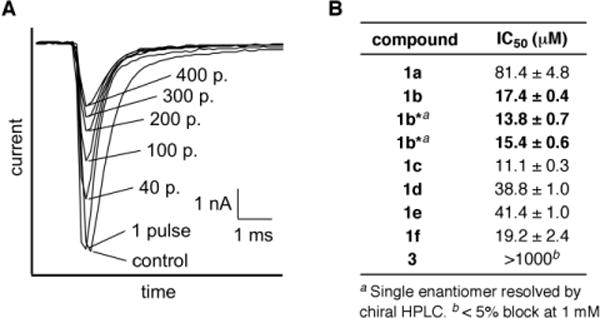
Assay results of BTX analogues against rNav1.4. (A) Use-dependent block of sodium ion current of rNaV1.4 by 1b (100 μM). Representative traces of superimposed Na+ currents are labeled with the number of repetitive pulses. (B) Structure-activity relationship studies on the ester group of the C/D/E core analogues 1a–1f and 3.
Other ester derivatives display similar inhibitory activities to 1a and 1b with the most potent having an IC50 value as low as 11.1 ± 0.3 μM (1c). Notably, block of Na+ current is also observed with the sterically large BODIPY® FL conjugate 1f (19.2 ± 2.4 μM). Assuming 1f shares a common receptor with BTX and other BTX analogues, this result suggests that a large open space may comprise the ligand binding site (vide infra). As with BTX, the absence of the ester group dramatically alters the affinity for channel binding.[13,18] Electrophysiology recordings with alcohol 3 show < 5% block at 1 mM concentrations. Use-dependent block of sodium ion conduction by 1a–1f markedly contrasts the activity of BTX and establishes these molecules as a new class of NaV inhibitors.
Subsequent investigations of BTX C/D/E analogues were performed with naphthoate 1b given the potency and ease of synthesis of this particular compound. Electrophysiology recordings with different NaV isoforms, which included rNaV1.2, hNaV1.5, and hNaV1.7, give measured IC50 values that vary between 8–30 μM (Figures 3, S2). The similar potency of 1b against channel subtypes is perhaps unsurprising in light of the nearly identical primary structure of the amino acids that are believed to comprise the inner pore.
Figure 3.
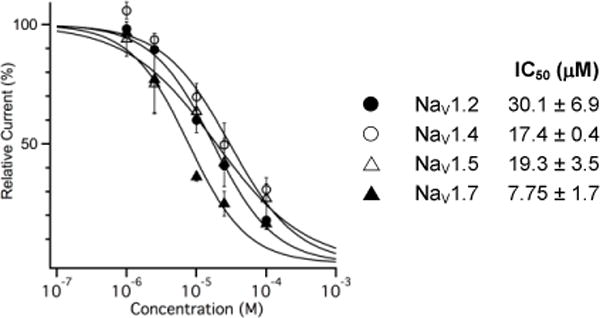
Dose-response curve of 1b against selected NaV isoforms. Normalized data were fit to the Hill equation.
Binding of BTX to NaV hyperpolarizes the voltage-dependence of channel activation by −45 mV, allowing a greater population of channels to open at resting membrane potentials.[3,9,10] In addition, BTX is known to inhibit fast inactivation. By contrast, electrophysiology experiments to test the influence of 1b on channel activation and inactivation reveal that this derivative has only a slight influence on the steady-state voltage dependence of activation and inactivation (Figure 4). Binding of 1b (25 μM) to rNaV1.4 shifts V0.5 of activation by +4.9 mV and the slope factor is increased by +2.0 mV. For steady-state inactivation, the midpoint voltage is shifted by −6.0 mV and the slope factor is not significantly changed (+0.7 mV).
Figure 4.
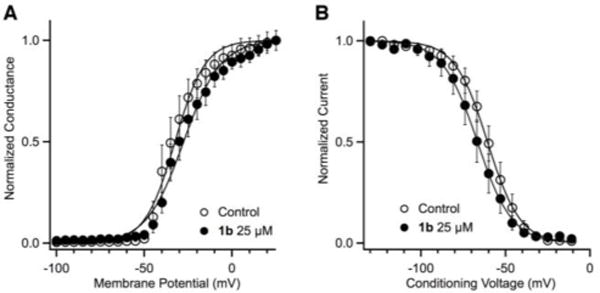
Effects on the voltage dependence of (A) activation and (B) inactivation of rNaV1.4 by 1b (25 μM). The normalized data were fit to a Boltzmann equation.
The obvious discrepancy in activity between BTX and C/D/E ring analogues such as 1b has motivated additional experiments to examine ligand-receptor binding interactions. Amino acid residues that comprise the inner channel pore were selected for initial single-point protein mutagenesis experiments, following previous studies of Site II ligands such as BTX and veratridine[20] and with the aid of a homology model comprising the S5, S6 and p-loop regions of the channel (Figures 5, S3). Ten amino acids positioned on the S6 helices of domains I–IV were changed to either lysine (K) or alanine (A). All mutant channels have been previously prepared, characterized and tested against BTX.[3] In most cases, lysine modification (N434K, N784K, F1280K, F1579K, N1584K) results in an increase (~2–10 fold) in the IC50 of naphthoate 1b, consistent with destabilization of ligand binding caused by Coulombic repulsion between the charged lysine and the protonated inhibitor. Alanine mutation at three sites (F1579A, N1584A, Y1586A) also influences the ability of 1b to block channel function, but the overall effect on IC50 is much less pronounced.
Figure 5.
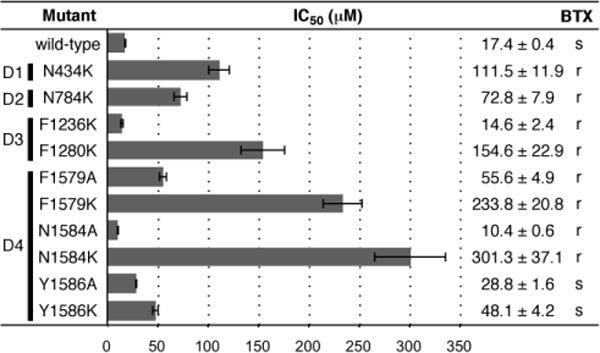
Inhibitory constants for 1b against single-point mutants of rNaV1.4 (corresponding domains are labeled D1–D4). The effect of 5 μM BTX on wild-type and mutant NaVs is shown as s (sensitive) or r (resistant).
With the exception of the tyrosine residue at position 1586, all amino acid mutations shown in Figure 5 have been found to abrogate the effect of BTX on channel function. The marked influence of N434K, N784K, F1280K, N1579K, and N1584K on binding of both 1b and BTX suggests some commonality in the receptor site for these two ligands. Differences between BTX and 1b are noted, however, with two BTX-destabilizing mutations, F1236K and N1584A; against these mutant isoforms, 1b shows a modest increase in affinity from that of wt-NaV1.4. Given the large volume size of the inner pore as estimated from NaV homology models, it is possible for BTX and 1b to bind to the open state of the channel in different spatial orientations. The lack of stereoselectivity for inhibition by either antipode of 1b as well as the action of alkaloids such as aconitine and veratridine at Site II are consistent with an inner pore domain that can accommodate sterically large, topologically unique structures. This conclusion is further supported by docking experiments[20] of 1b with a homology model of the channel pore[21] constructed from recent X-ray structures of bacterial NaVs[2] (Figures 6, S4, S5). In these poses, both enantiomers of 1b are positioned near residues N434, N784, L1280, F1579 and N1584 and the ammonium group faces the central pore, presumably blocking ion transport through charge repulsion. Future studies will examine these models in greater detail through additional mutagenesis and SAR experiments.
Figure 6.
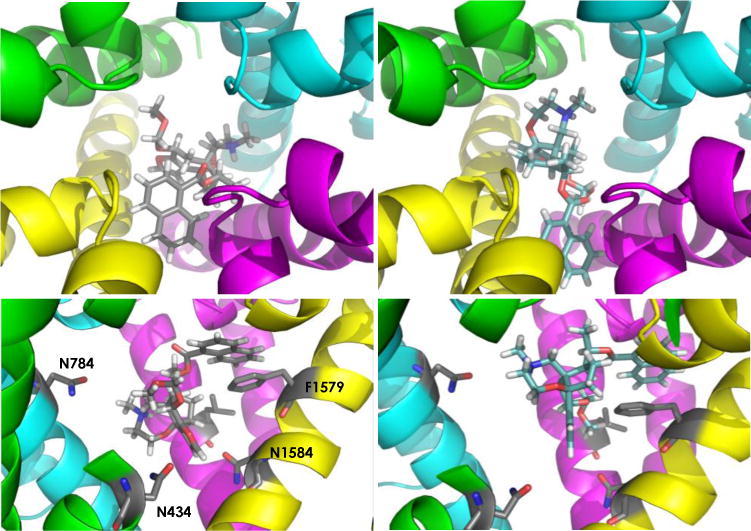
Enantiomers of 1b docked to a homology model of rNav1.4. Top panels: views down the pore axis; Bottom panels: side views (Domain I residues removed for clarity). Domain I = green; Domain II = cyan; Domain III = magenta; Domain IV = yellow. Bottom panels show residues N434, N784, L1280 (not labeled), F1579; N1584.
In sum, we have established that analogue structures of BTX possessing the C/D/E ring elements function as low micromolar inhibitors of NaV subtypes.[22] This activity is in striking contrast to the behavior of BTX as a channel agonist. Despite the evident functional differences, it appears from protein mutagenesis experiments that 1b, and by inference related compounds, overlap the binding site of BTX in the inner pore cavity of NaV. The availability of 1b and associated BTX structures is enabling subsequent investigations to understand how small molecules that bind the channel inner pore regulate ion gating.
Supplementary Material
Acknowledgments
We are grateful to Professor Merritt Maduke for allowing us use of her laboratory equipment and for many helpful discussions, to Dr. Allen Oliver (University of Notre Dame) for solving the crystal structure of alcohol 3 (Figure S6), and to Dr. Scott Virgil (Caltech) for his assistance with chiral chromatography. [Ki determinations, receptor binding profiles, agonist and/or antagonist functional data, HERG data, MDR1 data, etc. as appropriate] was generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract #HHSN-271-2013-00017-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA.
Funding sources
This work was supported in part by the National Institute of Health (R01NS045684) and Pfizer, Inc. TT is a Japan Society for the Promotion of Science (JSPS) Fellow for research abroad. FM was supported by NSERC (Canada) and by a Stanford University Dean’s Postdoctoral Fellowship award.
Footnotes
Supplementary figures (S1–S6), experimental details and homology models are available in the supporting online material. Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Tatsuya Toma, Graduate School of Pharmaceutical Sciences, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464−8601, Japan.
Matthew M. Logan, Gilead Sciences, Inc., Foster City, California 94404.
Frederic Menard, Department of Chemistry, University of British Columbia, Okanagan 3333 University Way, Kelowna, British Columbia Canada V1V 1V7.
A. Sloan Devlin, Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115.
J. Du Bois, Department of Chemistry, Stanford University, Stanford, CA 94305-5080.
References
- 1.Hille B. Ion Channels of Excitable Membranes. 3rd. Sinauer Associates; Massachusetts: 2001. pp. 1–22. [Google Scholar]
- 2.For a recent, comprehensive review of prokaryotic NaV structural biology, see:; Payandeh J, Minor DL., Jr Bacterial Voltage-Gated Sodium Channels (BacNaVs) from Soil, Sea, and Salt Lakes Enlighten Molecule Mechanisms of Electrical Signaling and Pharmacology in the Brain and Heart. J Mol Biol. 2015;427:3–30. doi: 10.1016/j.jmb.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Also see:; (a) Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Payandeh J, El-Din TMG, Scheuer T, Zheng N, Catterall WA. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–139. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang X, Ren W, DeCaen P, Yan C, Tao X, Tang L, Wang J, Hasegawa K, Kumasawa T, He J, Wang J, Clapham DE, Yan N. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–134. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) McCusker EC, Bagneris C, Naylor CE, Cole AR, D’Avanzo N, Nicholas CG, Wallace BA. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat Commun. 2012;3:1102. doi: 10.1038/ncomms2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Wang SY, Wang GK. Point mutations in segment I-S6 render voltage-gated Na+ channels resistant to batrachotoxin. Proc Natl Acad Sci U S A. 1998;95:2653–2658. doi: 10.1073/pnas.95.5.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang SY, Barile M, Wang GK. Disparate role of Na+ channel D2-S6 residues in batrachotoxin and local anesthetic action. Mol Pharmacol. 2001;59:1100–1107. doi: 10.1124/mol.59.5.1100. [DOI] [PubMed] [Google Scholar]; (c) Wang SY, Nau C, Wang GK. Residues in Na+ channel D3-S6 segment modulate both batrachotoxin and local anesthetic affinities. Biophys J. 2000;79:1379–1387. doi: 10.1016/S0006-3495(00)76390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tikhonov DB, Zhorov BS. Sodium channel activators: Model of binding inside the pore and a possible mechanism of action. FEBS Lett. 2005;579:4207–4212. doi: 10.1016/j.febslet.2005.07.017. [DOI] [PubMed] [Google Scholar]; (e) Wang SY, Wang GK. Batrachotoxin-resistant Na+ channels derived from point mutations in transmembrane segment D4-S6. Biophys J. 1999;76:3141–3149. doi: 10.1016/S0006-3495(99)77465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wang SY, Mitchell J, Tikhonov DB, Zhorov BS, Wang GK. How batrachotoxin modifies the sodium channel permeation pathway: Computer modeling and site-directed mutagenesis. Mol Pharmacol. 2006;69:788–795. doi: 10.1124/mol.105.018200. [DOI] [PubMed] [Google Scholar]; (g) Linford NJ, Cantrell AR, Qu Y, Scheuer T, Catterall WA. Interaction of batrachotoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc Natl Acad Sci U S A. 1998;95:13947–13952. doi: 10.1073/pnas.95.23.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Wang SY, Tikhonov DB, Zhorov BS, Mitchell J, Wang GK. Serine-401 as a batrachotoxin- and local anesthetic-sensing residue in the human cardiac Na+ channel. Pflugers Arch. 2007;454:277–287. doi: 10.1007/s00424-006-0202-2. [DOI] [PubMed] [Google Scholar]; (i) Du Y, Lee JE, Nomura Y, Zhang T, Zhorov BS, Dong K. Identification of a cluster of residues in transmembrane segment 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. Biochem J. 2009;419:377–385. doi: 10.1042/BJ20082082. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Vedantham V, Cannon SC. Rapid and slow voltage-dependent conformational changes in segment IVS6 of voltage-gated Na+ channels. Biophys J. 2000;78:2943–2958. doi: 10.1016/S0006-3495(00)76834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Wang SY, Tikhonov DB, Mitchell J, Zhorov BS, Wang GK. Irreversible block of cardiac mutant Na+ channels by batrachotoxin. Channels. 2007;1:179–188. doi: 10.4161/chan.4437. [DOI] [PubMed] [Google Scholar]; (l) Bosmans F, Maertens C, Verdonck F, Tytgat J. The poison dart frog’s batrachotoxin modulates NaV1.8. FEBS Lett. 2004;577:245–248. doi: 10.1016/j.febslet.2004.10.017. [DOI] [PubMed] [Google Scholar]; (m) Li H, Hadid D, Ragsdale DS. The batrachotoxin receptor on the voltage-gated sodium channel is guarded by the channel activation gate. Mol Pharmacol. 2002;61:905–912. doi: 10.1124/mol.61.4.905. [DOI] [PubMed] [Google Scholar]
- 4.Trainer VL, Brown GB, Catterall WA. Membranes and bioenergetics. J Biol Chem. 1996;271:11261–11267. doi: 10.1074/jbc.271.19.11261. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cellular Signaling. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]; (b) Catterall WA, Cestele S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]; (c) Stevens M, Peigneur S, Tytgat J. Neurotoxins and their biding areas on voltage-gated sodium channels. Front Pharmacol. 2011;2:1–13. doi: 10.3389/fphar.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai CX, Glaaser IW, Sawanobori T, Sunami A. Involvement of local anesthetic binding sites on IVS6 of sodium channels in fast and slow inactivation. Neurosci Lett. 2003;337:41–45. doi: 10.1016/s0304-3940(02)01288-0. [DOI] [PubMed] [Google Scholar]
- 7.For some leading references, see:; (a) de Lera Ruiz M, Kraus RL. Voltage-gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J Med Chem. 2015;58 doi: 10.1021/jm501981g. ASAP. [DOI] [PubMed] [Google Scholar]; (b) McCormack K, Santos S, Chapman ML, Krafte DS, Marron BE, West CW, Krambis MJ, Antonio BM, Zellmer SG, Printzenhoff D, Padilla KM, Lin Z, Wagoner PK, Swain NA, Stupple PA, de Groot M, Butt RP, Castle NA. Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. Proc Natl Acad Sci U S A. 2013;110:E2724–E2732. doi: 10.1073/pnas.1220844110. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nardi A, Damann N, Hertrampf T, Kless A. Advances in targeting voltage-gated sodium channels with small molecules. ChemMedChem. 2012;7:1712–1740. doi: 10.1002/cmdc.201200298. [DOI] [PubMed] [Google Scholar]; (d) Mantegazza M, Curia G, Biangini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–424. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- 8.(a) Marki F, Witkop B. The venom of the Colombian arrow poison frog Phyllobates bicolor. Experientia. 1963;19:329–338. doi: 10.1007/BF02152303. [DOI] [PubMed] [Google Scholar]; (b) Daly JW, Witkop B, Bommer P, Biemann K. Batrachotoxin. The active principle of the Colombian Arrow Poison Frog, Phyllobates bicolor. J Am Chem Soc. 1965;87:124–126. doi: 10.1021/ja01079a026. [DOI] [PubMed] [Google Scholar]; (c) Daly J, Witkop B. Batrachotoxin, an extremely active cardio- and neurotoxin from the Colombian arrow poison frog Phyllobates aurotaenia. Clin Toxicol. 1971;4:331–342. doi: 10.3109/15563657108990484. [DOI] [PubMed] [Google Scholar]; (d) Tokuyama T, Daly JW, Witkop B. Structure of batrachotoxin, a steroidal alkaloid from the Colombian arrow poison frog, phyllobates aurotaenia, and partial synthesis of batrachotoxin and its analogs and homologs. J Am Chem Soc. 1969;91:3931–3938. doi: 10.1021/ja01042a042. [DOI] [PubMed] [Google Scholar]; (e) Daly JW, Myers CW, Warnick JE, Albuquerque EX. Levels of batrachotoxin and lack of sensitivity to its action in poison-dart frogs (Phyllobates) Science. 1980;208:1383–1385. doi: 10.1126/science.6246586. [DOI] [PubMed] [Google Scholar]; (f) Tokuyama T, Daly J, Witkop B, Karle IL, Karle J. The structure of batrachotoxinin A, a novel steroidal alkaloid from the Colombian arrow poison frog, Pyllobates aurotaenia. J Am Chem Soc. 1968;90:1917–1918. doi: 10.1021/ja01009a052. [DOI] [PubMed] [Google Scholar]; (g) Karle IL, Karle J. The structural formula and crystal structure of the O-p-bromobenzoate derivative of batrachotoxinin A, C31H38NO6Br, a frog venom and steroidal alkaloid. Acta Crystallogr+, Sect B: Struct Crystallogr Cryst Chem. 1969;25:428–434. doi: 10.1107/s056774086900238x. [DOI] [PubMed] [Google Scholar]; (h) Gillardi RD. The absolute configuration of a steroidal substance, the O-p-bromobenzoate derivative of batrachotoxinin A. Acta Crystallogr+, Sect B: Struct Crystallogr Cryst Chem. 1970;26:440–441. [Google Scholar]
- 9.(a) Khodorov BI. Batrachotoxin as a tool to study voltage-sensitive sodium channels of excitable membranes. Prog Biophys Molec Biol. 1985;45:57–148. doi: 10.1016/0079-6107(85)90005-7. [DOI] [PubMed] [Google Scholar]; (b) Albuquerque EX, Daly JW, Witkop B. Batrachotoxin: Chemistry and pharmacology. Science. 1971;172:995–1002. doi: 10.1126/science.172.3987.995. [DOI] [PubMed] [Google Scholar]; (c) Brown GB. Batrachotoxin: A window on the allosteric nature of the voltage-sensitive sodium channel. Int Rev of Neurobiol. 1988;29:77–116. doi: 10.1016/s0074-7742(08)60084-7. [DOI] [PubMed] [Google Scholar]; (d) Garraffo HM, Spande TF. Discovery of batrachotoxin: The launch of the frog alkaloid program at the NIH. Heterocycles. 2009;79:195–205. [Google Scholar]; (e) Catterall WA. Activation of the action potential Na+ ionophore by neurotoxins. An allosteric model. J Biol Chem. 1977;252:8669–8676. [PubMed] [Google Scholar]
- 10.(a) Correa AM, Latorre R, Bezanilla F. Ion permeation in normal and batrachotoxin-modified Na+ channels in the squid giant axon. J Gen Physiol. 1991;97:605–625. doi: 10.1085/jgp.97.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Quandt FN, Narahashi T. Modification of single Na+ channels by batrachotoxin. Proc Natl Acad Sci U S A. 1982;79:6732–6736. doi: 10.1073/pnas.79.21.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mozhayeva GN, Naumov AP, Khodorov BI. A study of properties of batrachotoxin modified sodium channels. Gen Physiol Biophys. 1986;5:17–46. [PubMed] [Google Scholar]; (d) Wang GK, Wang SY. Inactivation of batrachotoxin-modified Na+ channels in GH3 cells. Characterization and pharmacological modification. J Gen Physiol. 1992;99:1–20. doi: 10.1085/jgp.99.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wasserstrom JA, Liberty K, Kelly J, Santucci P, Myers M. Modification of cardiac Na+ channels by batrachotoxin: effects on gating, kinetics, and local anesthetic binding. Biophys J. 1993;65:386–395. doi: 10.1016/S0006-3495(93)81046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Tanguy J, Yeh JZ. Batrachotoxin uncouples gating charge immobilization from fast Na inactivation in squid giant axons. Biophys J. 1988;54:719–730. doi: 10.1016/S0006-3495(88)83007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Mozhaeva GN, Naumov AP, Khodorov BI. Activation and inactivation of batrachotoxin-modified sodium-channels in the frog nerve-fiber membrane. Neirofiziologiya. 1984;16:14–26. [PubMed] [Google Scholar]; (h) Zubov AN, Naumov AP, Khodorov BI. Effect of batrachotoxin (BTX) on activation, inactivation and ion selectivity of sodium channels in clonal neuroblastoma cells. Gen Physiol Biophys. 1983;2:75–77. [Google Scholar]; (i) Wang GK, Wang SY. Modification of cloned brain Na+ channels by batrachotoxin. Pflugers Arch. 1994;427:309–316. doi: 10.1007/BF00374539. [DOI] [PubMed] [Google Scholar]; (j) Khodorov BI, Revenko SV. Further analysis of the mechanisms of action of batrachotoxin on the membrane of myelinated nerve. Neuroscience. 1979;4:1315–1330. doi: 10.1016/0306-4522(79)90159-3. [DOI] [PubMed] [Google Scholar]; (k) Leon LD, Ragsdale DS. State-dependent access to the batrachotoxin receptor on the sodium channel. Neuroreport. 2003;14:1353–1356. doi: 10.1097/01.wnr.0000077552.91466.08. [DOI] [PubMed] [Google Scholar]
- 11.Devlin AS, Du Bois J. Modular synthesis of the pentacyclic core of batrachotoxin and select batrachotoxin analogue designs. Chem Sci. 2013;4:1059–1063. doi: 10.1039/C2SC21723F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nau C, Wang SY, Wang GK. Point mutations at L1280 in NaV1.4 channel D3-S6 modulate binding affinity and stereoselectivity of bupivacaine enantiomers. Mol Pharm. 2003;63:1398–1406. doi: 10.1124/mol.63.6.1398. [DOI] [PubMed] [Google Scholar]
- 13.Synthetic A/B ring analogues of BTX derived with a pendant ester group have been shown to competitively block the action of BTX, although these compounds do not inhibit Na+ current, see:; Schow SR, Rossignol DP, Lund AE, Schnee ME. Batrachotoxin binding site antagonists. Bioorg Med Chem Lett. 1997;7:181–186. [Google Scholar]; For studies with semi-synthetic derivatives of BTX, see:; (a) Khodorov BI, Yelin EA, Zaborovskaya LD, Maksudov MZ, Tikhomirova OB, Leonov VN. Comparative analysis of the effects of synthetic derivatives of batrachotoxin on sodium currents in frog node of Ranvier. Cell Mol Neurobiol. 1992;12:59–81. doi: 10.1007/BF00711639. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Warnick JE, Albuquerque EX, Onur R, Jansson S, Daly J, Tokuyama T, Witkop B. The pharmacology of batrachotoxin. VII. Structure-activity relationships and the effects of pH. J Pharmacol Exp Therap. 1975;193:232–245. [PubMed] [Google Scholar]; (c) Mozhayeva GN, Naumov AP, Nosyreva ED. Modification of Na channels by synthetic dihydrobatrachotoxinin A-20α-benzoate. Gen Physiol Biophys. 1986;5:153–158. [PubMed] [Google Scholar]; (d) Brown GB, Daly JW. Interaction of batrachotoxinin-A benzoate with voltage-sensitive sodium channels: The effects of pH. Cell Mol Neurobio. 1981;1:361–371. doi: 10.1007/BF00716271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown GB, Tieszen SC, Daly JW, Warnick JE, Albuquerque EX. Batrachotoxinin-A 20α-benzoate: A new radioactive ligand for voltage sensitive sodium channels. Cell Mol Neurobiol. 1981;1:19–40. doi: 10.1007/BF00736037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Du Y, Garden DP, Wang L, Zhorov BS, Dong K. Identification of new batrachotoxin-sensing residues in segment IIIS6 of the sodium channel. J Biol Chem. 2011;286:13151–13160. doi: 10.1074/jbc.M110.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sunami A, Tracey A, Glaaser IW, Lipkind GM, Hanck DA, Fozzard HA. Accessibility of mid-segment domain IV S6 residues of the voltage-gated Na+ channel to methanethiosulfonate regents. J Physiol. 2004;561:403–413. doi: 10.1113/jphysiol.2004.067579. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cronin NB, O’Reilly A, Duclohier H, Wallace BA. Binding of the anticonvulsant drug lamotrigine and the neurotoxin batrachotoxin to voltage-gated sodium channels induces conformational changes associated with block and steady-state activation. J Biol Chem. 2003;278:10675–10682. doi: 10.1074/jbc.M208356200. [DOI] [PubMed] [Google Scholar]
- 16.Kurosu M, Marcin LR, Grinsteiner TJ, Kishi Y. Total synthesis of (±)-batrachotoxinin A. J Am Chem Soc. 1998;120:6627–6628. [Google Scholar]
- 17.(a) Shiina I, Ibuka R, Kubota M. A new condensation reaction for the synthesis of carboxylic esters from nearly equimolar amounts of carboxylic acids and alcohols using 2-methyl-6-nitrobenzoic anhydride. Chem Lett. 2002;31:286–287. [Google Scholar]; (b) Shiina I, Kubota M, Ibuka R. A novel and efficient macrolactonization of ω-hydroxycarboxylic acids using 2-methyl-6-nitrobenzoic anhydride (MNBA) Tetrahedron Lett. 2002;43:7535–7539. [Google Scholar]; (c) Shiina I, Kubota M, Osumi H, Hashizume M. An effective use of benzoic anhydride and its derivatives for the synthesis of carboxylic esters and lactones: A powerful and convenient mixed anhydride method promoted by basic catalysts. J Org Chem. 2004;69:1822–1830. doi: 10.1021/jo030367x. [DOI] [PubMed] [Google Scholar]; (d) Shiina I, Fukui H, Sasaki A. Synthesis of lactones using substituted benzoic anhydride as a coupling reagent. Nat Protoc. 2007;2:2312–2317. doi: 10.1038/nprot.2007.316. [DOI] [PubMed] [Google Scholar]
- 18.We have examined the stability of the ester moiety in 1b in buffered solution at pH 7.4 and have found no evidence of hydrolysis after 24 hours.
- 19.Wang GK, Wang SY. Veratridine block of rat skeletal muscle NaV1.4 sodium channels in the inner vestibule. J Physiol. 2003;548:667–675. doi: 10.1113/jphysiol.2002.035469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Docking experiments were performed with OMEGA, version 2.4.6 and OEDocking, version 3.0.1 (OpenEye Scientific Software, Santa Fe, NM, http://www.eyesopen.com).; (a) Hawkins PCD, Skillman AG, Warren GL, Ellingson BA, Stahl MT. Conformer generation with OMEGA: Algorithm and validation using high quality structures from the Protein Databank and Cambridge Structural Database. J Chem Inf Model. 2010;50:572–584. doi: 10.1021/ci100031x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hawkins PCD, Nicholls A. Conformer generation with OMEGA: Learning from the data set and analysis of failures. J Chem Inf Model. 2012;52:2919–2936. doi: 10.1021/ci300314k. [DOI] [PubMed] [Google Scholar]; (c) McGann M. FRED pose prediction and virtual screening accuracy. J Chem Inf Model. 2011;51:578–596. doi: 10.1021/ci100436p. [DOI] [PubMed] [Google Scholar]
- 21.Homology model was built by Modeller, version 9.11 (Accelrys, Inc., San Diego, CA, http://accelrys.com).; Sali A, Blundell TL. Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 22.Inhibition constants, Ki, of the naphthoate derivative, 1b, have been determined against l-type Ca2+ (rat) and hERG channels at 4.5 and 3.2 μM, respectively These data were obtained by the National Institute of Mental Health’s Psychoactive Drug Screening Program at the University of North Carolina, Chapel Hill, see: https://pdspdb.unc.edu/pdspWeb/for experimental details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


