Abstract
Purpose
Renal cell carcinoma is refractory to conventional radiation therapy but responds to higher doses per fraction. However, the dosimetric data and clinical factors affecting local control (LC) are largely unknown. We aimed to evaluate the safety and efficacy of stereotactic ablative radiation therapy (SAbR) for extracranial renal cell carcinoma metastases.
Methods and Materials
We reviewed 175 metastatic lesions from 84 patients treated with SAbR between 2005 and 2015. LC and toxicity after SAbR were assessed with Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Predictors of local failure were analyzed with χ2, Kaplan-Meier, and log-rank tests.
Results
In most cases (74%), SAbR was delivered with total doses of 40 to 60 Gy, 30 to 54 Gy, and 20 to 40 Gy in 5 fractions, 3 fractions, and a single fraction, respectively. The median biologically effective dose (BED) using the universal survival model was 134.5 Gy. The 1-year LC rate after SAbR was 91.2% (95% confidence interval, 84.9%–95.0%; median follow-up, 16.7 months). Local failures were associated with prior radiation therapy (hazard ratio [HR], 10.49; P<.0001), palliative-intent radiation therapy (HR, 4.63; P=.0189), spinal location (HR, 5.36; P=.0041), previous systemic therapy status (0–1 vs >1; HR, 3.52; P=.0217), and BED <115 Gy (HR, 3.45; P=.0254). Dose received by 99% of the target volume was the strongest dosimetric predictor for LC. Upon multivariate analysis, dose received by 99% of the target volume greater than BED of 98.7 Gy and systemic therapy status remained significant (HR, 0.12 and 3.64, with P=.0014 and P=.0472, respectively). Acute and late grade 3 toxicities attributed to SAbR were observed in 3 patients (1.7%) and 5 patients (2.9%), respectively.
Conclusions
SAbR demonstrated excellent LC of metastatic renal cell carcinoma with a favorable safety profile when an adequate dose and coverage were applied. Multimodality treatment with surgery should be considered for reirradiation or vertebral metastasis. A higher radiation dose may be required in patients who received previous systemic therapies.
Introduction
The standard of care for metastatic renal cell carcinoma (mRCC) is systemic therapy, whereas local treatment still remains controversial. A surgical series suggested that selected patients receiving curative-intent metastasectomy survived with a longer disease-free interval compared with patients who had not received it (1). Another series showed improved survival after resections of multiple limited metastases (2). The Mayo Clinic reviewed 887 mRCC patients and found improved cancer-specific survival with complete metastasectomy compared with less aggressive surgery, especially for pulmonary metastases (3). These findings suggested that selected patients with oligometastatic mRCC disease (4) can survive longer with improved quality of life when treated with complete metastasectomy (5).
Radiation therapy has historically been used for palliative purposes, and its practice in renal cell carcinoma (RCC) has been limited by perceived (radioresistance) to conventional fractionation (6). Radioresistance may be overcome with dose escalation, particularly by increasing the dose per fraction (7), as suggested by studies in preclinical models of human RCC xenografts (8). Stereotactic ablative radiation therapy (SAbR), or stereotactic body radiation therapy, has shown encouraging efficacy in mRCC (9, 10). SAbR relies on sophisticated image guidance and immobilization devices to deliver highly conformal ablative radiation doses to the tumor while sparing surrounding organs. However, factors associated with failure in mRCC are poorly understood. We report the safety and efficacy of SAbR and highlight its limitations for extracranial mRCC.
Methods and Materials
Patients
We retrospectively reviewed patients with extracranial mRCC treated with SAbR between 2005 and 2015 at our institution with >2 months’ follow-up (to obtain a scan to assess efficacy). Conventional fractionated radiation therapy and intracranial lesions were excluded. Pathologic confirmation was required to establish metastases. Patients treated with SAbR were divided into 2 radiation therapy intent categories: “curative,” in which all progressing lesions were treated, and “palliative,” in which only limited symptomatic disease was treated.
Treatment
Computed tomography (CT) simulation was conducted after patients were immobilized in a body frame and vacuum body bag for radiation therapy planning. Images were acquired with 4-dimensional CT if internal motion was suspected; abdominal compression was applied when appropriate. Gross tumors were identified by CT or magnetic resonance imaging and co-registered with a treatment planning scan. A 5-mm margin for the planning target volume (PTV) was typically applied to gross disease or internal target volume. Stereotactic planning was set to a single isocenter by use of multiple beams or a volumetric arc with multileaf collimators to provide adequate conformality and at least 95% PTV coverage. SAbR (8–60 Gy) was delivered in 1 to 5 fractions (Table E1, available online at www.redjournal.org) with cone beam CT guidance and with at least 36-hour intervals between fractions. The treating radiation oncologist selected the radiation therapy regimen, including dose, number of fractions, and interval between irradiations, using patient-specific characteristics and tumor-specific factors, as well as our institutional planning constraints (Table E2, available online at www.redjournal.org). The biologically effective dose (BED) was calculated by use of the A498 human RCC cell line parameters (a/b ratio, 2.63; final slope of the survival curve in Gy, 1.04; x-intercept of survival curve on multitarget model in Gy, 3.00; and transition dose at which linear quadratic model transitioned to multitarget model in Gy, 7.12) (11) applied to the universal survival model (12).
Outcome measures
Local control (LC) of each lesion was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Prognostic grouping was determined according to the International Metastatic Renal Cell Carcinoma Database Consortium model (13, 14). Toxicity was evaluated with Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
Categorical variables, including prognostic criteria, were analyzed by the χ2 contingency test. Continuous variables, such as age and BED, were compared by the t test. The Kaplan-Meier method was used to estimate LC. Log-rank tests were used to evaluate differences in LC by categorical parameters. Univariate analysis for each dosimetric factor as a continuous variable was also performed separately to ensure validity of the interpretation (Table E3, available online at www.redjournal.org). Optimal threshold cutoffs for continuous dosimetric parameters were determined by examining the deciles for the best model fit according to the score χ2 statistics. After univariate analysis of clinical factors, the Cox proportional hazards model was applied for multivariate analysis using all significant covariables to calculate the adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) as independent predictors of survival. All analyses were completed at a .05 two-sided significance level using SAS software (version 9.4; SAS Institute).
Results
Tumor characteristics and radiation therapy regimens
Lesions (N=175) were identified from 84 patients treated over 124 SAbR sessions (Table 1). Most patients (72.6%) had localized disease at initial presentation but later had metastases develop. Most lesions (90%) were detected in patients with favorable or intermediate International Metastatic Renal Cell Carcinoma Database Consortium prognosis groups at the time of diagnosis with metastatic disease. The median dose per fraction was 11 Gy, and the median fraction number was 3. The median target volume was 26.55 cm3 (range, 0.19–557 cm3). The median volume covered by the prescription dose was 95% of target volume (67.6–100). The median BED was 134.54 Gy, corresponding to 24 Gy ×1 fraction, 10 Gy ×3 fractions, or 7.2 Gy ×5 fractions. Lesions receiving curative-intent irradiation had a higher median dose than lesions treated with palliative intent (108.95 Gy vs 160.12 Gy, P<.0001). Curative-intent irradiation was also more commonly administered to patients with ≤3 lesions at the time of SAbR (86.0% vs 58.5%, P=.005). Curative-intent irradiation was more commonly delivered in older patients (median, 66.6 years vs 60.0 years), patients with a better performance status (Karnofsky Performance Status ≥80), patients with localized disease at initial diagnosis, and patients with time from diagnosis to SAbR >1 year (Table 1). Lesions with a higher grade at initial presentation, poor prognostic grouping, more advanced systemic treatment, and a spinal location were more likely to be treated with palliative-intent radiation therapy (Table 1). Tumor size did not differ between curative- and palliative-intent SAbR.
Table 1.
Baseline lesion characteristics and treatment parameters
| Characteristic | Total (N=175) | Curative (n=75) | Palliative (n=100) | P value |
|---|---|---|---|---|
| Tumor volume, median (range), cm3 | 12.95 (0.10–769.0) | 11.32 (0.60–769.0) | 14.62 (0.10–265.5) | .9465 |
| Histology | ||||
| Clear cell | 141 (80.6%) | 54 (72.0%) | 87 (87.0%) | .0316 |
| Papillary | 18 (10.3%) | 12 (16.0%) | 6 (6.0%) | |
| Other | 8 (4.6%) | 6 (8.0%) | 2 (2.0%) | |
| Unavailable | 8 (4.6%) | 3 (4.0%) | 5 (5.0%) | |
| Grade | ||||
| 1 | 7 (4.0%) | 5 (6.7%) | 2 (2.0%) | .0273 |
| 2 | 17 (9.7%) | 6 (8.0%) | 11 (11.0%) | |
| 3 | 85 (48.6%) | 28 (37.3%) | 57 (57.0%) | |
| 4 | 44 (25.1%) | 22 (29.3%) | 22 (22.0%) | |
| Unavailable | 22 (12.6%) | 14 (18.7%) | 8 (8.0%) | |
| Performance status | ||||
| ≥80 | 158 (90.3%) | 73 (97.3%) | 85 (85.0%) | .0064 |
| <80 | 17 (9.7%) | 2 (2.7%) | 15 (15.0%) | |
| Initial M stage | ||||
| M0 | 135 (77.1%) | 65 (86.7%) | 70 (70.0%) | .0273 |
| M1 | 40 (22.9%) | 10 (13.3%) | 30 (30.0%) | |
| Time from Dx to SAbR | ||||
| ≥1 y | 121 (69.1%) | 53 (70.7%) | 62 (62.0%) | .0253 |
| <1 y | 54 (30.9%) | 17 (22.7%) | 37 (37.0%) | |
| Undetermined | 6 (3.4%) | 5 (6.7%) | 1 (1.0%) | |
| Prognostic group at SAbR | ||||
| Favorable (0) | 35 (20.0%) | 25 (33.3%) | 10 (10.0%) | <.0001 |
| Intermediate (1, 2) | 123 (70.3%) | 43 (57.3%) | 80 (80.0%) | |
| Unfavorable (3–6) | 7 (4.0%) | 0 (0.0%) | 7 (7.0%) | |
| Incomplete | 10 (5.7%) | 7 (9.3%) | 3 (3.0%) | |
| Location | ||||
| Bone | <.0001 | |||
| Spine | 42 (24.0%) | 6 (8.0%) | 36 (36.0%) | |
| Nonspine | 25 (14.3%) | 9 (12.0%) | 16 (16.0%) | |
| Thorax | 35 (20.0%) | 18 (24.0%) | 17 (17.0%) | |
| Abdomen | 49 (28.0%) | 36 (48.0%) | 13 (13.0%) | |
| Kidney | 5 (2.9%) | 4 (5.3%) | 1 (1.0%) | |
| Soft tissue | 16 (9.1%) | 2 (2.7%) | 14 (14.0%) | |
| Spinal cord | 3 (1.7%) | 0 (0.0%) | 3 (3.0%) | |
| BED, median (range), Gy | 134.5 (32.0–288.4) | 160.1 (72.5–288.4) | 109.0 (32.0–230.7) | <.0001 |
| Prior RT status | ||||
| Reirradiation | 8 (4.6%) | 1 (1.3%) | 7 (7.0%) | .0757 |
| No prior RT | 167 (95.4%) | 74 (98.7%) | 93 (93.0%) | |
| Systemic therapy status | ||||
| No prior therapy | 83 (47.4%) | 40 (53.3%) | 43 (43.0%) | .0248 |
| First line | 32 (18.3%) | 16 (21.3%) | 16 (16.0%) | |
| Second line | 25 (14.29%) | 12 (16.0%) | 13 (13.0%) | |
| More than second line | 35 (20.0%) | 7 (9.3%) | 28 (28.0%) | |
| Type of systemic agent | ||||
| None | 83 (47.4%) | 40 (53.3%) | 43 (43.0%) | .2422 |
| VEGF pathway | 64 (36.6%) | 23 (30.7%) | 41 (41.0%) | |
| mTOR pathway | 24 (13.7%) | 9 (12.0%) | 15 (15.0%) | |
| Other or unknown | 4 (2.3%) | 3 (4.0%) | 1 (1.0%) |
Abbreviations: BED = biologically equivalent dose; Dx = diagnosis; mTOR = mammalian target of rapamycin; RT = radiation therapy; SAbR = stereotactic ablative radiation therapy; VEGF = vascular endothelial growth factor.
LC and dosimetric analysis
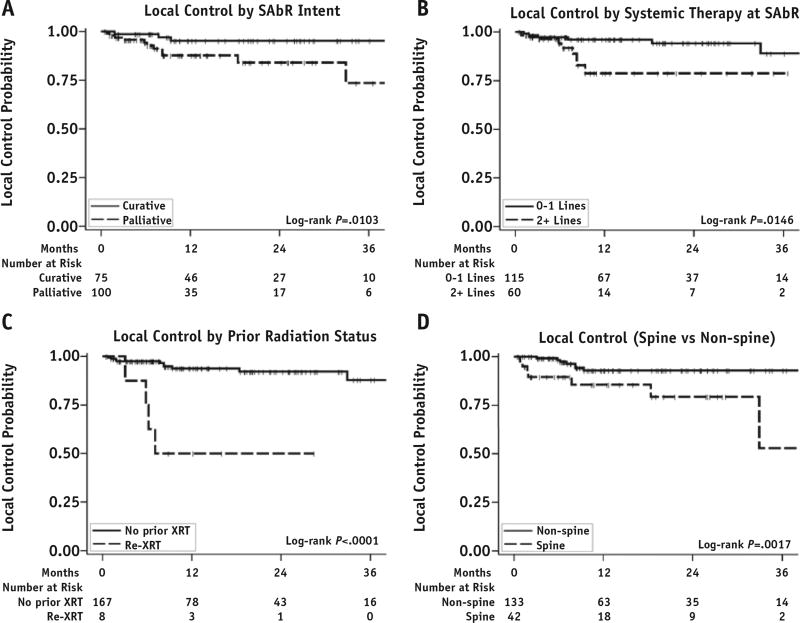
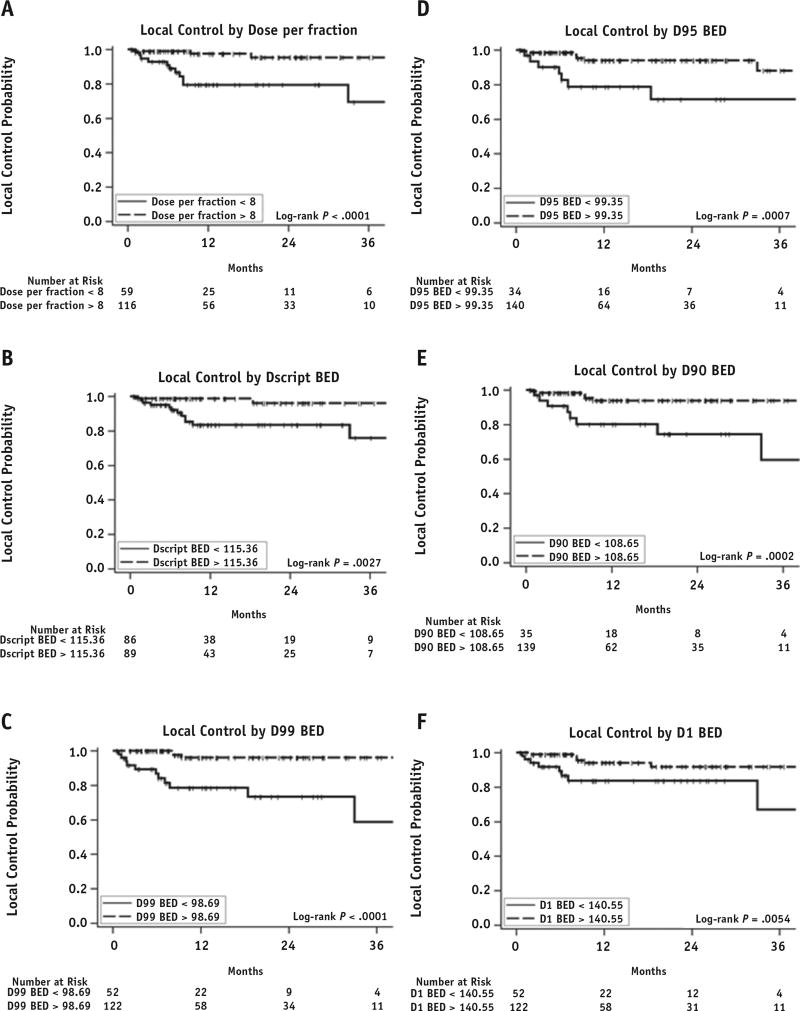
The median follow-up period after treatment based on available imaging studies was 10.5 months for lesions and 16.7 months for individual patients. The 1-year LC rate was 91.2% (95% CI, 84.9%–95.0%). The local failure (LF) rate was conservatively overestimated without accounting for competing-risk analysis. We maintained excellent follow-up in >90% of treated lesions and tracked LC independently of competing events at other sites. LF occurred in 15 lesions (8.6%), with a median time to failure of 7.1 months after SAbR. Upon univariate analysis, LFs were associated with the lesions that had prior irradiation (HR, 10.49; P=.0001), were treated with palliative-intent radiation therapy (HR, 4.63; P=.0189), had a spinal location (HR, 5.36; P=.0041), and received >2 lines of systemic therapy prior to irradiation (HR, 3.52; P=.0217) (Fig. 1). Dosimetric data for individual lesions were analyzed for their effect on LC. The median and range of prescription dose, maximum dose to 1% of the target volume, minimum dose to 99% of the target volume (D99), minimum dose to 95% of the target volume (D95), and minimum dose to 90% of the target volume were converted to BED values and are reported in Table 2. Dose per fraction, prescription dose, D99, D95, minimum dose to 90% of the target volume, and maximum dose to 1% of the target volume were all significantly associated with LC upon univariate analysis (Fig. 2). The number of fractions did not affect LC (P=.1276). Each of these parameters was further analyzed to identify threshold doses predicting LF (Table 2). The controlled lesions exhibited a significantly higher BED than the lesions that failed (median BED, 134.5 Gy vs 98.4 Gy; P=.0012). Because all dosimetric measures were highly correlated with each other and each dosimetric measurement could also be related to the dose per fraction at any given fraction, only D99 (which had the strongest univariate association with LC) and clinical factors were entered into the multivariate model. Upon multivariate analysis, D99 >98.69 Gy and use of >1 systemic therapy were independent predictors of LC (Table 2). Of 8 reirradiated lesions, 4 showed LF. The BED to lesions with prior irradiation was significantly lower than the BED to lesions without it (median BED, 50.4 Gy vs 134.5 Gy; P<.0001). The difference in fractionation was not associated with LF (1-year LC rate, 96.7% [95% CI, 87.0%–99.1%] and 87.7% [95% CI, 78.2%–93.3%] for single fraction and multifraction, respectively; P=.1070). Among the lesions that failed, 3 were treated with 1 fraction, 1 with 3 fractions, 1 with 4 fractions, and the remaining with 5 fractions. Of 15 lesions, 11 were identified in osseous sites, mostly involving the spine (8 lesions). The 1-year LC rates were 85.5% (95% CI, 67.9%–93.8%) and 92.9% (95% CI, 85.6%–96.6%) for spinal and nonspinal metastasis, respectively (P=.0017). Of 15 patients with LF, 13 also showed systemic progression.
Fig. 1.
Local control of stereotactic ablative radiation therapy (SAbR) separated by radiation therapy intent, lines of systemic therapy, prior radiation therapy status, and location. (A) The 1-year local control rates were 95.2% and 87.7% for curative-intent (eg, all lesions treated) and palliative-intent radiation therapy, respectively (P=.0103). (B) The 1-year local control rates were 96.2% and 78.8% for 0 to 1 line of systemic therapy and >2 lines of systemic therapy, respectively (P=.0146). (C) The 1-year local control rates were 93.8% and 50.0% for no prior radiation therapy (XRT) and reirradiation (Re-XRT), respectively (P<.0001). (D) The 1-year local control rates were 85.5% and 94.5% for spinal and non-spinal sites, respectively (P=.0013).
Table 2.
Univariate and multivariate analysis of local control
| Univariate analysis
|
Multivariate analysis
|
|||||
|---|---|---|---|---|---|---|
| Local control | Median (range) | Cutoff | HR (95% CI) | P value | HR (95% CI) | P value |
| Dosimetric factors, Gy | ||||||
| Dose per fraction | 11 (4–40) | 8 | 0.12 (0.03–0.43) | .0014 | ||
| Multifraction vs single fraction | 3 (1–5) | NA | 0.37 (0.11–1.33) | .1276 | ||
| Dscript BED | 134.54 (32–288.38) | 115.36 | 0.29 (0.10–0.86) | .0254 | ||
| D99 BED | 114.98 (0.42–264.22) | 98.69 | 0.09 (0.03–0.32) | .0002 | 0.12 (0.03–0.43) | .0014 |
| D95 BED | 134.54 (32–289.73) | 99.35 | 0.20 (0.07–0.56) | .0022 | ||
| D90 BED | 141.29 (33.63–313.97) | 108.65 | 0.17 (0.06–0.49) | .0010 | ||
| D1 BED | 173.93 (54.52–512.69) | 140.55 | 0.26 (0.09–0.72) | .0099 | ||
| Clinical factors | ||||||
| Re-treated vs first treatment | 10.49 (3.20–34.38) | .0001 | 1.63 (0.38–6.96) | .5122 | ||
| Palliative vs curative intent | 4.63 (1.29–16.68) | .0189 | 1.51 (0.36–6.33) | .5750 | ||
| Spinal vs nonspinal | 5.36 (1.70–16.90) | .0041 | 1.51 (0.45–5.05) | .5026 | ||
| ≥2 lines vs 0–1 lines | 3.52 (1.20–10.32) | .0217 | 3.64 (1.02–13.00) | .0472 | ||
Abbreviations: CI = confidence interval; D1 = maximum dose to 1% of target volume; D90 = minimum dose to 90% of target volume; D95 = minimum dose to 95% of target volume; D99 = minimum dose to 99% of target volume; Dscript = prescription dose; HR = hazard ratio; NA = not applicable.
Fig. 2.
Dosimetric analysis for local control. Abbreviations: BED=biologically equivalent dose; D1=maximum dose to 1% of target volume; D90 = minimum dose to 90% of target volume; D95 = minimum dose to 95% of target volume; D99 = minimum dose to 99% of target volume; Dscript = prescription dose.
Toxicity
Most lesions (98.9%) were assessed for toxicity, with a median follow-up time of 16.4 months. Eighteen treatments (10.4%) of grades between 1 and 2 (15 of 18) were associated with acute toxicity within 3 months. Of the 3 patients with acute grade 3 toxicity, 1 was admitted to the hospital for a urinary tract infection caused by Pseudomonas aeruginosa after treatment of a kidney lesion, while 2 had progressive pain requiring inpatient pain control after treatment of spinal lesions (L2 and T11, respectively). It is unclear whether these acute grade 3 toxicities were related to SAbR. Late toxicities were uncommon (4.5% for all grades and 1.9% for grade 3 or higher at 1 year), with a median time to toxicity development of 5.2 months (range, 2.1–25.1 months); of these, 8 were grade 1 or 2 and 5 were grade 3. Grade 3 late toxicities included 2 cases of gastrointestinal bleeding requiring surgical intervention and transfusion upon resuming targeted therapies, 2 compression fractures requiring kyphoplasty, and 1 persistent debilitating radiculopathy (Table E4, available online at www.redjournal.org).
Discussion
We conducted a detailed analysis of our institutional SAbR experience for extracranial mRCC and showed satisfactory LC rates, confirming SAbR’s safety and efficacy as a local therapy. More than half of the analyzed lesions were located in regions with significant internal motion (eg, chest, abdomen, or kidney). Despite the internal motion, adequate LC was achieved, indicating that modern radiation therapy techniques can accurately treat moving targets. A recent experience from the University of Colorado showed that SAbR leads to better radiologic and symptomatic LC compared with conventional fractionation (9). In a series of 105 mRCC lesions, Memorial Sloan Kettering Cancer Center reported a 3-year LF-free survival rate of 88% in patients who had received 24 Gy ×1 fraction but only 17% to 21% for hypofractionated SAbR, despite a lower BED in the hypofractionated arm (10).
In our series, the most important factor predicting LC was D99 BED. This suggests that LC of mRCC by SAbR can be achieved by an adequate radiation dose and tumor coverage. A lower BED was associated with reirradiation, palliative-intent radiation therapy, and a spinal location, which led to the compromised SAbR efficacy observed in those cohorts. Of the 175 lesions, 15 failed locally following SAbR (Table 3). The median prescription BED for the controlled lesions was significantly higher than that for the lesions that failed. Eight failed lesions had received inadequate palliative doses (4–6 Gy ×5 fractions and 8 Gy ×1 fraction). A lung lesion that progressed after receiving 10 Gy ×3 fractions was >10 cm in diameter. Tumor size is a known predictor of LF following SAbR (15); larger tumors are generally more difficult to control by radiation therapy because of the presence of more radioresistant hypoxic cells and smaller fractions of proliferating cells (16), suggesting that a higher radiation dose may be needed (17). The optimal threshold cutoff from dosimetric analysis indicated D99 BED >98.69 Gy, which is equivalent to 18 Gy ×1 fraction, 8 Gy ×3 fractions, and 6 Gy ×5 fractions. Although this dose is likely an underestimate because of the short follow-up, D99 requires nearly complete tumor coverage, making it a challenging dosimetric constraint to achieve in clinical practice. We performed a correlation analysis showing that there was a strong association between D99 and D95 (R2=0.90 with P<.0001 by analysis of variance; Fig. E1, available online at www.redjournal.org). In our study, the median BED that was prescribed for lesions without LF was 134 Gy, which lies within the 95% CI when correlated to D99 (Fig. E1, available online at www.redjournal.org). Therefore, we recommend at least 24 Gy ×1 fraction, 12 Gy ×3 fractions, or 8 Gy ×5 fractions with adequate (>95%) target coverage while trying to achieve at least D99 BED >100 Gy to provide sufficient LC for most mRCC lesions. These regimens are supported by other series (10, 18, 19). We have often found it easier to achieve this dose distribution using an integrated boost technique with a higher dose prescription to the gross tumor volume (BED >134 Gy with 95% coverage) compared with the clinical target volume or PTV (BED >100 Gy with 99% coverage). An interesting finding of this study was that patients who received >1 line of systemic therapy exhibited a higher risk of LF that was independent of the D99 BED cutoff of 98.69 Gy upon multivariate analysis (Table 2). This suggests a selection of more therapy-resistant disease in patients in whom previous systemic therapy failed and who may require a higher dose to reach adequate LC.
Table 3.
Characteristics of 15 lesions with local progression after SAbR
| Lesion | Tx site | Site description | Regimen | BED by USC | BED by LQ | Time from Dx to SBRT |
Prognostic group |
|---|---|---|---|---|---|---|---|
| 1 | Osseous, nonspine | Sacrum | 8 Gy × 1 fx | 32.02 | 32.33 | >1 y | Intermediate |
| 2 | Osseous, spine | T9 spine | 20 Gy × 1 fx | 108.95 | 172.09 | >1 y | Intermediate |
| 3 | Osseous, spine | T10 spine | 20 Gy × 1 fx | 108.95 | 172.09 | >1 y | Intermediate |
| 4 | Thorax | Lung RUL | 10 Gy × 3 fx | 134.54 | 144.07 | >1 y | Intermediate |
| 5 | Osseous, spine | T5 spine | 4 Gy × 4 fx | 40.32 | 40.33 | <1 y | Unfavorable |
| 6 | Osseous, nonspine | L mandible | 4 Gy × 5 fx | 50.40 | 50.42 | >1 y | Favorable |
| 7 | Soft tissue | T9 paraspinal | 4.5 Gy × 5 fx | 60.98 | 61.00 | >1 y | Intermediate |
| 8 | Osseous, nonspine | Sacrum | 5 Gy × 5 fx | 72.50 | 72.53 | >1 y | Intermediate |
| 9 | Osseous, spine | T9 spine | 5 Gy × 5 fx | 72.50 | 72.53 | >1 y | Favorable |
| 10 | Osseous, spine | T12 spine | 6 Gy × 5 fx | 98.40 | 98.44 | <1 y | Intermediate |
| 11 | Osseous, spine | T4 spine | 6 Gy × 5 fx | 98.40 | 98.44 | <1 y | Intermediate |
| 12 | Osseous, spine | T7-T8 spine | 8 Gy × 5 fx | 160.12 | 161.67 | >1 y | Intermediate |
| 13 | Soft tissue | R neck | 8 Gy × 5 fx | 160.12 | 161.67 | >1 y | Intermediate |
| 14 | Soft tissue | Occipital | 8 Gy × 5 fx | 160.12 | 161.67 | >1 y | Intermediate |
| 15 | Osseous, spine | L1-L2 spine | 8 Gy × 5 fx | 160.12 | 161.67 | >1 y | Intermediate |
| Systemic therapy |
Prior irradiation |
All lesions Tx’d at SAbR |
Systemic disease progression |
Additional information |
|---|---|---|---|---|
| ≥2 | Yes | No | Yes | Palliative dose |
| 1 | No | No | Yes | Inadequate coverage (invading spinal canal) |
| ≥2 | No | No | Yes | Inadequate coverage (proximity to cord) |
| 2 | No | Yes | No | Large tumor size |
| 0 | No | No | Yes | Palliative dose |
| ≥2 | Yes | No | Yes | Palliative dose |
| 1 | Yes | No | Yes | Palliative dose |
| ≥2 | Yes | No | Yes | Palliative dose |
| 1 | No | No | Yes | Palliative dose |
| 0 | No | No | Yes | Palliative dose |
| 1 | No | No | Yes | Palliative dose |
| 0 | No | Yes | Yes | Inadequate coverage (invading spinal canal) |
| 2 | No | No | Yes | Inadequate coverage (reduced PTV margin), ≥10 lesions |
| 2 | No | No | Yes | Inadequate coverage (reduced PTV margin), ≥10 lesions |
| 2 | No | Yes | No | Inadequate coverage (overlapping prior irradiation field) |
Abbreviations: BED = biologically equivalent dose; Dx = diagnosis; fx = fraction; L = left; LQ = linear quadratic model; PTV = planning target volume; R = Right; RUL = right upper lobe; SAbR = stereotactic ablative radiation therapy; SBRT = stereotactic body radiation therapy; Tx = treatment; Tx’d = treated; USC = universal survival model.
Dosimetric evaluations of the radiation planning were performed on all lesions that had failed locally, showing an inadequate dose for reirradiated sites (median BED of 50.4 Gy) and insufficient PTV coverage for several spinal lesions (Table 3); these factors likely contributed to the failures in these settings. However, LF may be detrimental for lesions located in critical locations, such as the spine. A series from MD Anderson using SAbR to treat 40 spinal lesions in 37 RCC patients showed an increased risk of death in patients with LF, even after correction for other competing factors including performance status, neurologic deficits, and systemic disease status (20). Therefore, the risks of LF will have to be carefully balanced with the risks of overdosing the spinal cord. The Princess Margaret Cancer Centre reported a comparable 1-year LC rate of 83% in 71 spinal lesions in 37 patients after SAbR, with significant portions of reirradiation (15%) and postoperative treatment (14%) (21). Although most patients were treated with 18 to 24 Gy ×1 fraction, those treated with hypofractionation received lower doses (8–9 Gy ×3 fractions and 6–7 Gy ×5 fractions) that may have contributed to poor LC. Although recurring spinal tumors have been treated with aggressive SAbR regimens with acceptable LC (22), these 2 settings of reirradiation and spinal location require a more aggressive treatment regimen in the form of a higher BED, surgical intervention, or radiosensitization. Surgical intervention should be considered over SAbR whenever target underdosing arises as a possibility given the proximity of the lesion to critical radiosensitive organs such as the spinal cord. Multimodality evaluations including surgery, radiation therapy, prophylactic vertebroplasty, and other local therapies are required to select the optimal approach for these patients.
In our series, late toxicities were low and <3% were high grade. Gastrointestinal bleeding developed in 2 of the patients, requiring surgical intervention, while another presented with persistent debilitating radiculopathy after resuming systemic therapy. A careful review of the treatment plan according to our institutional normal tissue constraints (Table E2, available online at www.redjournal.org) was performed. Dosimetric data for patients having grade 3 or higher late toxicities are included in Table E4 (available online at www.redjournal.org). A patient with a tumor involving the L2-L3 foramina received an excessive dose to the nerve root and had late grade 3 radiculopathy. A second patient, with a vertebral compression fracture, had a tumor involving >40% of the vertebral body, which is a known risk factor for compression fracture after SAbR (23). Another patient, with mild vertebral body height loss of the treated site, received vertebroplasty to multiple levels at the time of systemic disease progression. Two patients who were undergoing vascular endothelial growth factor/tyrosine kinase inhibitor targeted therapy had grade 3 gastrointestinal bleeding, in which the maximal point dose to the stomach exceeded the planning constraints. Although it was unclear whether the location of the bleed correlated to the region receiving the maximum point dose in these patients, vascular endothelial growth factor targeted therapy, such as axitinib, has been shown to sensitize the radiation effect to high single-dose radiation therapy, likely via endothelial apoptosis (24). As targeted therapy becomes more common in the management of mRCC, and particularly when considering SAbR for oligo-progressive lesions, the interaction of SAbR with targeted therapy should be taken into account. If this type of interaction is suspected, systemic therapy can be withheld for a few days before and after SAbR (depending on the clearance of the drug), or SAbR can be delivered during the scheduled breaks in systemic therapy (eg, sunitinib). More safety data on the combination are clearly required.
Limitations of this study include its retrospective nature and the relatively short follow-up. The retrospective nature likely led to a selection bias wherein patients with unfavorable metastatic locations (ie, near the bowel or spinal cord) were less likely selected for SAbR. Short follow-up periods typically overestimate the LC and underestimate toxicity. With the approval of more effective systemic therapies (eg, immunotherapy) leading to improved survival of mRCC patients, long-term toxicity and SAbR LC will have to be evaluated, and the dose adequacy of the recommended SAbR regimens will need to be reassessed.
Conclusions
SAbR exerts favorable LC with minimum acute and late complications and should be considered a treatment modality in selected RCC patients with limited metastases. No failures were observed when SAbR regimens of 24 Gy in 1 fraction, 12 Gy in 3 fractions, or 8 Gy in 5 fractions were used with ≥95% PTV coverage. These regimens may have been underestimated, as longer follow-up periods may result in additional failures. Lesions in patients in whom systemic therapy failed may require a higher dose. The most critical factors affecting LC of mRCC after SAbR are adequate radiation dose and appropriate target coverage. Spinal lesions or lesions that had received prior irradiation, in which the proximity to critical organs may compromise adequate radiation delivery, require multimodality management in which surgery may be preferred over SAbR.
Supplementary Material
Summary.
Stereotactic ablative radiation therapy demonstrated excellent local control with a favorable toxicity profile for metastatic renal cell carcinoma when an adequate dose and coverage were applied. Radiation dose received by 99% of the target volume greater than 98.7 Gy was the strongest dosimetric predictor for local control. Multiple systemic therapy status was independently associated with a higher local failure rate, suggesting the need for a higher radiation dose. Challenges exist for reirradiation and spinal lesions for which surgery may be preferred.
Acknowledgments
The authors thank Dr. Damiana Chiavolini for scientific editing of the manuscript. I.P., V.M., J.C., A.S., X-J.X., and J.B. are supported by NIH grant P50CA196516.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Kavolius JP, Mastorakos DP, Pavlovich C, et al. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261–2266. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 2.van der Poel HG, Roukema JA, Horenblas S, et al. Metastasectomy in renal cell carcinoma: A multicenter retrospective analysis. Eur Urol. 1999;35:197–203. doi: 10.1159/000019849. [DOI] [PubMed] [Google Scholar]
- 3.Alt AL, Boorjian SA, Lohse CM, et al. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117:2873–2882. doi: 10.1002/cncr.25836. [DOI] [PubMed] [Google Scholar]
- 4.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 5.Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: A systematic review. Lancet Oncol. 2014;15:e549–e561. doi: 10.1016/S1470-2045(14)70235-9. [DOI] [PubMed] [Google Scholar]
- 6.Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34:251–266. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 7.DiBiase SJ, Valicenti RK, Schultz D, et al. Palliative irradiation for focally symptomatic metastatic renal cell carcinoma: Support for dose escalation based on a biological model. J Urol. 1997;158:746–749. doi: 10.1097/00005392-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Walsh L, Stanfield JL, Cho LC, et al. Efficacy of ablative high-dose-per-fraction radiation for implanted human renal cell cancer in a nude mouse model. Eur Urol. 2006;50:795–800. doi: 10.1016/j.eururo.2006.03.021. [discussion 800] [DOI] [PubMed] [Google Scholar]
- 9.Amini A, Altoos B, Bourlon MT, et al. Local control rates of metastatic renal cell carcinoma (RCC) to the bone using stereotactic body radiation therapy: Is RCC truly radioresistant? Pract Radiat Oncol. 2015;5:e589–e596. doi: 10.1016/j.prro.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1744–1748. doi: 10.1016/j.ijrobp.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ning S, Trisler K, Wessels BW, et al. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer. 1997;80(Suppl 12):2519–2528. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2519::aid-cncr26>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 13.Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 15.McDonald R, Probyn L, Poon I, et al. Tumor response after stereotactic body radiation therapy to nonspine bone metastases: An evaluation of response criteria. Int J Radiat Oncol Biol Phys. 2015;93:879–881. doi: 10.1016/j.ijrobp.2015.07.2288. [DOI] [PubMed] [Google Scholar]
- 16.Dubben HH, Thames HD, Beck-Bornholdt HP. Tumor volume: A basic and specific response predictor in radiotherapy. Radiother Oncol. 1998;47:167–174. doi: 10.1016/s0167-8140(97)00215-6. [DOI] [PubMed] [Google Scholar]
- 17.Leith JT, Cook S, Chougule P, et al. Intrinsic and extrinsic characteristics of human tumors relevant to radiosurgery: Comparative cellular radiosensitivity and hypoxic percentages. Acta Neurochir Suppl. 1994;62:18–27. doi: 10.1007/978-3-7091-9371-6_5. [DOI] [PubMed] [Google Scholar]
- 18.Salama JK, Hasselle MD, Chmura SJ, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: Final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 19.Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: Impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sellin JN, Reichardt W, Bishop AJ, et al. Factors affecting survival in 37 consecutive patients undergoing de novo stereotactic radiosurgery for contiguous sites of vertebral body metastasis from renal cell carcinoma. J Neurosurg Spine. 2015;22:52–59. doi: 10.3171/2014.9.SPINE1482. [DOI] [PubMed] [Google Scholar]
- 21.Thibault I, Al-Omair A, Masucci GL, et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: Analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine. 2014;21:711–718. doi: 10.3171/2014.7.SPINE13895. [DOI] [PubMed] [Google Scholar]
- 22.Hashmi A, Guckenberger M, Kersh R, et al. Re-irradiation stereotactic body radiotherapy for spinal metastases: A multi-institutional outcome analysis. J Neurosurg Spine. 2016:1–8. doi: 10.3171/2016.4.SPINE151523. [DOI] [PubMed] [Google Scholar]
- 23.Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao SS, Thompson C, Cheng J, et al. Axitinib sensitization of high single dose radiotherapy. Radiother Oncol. 2014;111:88–93. doi: 10.1016/j.radonc.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.