Abstract
Cell-derived nanoparticles (CDNPs) containing cytosolic proteins and RNAs/DNAs can be isolated from stressed eukaryotic cells. Previously, CDNPs isolated from cultured cells, exerted immunomodulatory activities in different infections. Here, we sought to elucidate the role of CDNPs using a murine model of cecal ligation and puncture (CLP). We hypothesized that CDNPs influence the immune response at the site of infection, where severe cellular stress occurs. We observed early CDNP accumulation in the peritoneum after 4 hours and continued CDNP presence 24 hours after CLP. To determine whether CDNPs influence the host response to sepsis, we isolated CDNPs from a murine fibroblast cell line stressed by nutrient-deprivation, and injected them into septic mice. CDNP-treated mice demonstrated decreased peritoneal IL6-levels and an approximately 2-log lower bacterial load compared to control mice 24 hours after CLP. Additionally, a 20% CFU reduction was observed when incubating CDNPs with Pseudomona aeroginosa, indicating that CDNPs are bactericidal. To identify CDNP-responsive cells, CFSE-labeled CDNPs were injected into mice at the time of CLP. We observed that CDNPs were preferentially ingested by F4/80+ macrophages, and to a lesser degree, associated with inflammatory monocytes and neutrophils. Strikingly, CDNP-ingesting cells demonstrated elevated CD11b and MHCII expression compared to control cells. Altogether, our data indicate that CDNPs enhance the immune response at the site of infection and promote bacterial clearance, by 1) direct bacterial killing and 2) increasing phagocyte activation. Thus, CDNPs represent a novel, unexplored endogenous sepsis modulator with therapeutic potential.
Keywords: Sepsis, CDNP, nanoparticles, Annexin A5, stress, immunosuppression
Introduction
Sepsis is a severe medical condition characterized by a dysregulated, systemic host response to infection, leading to multiple organ failure and death (1). The global incidence of 31.5 million cases, is predicted to grow even further due to aging populations and the continued spread of antimicrobial resistance (2). Despite extensive research in the field of sepsis and long lasting efforts in the identification of septic modulators, all clinical trials conducted so far have failed to translate into successful therapeutic medications (3, 4). The urgent need for development of therapeutic innovations is emphasized by the unacceptably high mortality rates of 20-30% in sepsis (2).
Sepsis proceeds in two phases: an initial inflammatory immune response that is chiefly characterized by massive cellular activation and the subsequent immunosuppressed state (5). While most patients survive the early hyper-inflammation, the immunosuppressed phase is regarded as the major cause of death among septic patients (6, 7). Activated monocytes, macrophages, and neutrophils at the infection site normally secrete pro-inflammatory mediators, promote bacterial clearance, and activate adaptive immune cells via antigen presentation in the context of major histocompatibility complex class II (MHC II). However, under the immunosuppressed state, these effector cell functions are heavily impaired (7, 8). As a result, immune cells exhibit reduced surface expression of activation markers, limited phagocytosis and bacterial clearance, as well as downregulated antigen presentation. Moreover, there is excessive release of the anti-inflammatory cytokine IL-10, which further suppresses pro-inflammatory immune responses (9). Paralyzed effector cells are unable to eradicate the primary infection, rendering the immune system highly susceptible to secondary infections (10). Thus, therapeutic concepts in sepsis should focus on the enhancement of immune cell functions.
One potential immunogenic mediator of sepsis might be the cell-derived nanoparticle (CDNP). CDNPs are described as endogenous immunomodulatory nanoparticles, and are characterized by size, particularity, and composition (11, 12). CDNPs mainly contain intracellular proteins such as annexins, actin, histones, heat shock proteins, myosin, peroxiredoxines and vimentin and small traces of nucleic acids, with annexin A5 (AnxA5) being one of the most abundant CDNP components (12). This discriminates them from stress granules and processing bodies, which lack AnxA5 and mainly contain RNA binding proteins (13). CDNPs contain proteins at concentrations of approximately 150 µl/ml, DNA at 2 µg/ml and RNA at 4 µg/ml. Even though it cannot be excluded that CDNP comprise traces of lipids, they are resistant to treatment with chloroform or detergent. Therefore, it seems very unlikely that CDNPs are surrounded by a lipid membrane. The lack of a surrounding lipid membrane and a size ranging between 50 and 200 nm (12) distinguishes CDNPs from other cell derived particles, such as exosomes (<100 nm), microparticles (>200 nm) and apoptotic bodies (>1 µm), which are sensitive to treatment with detergent (14).
Although the mechanism of action of CDNPs still remains unknown, they have been shown to be taken up by macrophages and modulate their cytokine expression in vitro (unpublished data). Functionally, CDNPs were shown to modulate the immune response to intracellular and viral infections (15). Moreover, in a murine model of cancer, which is known to induce an immunosuppressive state similar to what is observed in sepsis, CDNPs have been demonstrated to exert a protective effect (16). Thus, CDNPs are a promising candidate for the immune-enhancing modulation of sepsis.
Since CDNPs were shown to be released in vitro and ex vivo as a response to cellular stress caused by nutrient deprivation or viral infection (11, 12), endogenously produced CDNPs are likely present at the infection site in sepsis, where severe cellular stress occurs. Therefore, the aim of the current study was to determine whether immunogenic CDNPs are produced in septic mice. We hypothesized that CDNPs are present at the infection site in sepsis and enhance the immune response in this setting.
Materials and Methods
Mice
Male C57BL/6J mice from ENVIGO (Indianapolis, IN) aged 7-8 weeks were utilized for all experiments. The mice were housed in standard environmental conditions and were fed a standard laboratory mouse diet with water ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC # 08-09-19-01) of the University of Cincinnati.
CDNP isolation and preparation
CDNPs were isolated from two different sources: fibroblast cell cultures and peritoneal lavages. Cell-culture derived CDNPs were produced as described previously (12). Briefly, CDNP were isolated from the embryonic fibroblast cell line MC3T3-E1 (DSMZ, Braunschweig, Germany) after culture in nutrient-deprived media for 2 days. Cell sonicates were prepared, centrifuged at 50,000 × g and the supernatants were subjected to chloroform treatment for exosome removal. Quality control was performed by SDS-PAGE (Supplemental Figure 1). To identify the nature of the nucleic acids, CDNP were treated with DNase and RNase. For nuclease treatment, 9 µl CDNP were mixed with 1 U RQ1 RNase-free DNase or 10 µg RNase A (both Promega GmbH, Germany), respectively, in a final volume of 10 µl and visualized on an agarose gel (Supplemental Figure 2). For identification of proteins, two-dimensional gel electrophoresis with subsequent MALDI-TOF analyses were performed as described (17). Exemplary proteins are shown in Supplementary Table 1. Endotoxin levels of cell-culture derived CDNPs were assessed using the LAL Chromogenic Endotoxin Quantitation Kit (Pierce, Waltham, MA). The endotoxin content of 0.016 EU/µg CDNPs was considered negligible.
Mouse-derived CDNPs were isolated using a modification of this protocol. Peritoneal fluid was collected via standard peritoneal lavage technique. Cell-free supernatant was obtained after centrifugation at 450 × g for 10 minutes followed by a second centrifugation at 25,000 × g for 30 minutes. The pellet, composed of apoptotic bodies and microparticles, was discarded and the supernatant was incubated with 1% Triton X-100 (Bio-Rad, Hercules, CA) for 10 minutes. Triton X-100 disrupts cell-membranes to remove exosomes as well as chloroform, but without having the negative side effect of denaturing and aggregating proteins (18). The sample was then centrifuged at 100,000 × g for 60 min to pellet CDNPs. CDNPs were suspended in HBSS (Grand Island, NY) and quantified using nanoparticle tracking analysis (NTA, Malvern, United Kingdom) and scanning electron microscopy (SEM). SEM samples were settled on thermanox coverslips (Nunc, Rochester, NY) and were fixed with Monti-Graziadei solution (2% glutaraldehyde, 0.6% paraformaldehyde in 0.1M cacodylate buffer, pH 7.2) for 2 h at 22°C. Then samples were dehydrated in a rising alcohol series and sputtered with gold. Samples were analyzed on the EVO HD 15-LS (Zeiss, Germany).
Cecal ligation and puncture
The cecal ligation and puncture (CLP) model was used to induce polymicrobial sepsis as described previously (19). Briefly, mice were anesthetized with isoflurane and a small mid-abdominal incision was made. The cecum was exteriorized and the latter third of the cecum was ligated with a 4-0 silk suture without obstructing bowel continuity. The cecum was then punctured with a 23-guage needle and gently squeezed to extrude a small amount of stool. After re-placing the cecum in the abdomen, vehicle or CDNPs were injected into the peritoneum. If not otherwise stated, 5µg CDNPs were applied per mouse. The abdominal incision was then closed in two-layers with 4-0 silk suture. Finally, animals were resuscitated with 1 mL of 0.9% sterile saline and allowed to recover on a heating blanket for one hour.
CFSE-labeling of CDNPs
To assess CDNP internalization by peritoneal cells, cell-culture derived CDNPs were labeled with carboxyfluorescein succinimidyl ester (CFSE). CDNPs were adjusted to a concentration of 100µg/ml with sterile PBS and incubated with 10µM CFSE (Biolegend, San Diego, CA) according to the manufacturer instructions. Briefly, CFSE was added to CDNPs or a PBS control, mixed and incubated for 20 min at 37°C. An equal volume of RPMI containing 10% FBS, (Sigma Aldrich, Germany) was added to capture excessive CFSE and the sample were incubated for 10 additional minutes at 37°C.
Western blot analysis
Annexin A5 content in mouse-derived CDNPs was evaluated by Western blot. 15µl isolated CDNPs were mixed with 5µl 4× Laemmli buffer and heated at 60°C for 20 min. Samples were then run on a 10% Bis-Tris polyacrylamide gel (Thermo Fisher Scientific, Carlsbad, CA) in MES buffer (Bio-Rad, Hercules, CA). After transferring the protein to a PVDF membrane for 60 min at 75 mA, the membrane was blocked with 3% BSA-solution for 60 min at room temperature. The first, second and third antibodies were diluted in 3% BSA as well. The primary Anti-Annexin A5 antibody (GeneTex, Irvine, CA) was applied in a 1:300 dilution and incubated overnight at 4°C. The membrane was washed with Tween-PBS and incubated with the second FITC-conjugated goat anti rabbit IgG antibody (1:200; Jackson Immuno Research, West Grove, PA) for 90 minutes at room temperature. After a washing step, the membrane was incubated for 60 min with the tertiary alkaline phosphatase-conjugated mouse anti-FITC antibody (1:5000; Jackson Immuno Research, West Grove, PA) at room temperature. Finally, washed membranes were developed using 1 ml ECF substrate (GE Healthcare, Little Chalfont, United Kingdom).
Determination of bacterial loads
Peritoneal lavages were aseptically collected in sterile 0.9% saline, and serially diluted 1:10 to 1:10000 in PBS. Samples were plated on tryptic soy agar plates (Fisher Scientific, Waltham, MA) and incubated at 37°C for 24 hours. Colony-forming units (CFUs) were counted in all non-confluent grown plates.
Bacteria growth assay
To determine the direct effect of CDNPs on bacteria growth and survival, bacterial growth assays were performed as described (20). Briefly, the Pseudomonas aeroginosa (P. aeruginosa) strain PA01 was grown for 14 hours on tryptic soy agar plates, collected and diluted to a density of 3 × 103 CFU/ml in PBS. Indicated amounts of CDNPs were added to the bacteria and incubated for 2 h at 37°C under shaking at 125 rpm. Incubations with buffer alone served as controls. 100 µl Aliquots of the bacterial suspensions were then plated on LB agar plates and incubated overnight at 37°C followed by the counting of CFUs.
In vitro activation of peritoneal macrophages
1×105 cells from by peritoneal lavages from naïve C57BL/6J mice were incubated in complete RPMI medium (Sigma Aldrich, St. Louis, MS) for 2 h at 37°C to allow macrophages to adhere. The supernatant containing non-adherent lymphocytes was removed and 100µl medium with 1 ng/ml LPS (Sigma Aldrich) and 50µg/ml CDNPs were added. Cells and supernatants were collected after 24 h of incubation at 37°C.
Analysis of cytokine secretion levels
IL-6 levels in the supernatant of in vitro cultivated peritoneal macrophages were determined using Ready-SET-Go! ELISA kits (eBioscience, San Diego, CA) according to the manufacturer's instructions.
For in vivo cytokine levels, peritoneal lavages from septic mice were collected and IL-6 and IL-10 were measured using a Cytometric Bead Array analysis kit (BD Biosciences, San Jose, CA) according to the manufacturer's protocol.
Real-time polymerase chain reaction
Expression of IL-6 was quantified in in vitro cultured macrophages using real-time PCR. Briefly: Total nucleic acids were isolated from peritoneal macrophage cell cultures using the innuPREP DNA/RNA mini kit (Analytik Jena, Jena, Germany) according to the manufacturer's protocol. DNA was degraded, cDNA was synthesized and real-time polymerase chain reaction performed as described previously (21). IL-6 mRNA expression was detected using the TaqMan Real-Time PCR Master Mix (Life Technologies, Carlsbad, CA) and following primers and TaqMan probe (Biomers, Ulm, Germany): Forward: 5′ CTCCCAACAGACCTGTCTATAC, Reverse: 5′GTGCATCATCGTTGTTCATAC, Probe: 5′TGCCATTGCACAACTCTTTTCTCATTTCCACG on a ABI Prism 7900 Real-Time PCR system (Applied Biosystems, Foster City, CA). Concentrations used were 500 nM for both the forward and reverse primer and 200 nM for the TaqMan probe. IL-6 expression was normalized to expression of the house keeping gene MLN51 using the comparative CT method (21).
Flow cytometry
To identify CDNP-ingesting cells and determine their activation status, CFSE-labeled CDNPs were injected into CLP mice as described above. Peritoneal lavages were performed after 4 h of incubation and cells were pelleted by centrifugation at 450 g for 10 minutes. Cells were resuspended in 50µl FACS buffer (PBS with 1% bovine albumin and 0.1% azide) and nonspecific antibody binding to Fc receptors was prevented by a 10-minute incubation at room temperature with 2.5µl rat serum (Invitrogen, Life Technologies, Grand Island, NY) and 1µl rat anti-mouse CD16/CD32 antibody (BD Pharmingen, San Jose, CA). Peritoneal cells were stained for 20 min at 4°C using rat anti-mouse APC-Cy 7 CD11b (clone M1/70), mouse anti-mouse PE I-Ab (clone AF6-120.1), rat anti-mouse APC or APC-Cy7 Ly6C (clone AL-21), rat anti-mouse PerCP-Cy5.5 Ly6G (clone 1A8) (all BD Pharmingen, San Jose, CA), rat anti-mouse Alexa Fluor 647 F4/80 (clone CI:A3-1; AbD Serotec, Raleigh, NC) and PE anti-mouse F4/80 (clone BM8; BioLegend, San Diego, CA). Neutrophils were identified as Ly6G+ Ly6C+, inflammatory monocytes were gated as Ly6Chi Ly6G- and macrophages were characterized by F4/80hiLy6Cint expression. Association of CDNPs and cells was determined by quantifying CFSE expression. To determine internalized CDNPs, samples were treated with trypan blue to quench surface fluorescence and re-analyzed by FACS (22). Cells still expressing CFSE were regarded as CDNP-ingesting. Flow cytometry data acquisition and analysis were performed on an Attune acoustic focusing cytometer (Life Technologies, Grand Island, NY) and analyzed with FlowJo 10 (Tree Star, Ashland, OR).
Statistical analysis
Data was analyzed using Prism 5.0 (GraphPad, La Jolla, CA), and presented as mean ± SEM. For comparison of two groups, Student's t-test or Wilcoxon Signed Rank test were used. Three or more groups were compared using standard or repeated-measurements ANOVA with Bonferroni post-hoc tests. A P value of ≤ 0.05 was considered statistically significant.
Results
CDNPs accumulate at the infection site in sepsis
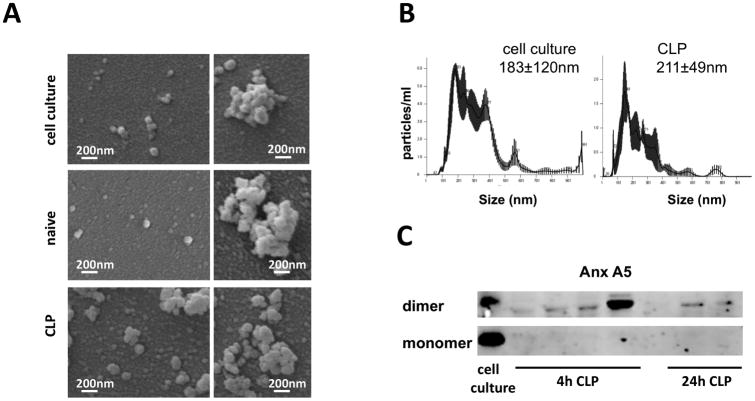
Previous studies demonstrated that CDNPs are produced as a specific response to stress in vitro and ex vivo (11). As sepsis is associated with severe cellular stress including hypoxia, metabolic and oxidative stress, we hypothesized that CDNPs would be present at the infection site of sepsis. To test this, we induced polymicrobial sepsis using the CLP model. After 4 or 24 hours, peritoneal lavages were collected, and CDNPs isolated from cell-free fractions by differential centrifugation. High centrifugation speeds and Triton-X treatment guaranteed the exclusion of apoptotic bodies, microparticles, and exosomes, respectively. CDNPs were identified due to their particularity, size, and protein composition using nanoparticle tracking analysis (NTA), SEM and Western Blot techniques. Well-characterized cell culture derived CDNPs isolated according to established protocols (12) served as prototypic CDNPs. Under the SEM, both CLP- and cell culture-derived CDNPs appear as similar spherical structures of approximately 100 nm diameters which exists as single particles or aggregates (Fig. 1 A). Interestingly, peritoneal lavages from naïve mice analyzed by SEM also contained CDNPs, albeit in very low amounts as compared to CLP-derived CDNPs (Fig. 1 A, central panel), suggesting that CDNPs are highly enriched upon sepsis induction. A size distribution between 100 and 500nm with a mean CDNP size of approximately 200nm (183nm for cell culture derived and 211nm for CLP-derived CDNPs, Fig. 1 B) was confirmed using NTA. A mean of 100-200 nm is in good accordance with previous reports (11), although the wider particle size range detected in our experiment indicates a higher amount of CDNP aggregation. We next set out to test whether CLP-derived CDNPs contained annexin A5, one of the major compounds of CDNPs (12). Indeed, annexin A5 dimers were present in CDNPs isolated 4 and 24 hours after CLP (Fig. 1 C) with varying levels in individual mice. These results demonstrate that CDNP are released at the infection site in sepsis.
Figure 1. CDNPs accumulate at the infection site of sepsis.
Characterization of CDNPs isolated from the cell-free fraction of peritoneal lavages from septic mice post CLP induction compared to well characterized cell culture derived CDNPs. A) Scanning electron micrographs of CDNPs derived from cell cultures, naïve mice or mice 3h post CLP. Left panels show single CDNPs, right panels depict CDNP aggregates. Samples from naïve mice contained very small amounts of CDNPs; depicted are some of the few CDNPs found. B) Particularity and size distribution of CDNPs as assessed by NTA. Representative plots for CDNP preparations from 4 individual mice 4h post CLP are shown. C) Annexin A5 content of CDNP isolates from 6 individual mice was determined by Western Blot using annexin A5-specific Antibodies.
CDNPs reduce peritoneal bacterial loads and IL-6 levels in septic mice
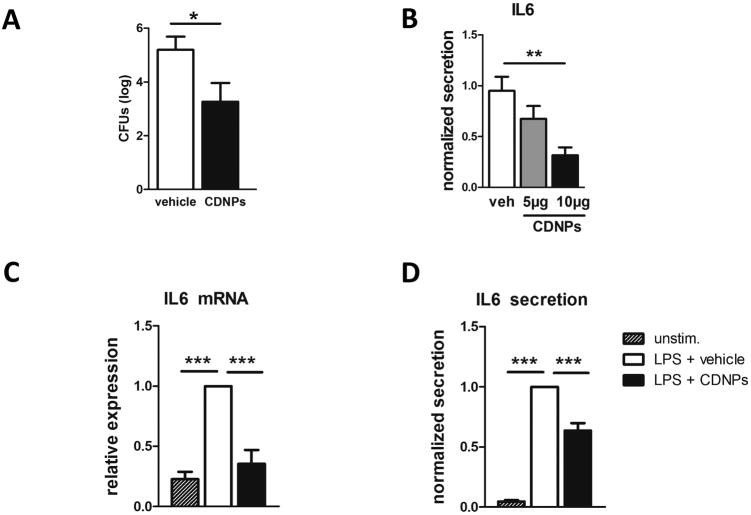
We next investigated whether CDNPs can influence the hosts' immune response during sepsis. Based on previous reports suggesting a protective role for CDNPs in virus infection and cancer, we hypothesized that CDNPs would improve the course of sepsis. Due to the low amounts of CDNPs isolated from CLP mice and in order to avoid a transfer of endotoxins likely present in CLP-derived isolates, we used CDNPs derived from a murine fibroblast cell line stressed by nutrient-deprivation for treatment in all subsequent experiments. WT mice were injected with vehicle or 5 µg CDNPs at the time of CLP-induction. 24 hours following CLP, peritoneal cavities were lavaged and peritoneal fluids analyzed for bacterial loads and levels of IL-6, a known sepsis mediator negatively correlated with the severity of sepsis (23). In fact, peritoneal bacterial loads were drastically reduced after CDNP-treatment by approximately two logs (Fig. 2 A). Corresponding to this finding, peritoneal IL-6 levels were decreased by 30% when the same dose of CDNPs was applied. Dosage duplication significantly diminished peritoneal IL-6 levels by another 30%, clearly showing a dose-dependent IL-6 reduction by CDNP-treatment (Fig. 2 B). To confirm that CDNPs are mediating this effect directly, LPS-stimulated peritoneal macrophages, the main IL-6 producers among innate effector cells, were obtained from naive mice and were incubated with 5µg CDNP for 24 hours. Cells and supernatants were then collected and analyzed for IL-6 mRNA and protein expression, respectively. Strikingly, CDNP-treated macrophages expressed less IL-6 mRNA reaching levels comparable to unstimulated cells (Fig. 2 C). Similarly, IL-6 protein levels were markedly reduced by ∼40% in CDNP- vs. vehicle-treated macrophages (Fig. 2 D), indicating that CDNPs modulate macrophage IL-6 gene and protein expression. Taken together, this data strongly suggests that CDNP-treatment is beneficial during sepsis.
Figure 2. CDNPs reduce peritoneal bacterial loads and IL-6 levels in septic mice.
A) Mice were treated with 5µg CDNPs at the time of CLP induction and peritoneal bacterial loads were determined 24h later. Pooled data from 3 independent experiments with n=5-8 mice per group. B) Mice were treated with 5 µg or 10 µg PNACs, respectively, at the time of CLP induction and peritoneal concentrations of IL-6 was determined using cytometric bead assay. C) mRNA expression and D) protein secretion of IL6 by peritoneal macrophages after 24h LPS stimulation in vitro. Data are expressed as mean ± SEM. Significance was determined using Student's t-test for comparison of two samples and standard ANOVA (B) or ANOVA for repeated measurements (C and D) with Bonferroni post-hoc test for multi-comparison analysis. *p<0.05, **p<0.01, ***p<0.001.
CDNPs directly reduce bacterial growth in vitro
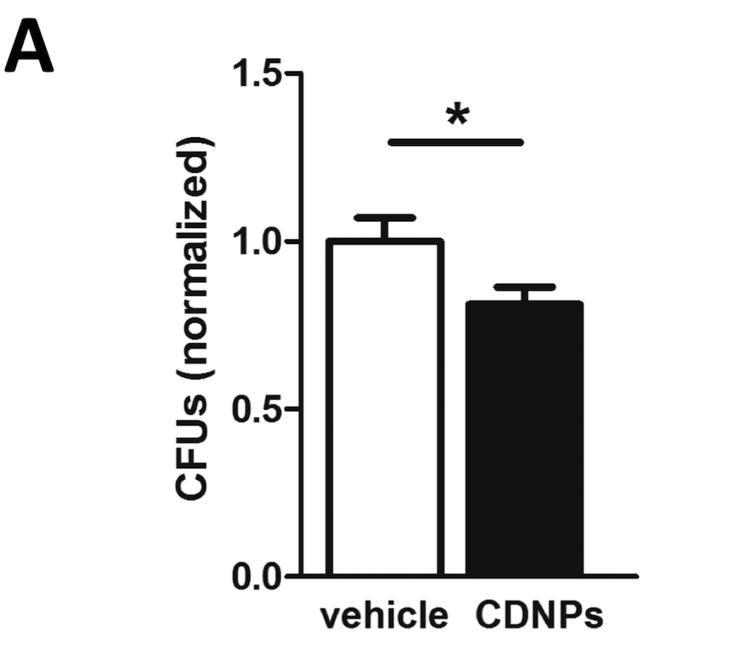
The reduced bacterial loads after CDNP-treatment observed above prompted us to speculate whether CDNPs might directly inhibit bacterial growth. To this end, we pre-incubated P. aeruginosa cultures with small amounts of CDNPs, plated aliquots of this mixture on agar plates and incubated them over night. Surprisingly, P. aeruginosa cultures treated with only 1/5th of the CDNP dose used in vivo exhibited significantly reduced CFU counts (Fig. 3), indicating that CDNPs directly impair bacterial growth and/or survival.
Figure 3. CDNPs directly reduce bacterial growth in vitro.
25µg of CDNPs were pre-incubated with P. aeruginosa and then plated on ager plates. CFUs were counted after 24h of incubation. Pooled data from 4 independent experiments with n=3-4. Data are expressed as means ± SEM. Significance was determined using Student's t-test. *p<0.05.
CDNPs are ingested by effector cells at the infection site of sepsis
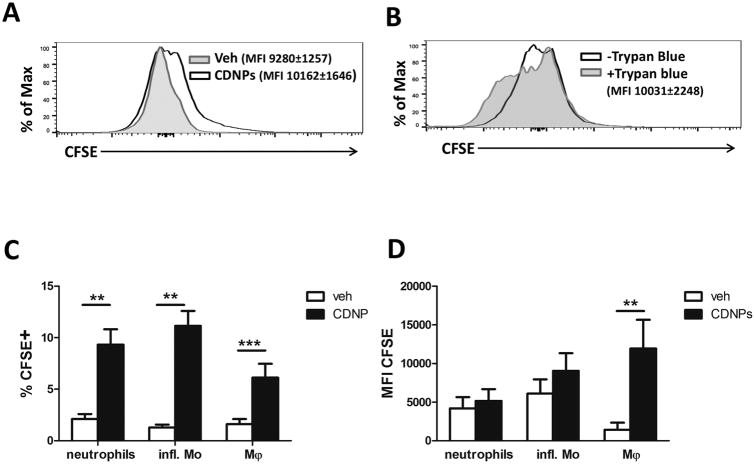
To identify CDNP-responsive cells in the setting of sepsis, we next labeled CDNPs with CFSE and evaluated their ingestion by effector cells at the infection site. Mice were treated with 5 µg of CFSE-labeled CDNPs at the time of CLP and peritoneal lavages were isolated 4 hours following treatment. Association of CDNPs with peritoneal cells was determined by positive CFSE expression. As expected, ungated peritoneal cells showed significantly increased median fluorescence intensity (MFI) levels of CFSE in CDNP- vs. vehicle-treated mice (Fig. 4 A). In order to discriminate between CDNPs bound to the cell surface and truly ingested CDNPs, we quenched all surface fluorescence using trypan blue and re-analyzed each sample for intracellular CFSE expression (22). Intriguingly, almost 98% of CFSE MFI remained after trypan blue quenching (10,031±2,248 quenched vs. 10,162±1,646 unquenched, Fig. 4 B). Given that the great majority of CDNPs was located inside peritoneal cells, we next evaluated which cell types ingest CDNPs by surface marker-based identification of CFSE positive cells in unquenched samples. We focused on the three most abundant cell populations at the infection site 4 hours after CLP: neutrophils, inflammatory monocytes, and F4/80+ macrophages. Interestingly, 5-10% of cells within each population exhibited CDNP ingestion (Fig. 4 C). Particularly, F4/80+ macrophages ingested the highest amounts of CDNPs as indicated by 20-fold increased CFSE levels vs. vehicle-treated mice (Fig. 4 D). Thus, neutrophils, inflammatory monocytes, and F4/80+ macrophages at the infection site of septic mice all ingest CDNPs, with F4/80+ macrophages accounting for the highest uptake.
Figure 4. CDNPs are ingested by neutrophils, inflammatory monocytes and macrophages at the infection site.
Mice were treated with 5µg CFSE-labeled CDNPs at the time of CLP induction and peritoneal cells were analyzed 4h later. Pooled data from 2 independent experiments with n =8-9 mice per group. A) Association of CDNPs with peritoneal cells was determined by CFSE fluorescence. B) Ingestion of CDNPs was assessed after surface fluorescence quenching with trypan blue. C) Percentages of CFSE positive cells within distinct peritoneal cell subsets. D) MFI of CFSE within distinct peritoneal cell subsets (D). A and B show representative FACS plots gated on all cells. Data in C and D are expressed as means ± SEM. Significance was determined using Student's t-test. **p<0.01, ***p<0.001.
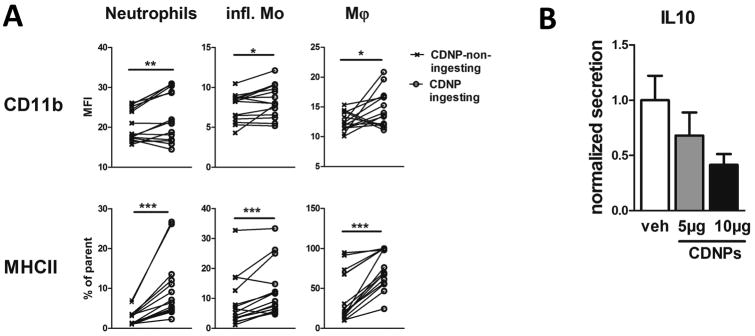
CDNPs increase effector cell activation and decrease IL-10 secretion in septic mice
To assess whether the ingestion of CDNPs by neutrophils, inflammatory monocytes, and F4/80+ macrophages has functional consequences, we measured surface expressions of the surrogate activation marker CD11b and MHCII in CDNP-treated and control mice 4 hours after CLP. To specifically address the effect of CDNP ingestion, expression of CD11b and MHCII was compared between cells that ingested (CFSE+) and cells that did not ingest CDNPs (CFSE-). Importantly, both CD11b and MHCII expression by cells that ingested CDNPs were significantly increased compared to non-ingesting cells in all three cell types (Fig. 5 A). The highest CD11b increase was observed on macrophages (approximately 30%), followed by neutrophils (16%), and inflammatory monocytes (11%). Regarding MHCII, 3.6-fold more neutrophils expressed MHCII after ingesting CDNPs as compared to 2-fold more macrophages and 1.5-fold more inflammatory monocytes (Fig. 5 A). Undoubtedly, CDNP treatment improved the immunosuppressed conditions characteristic for sepsis. Since immunosuppression is associated with excessive IL-10 release (9), we next determined the impact of CDNPs on peritoneal IL-10 secretion 24 hours post CLP. Corresponding to the increased expression of activation marker, mice treated with CDNPs at the time of CLP demonstrated a dose-dependent decrease in IL-10 levels ranging from 30 to 60% (Fig. 5 B). These findings are consistent with CDNP-mediated reduced bacterial loads and decreased peritoneal IL-6 levels described above, and suggest that CDNPs beneficially enhance the immune response in septic mice.
Figure 5. CDNPs enhance the immune response at the site of infection.
Mice were treated with 5µg or 10 µg CFSE-labeled CDNP at the time of CLP induction and peritoneal lavages were analyzed 4h (A) or 24h (B) later. Pooled data from 2-4 independent experiments with n =5-9 mice per group. A) Surface expression of activation marker on CDNP-ingesting (CFSE+) and non-ingesting (CFSE-) peritoneal cell subsets. B) Peritoneal concentrations of IL-10 were determined using a cytometric bead assay. Data in B is expressed as mean ± SEM. Significance was determined using Wilcoxon-Signed Rank test or ANOVA with Bonferroni post-hoc test. *P<0.05, **P<0.01, ***p<0.001.
Discussion
The immunosuppressed state induced by sepsis remains a life-threatening condition with very limited treatment options (24). In the present study, we examined the impact of potentially immunogenic CDNPs (15, 16), on the immune response to sepsis. First, we confirmed that endogenous CDNPs accumulate at the infection site in septic mice. Our data indicate that CDNPs are taken up by macrophages, inflammatory monocytes, and neutrophils at the infection site. CDNP ingestion leads to cell activation as reflected by increased surface expression of CD11b and MHCII, and reduced levels of peritoneal IL-10. Moreover, CDNP treatment considerably improved bacterial clearance in the peritoneum of septic mice. Surprisingly, in vitro incubation of CDNPs with P. aeruginosa suggests that CDNPs are able to inhibit bacterial growth directly. Altogether, we have demonstrated that CDNPs are endogenous modulators of sepsis that conquer the immune-paralysis in sepsis by enhancing the inflammatory response and augmenting bacterial clearance.
Our finding of a beneficial effect of CDNPs in sepsis is consistent with previous studies showing that CDNPs are protective in viral infections and cancer (15, 16). Although, these studies have not investigated the mechanism underlying this effect, an augmented immune response was certainly contributing to the protective effect. The sole available report addressing the mode of action of CDNPs suggests that CDNPs modulate the expression of cellular adhesion molecules ICAM-1 and VCAM-1 via NF-κB (12). Here, we demonstrate that CDNPs also promote CD11b and MHCII surface expression on CDNP-ingesting neutrophils, inflammatory monocytes, and macrophages at the site of infection (Fig. 5 A). CD11b is a subunit of the leukocyte adhesion receptor Mac-1 (complement receptor 3), which is expressed by phagocytes rapidly after cell activation (25). Since CD11b is involved in leukocyte recruitment and receptor-mediated phagocytosis, increased CD11b expression is likely beneficial in sepsis, while Mac-1 deficiency is associated with impaired bacterial clearance and reduced survival (26). Increased MHCII expression observed after CDNP treatment (Fig. 5 A) indicates increased antigen presentation, which in turn enhances the activation of adaptive effector cells. In fact, decreased HLA-DR expression is characteristic for septic patients and low expression levels are associated with higher mortality (27).
The finding of CDNP-driven cell activation was supported by decreased levels of the anti-inflammatory cytokine IL-10 at the infection site (Fig. 5 B). IL-10 has been shown to be one of the key modulators in the setting of sepsis (28). Its release suppresses the secretion of pro-inflammatory cytokines, impairs phagocytic capacities, and respiratory bursts of APCs (28). Notably, high systemic IL-10 levels have been shown to increase mortality (29), while inhibition of IL-10 expression using the immunomodulator AS101 improved bacterial clearance and increased survival in a clinically relevant mouse model of sepsis (30).
The results presented here raise the question of how CDNPs mechanistically activate immune cells. The mode of action of CDNPs was not assessed in our study; however, there are a number of possible mechanisms by which CDNPs may promote immune cell activation. Injection of CFSE-labeled CDNPs revealed that CDNPs are ingested by effector cells at the infection site (Fig. 4 A-D), suggesting that CDNPs might activate intracellular receptors. We speculate that small traces of DNA and RNA present in CDNPs (12), might activate intracellular Toll-like receptors, thereby inducing inflammation. Alternatively, proteins present in CDNPs might have immunomodulatory capacities. AnxA5, for instance, was shown to improve survival in an endotoxemia model of sepsis (31). Clearly, further studies are required to determine the mechanisms involved in CDNP-mediated cell activation.
A major finding of the present study is the 2 log-fold reduction in intraperitoneal CFU counts after CDNP treatment (Fig. 2 A). Reduced bacterial loads can be attributed to the increased activation of effector cells discussed above. CDNP-driven down-regulation of peritoneal IL-6 levels in vivo and direct impairment of IL-6 expression and secretion in vitro would support this hypothesis. Although, IL-6 is considered a pro-inflammatory cytokine with IL-6 deficient mice being unable to control infection (23, 32), it also has been reported that IL-6 can have anti-inflammatory effects via promoting the polarization to alternatively activated M2 macrophages (33), thereby limiting inflammation and bacterial clearance. Along these lines the failure to clear bacteria correlates with increased IL-6 release (34) and IL-6 was shown to suppress inflammation in a murine model of alveolar endotoxemia (35). Thus, the authors suggest that IL-6 might have pro-inflammatory effects in the first hyperinflammatory state, but switch to an anti-inflammatory mediator in the secondary suppressive state. Alternatively, AnxA5 from CDNPs might bind to LPS and prevent its interaction with TLR4 as shown by Arnold et al. (31), thereby reducing the secretion of pro-inflammatory IL-6. However, independent of the exact role of IL-6, there is abundance evidence on the clinical importance of decreased IL-6 levels as a predictive marker for survival in sepsis (23, 36). Consequently, Barkhausen et. al. observed improved survival in CLP-induced sepsis after selectively blocking pro-inflammatory IL-6 trans-signaling (37).
Surprisingly, we also found a direct antimicrobial effect of CDNPs in vitro (Fig. 3). Although we did not further investigate the underlying mechanisms, we speculate that annexin A5 might be involved. Rand et. al. reported that high-affinity binding of annexin A5 to the lipid A domain of LPS from the outer membrane of P. aeruginosa (38). It seems possible that the multiple annexins present in CDNPs bind LPS on several bacteria at once, thereby clumping them together and inducing a contact-dependent growth-inhibition. Alternatively, histone 4, present in CDNPs (Supplemental Table 1), might mediate the direct anti-inflammatory effect of CDNPs as described by Lee et al. (39).
Given that reduced bacterial clearance due to immunosuppression is one of the major causes of death in septic patients (6, 7) and the recovery of effector cells is associated with improved survival (40), we speculate that immune enhancing CDNP treatment likely will result in increased survival rates in sepsis. In support of this hypothesis, decreased bacterial loads, lower IL-6 and IL-10 levels, as well as higher CD11b and MHCII expression seen in CDNP treated mice, were all shown to be predictive for enhanced survival (27, 29, 36). We are currently improving our methods to generate sufficient amounts of cell culture-derived CDNPs in order to test this hypothesis.
Previous studies observing CDNP generation ex vivo hypothesized that CDNPs are endogenous immunomodulatory molecules produced by stressed cells (11). The present study indeed confirmed the accumulation of CDNPs in vivo using the setting of sepsis, where severe cellular stress occurs (Fig. 1 A-C). Since CDNPs were isolated from cell-free fractions of peritoneal fluids, we speculate that CDNPs might be actively released by stressed cells as part of a stress observation system (41) similar to microparticles and exosomes. Although the mechanisms of CDNP release are not known, a non-classical secretion pathway via secretary endolysosomes fusing with the plasma membrane might be involved (42). This possibility would be supported by Solisch et. al., who observed that intracellularly located CDNPs are sometimes surrounded by a membrane (11). Another intriguing possibility for CDNP-release would be the annexin A5-mediated binding to PS within the inner leaflet of the plasma membrane and subsequent PS “flipping” to the cell surface with its annexin cargo attached (43). A stress-induced increase of flippase activity responsible for PS translocation would explain why CDNP release is coupled to cellular stress. Alternatively, it is tempting to speculate that especially during sepsis CDNPs are released as part of neutrophil extracellular traps (NETs) by activated neutrophils (44). Even though NETs do not contain AnxA5, which is the signature protein of CDNPs, it will be important in further experiment to study the role of neutrophils in the formation of CDNPs (45).
Another task for the future will be to identify the active components contained in CDNPs. Western blot analysis revealed that both, cell culture- and CLP-derived CDNPs contain AnxA5 (Fig. 1C). In addition, initial proteomic analysis demonstrated that besides AnxA5 cytoskeleton proteins such as actins and myosins and the stress protein heat shock protein 70 were present in both cell culture and peritoneal-derived CDNPs (data not shown).
In conclusion, our study is the first to demonstrate that CDNPs are endogenously produced at the infection site in sepsis. Since CDNP-treated mice showed partially restored immunosuppression and increased bacterial clearance, modulation of endogenous CDNP levels or exogenous application of CDNPs might be promising therapeutic strategies for the treatment of sepsis.
Supplementary Material
Figure S1. CDNPs isolated from cell cultures contain a highly reproducible mixture of proteins. A) CDNPs were isolated from 4 distinct cell cultures of mouse fibroblasts, run on 4-12% Gel and stained with Coomassie blue. MALDI-TOF and Western blot analysis revealed that the three major bands are annexins, mainly AnxA5 and AnxA1. B) To compare CDNP preparations among different species, CDNPs were isolated from porcine and bovine embryonic porcine kidney cells (EFN-R, KN-R1, both Friedrich-Loeffler-Institute, Insel Riems, Germany) and mouse embryonic fibroblast cells MC3T3-E1 (DSMZ, Braunschweig, Germany) and analyzed by SDS-PAGE.
Figure S2. CDNPs isolated from cell cultures contain small amounts of RNA and DNA. CDNPs were isolated from cell cultures, digested with DNase (left panel) or RNase (middle panel) and run on 1% Agarose gel. No nuclease was added to mock controls (right panel).
Acknowledgments
We thank H. Goetzman, L. England, S. Allan and C. Örün for their excellent technical assistance.
Sources of Support: This study was funded by the German Research Foundation (IRTG 1911 project B3 to K.K.) and by Award Number R01 GM100913 (CCC) from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Abbreviations
- AnxA5
annexin A5
- APC
antigen-presenting cell
- CDNP
cell-derived nanoparticle
- CFSE
carboxyfluorescein succinimidyl ester
- CFU
colony forming unit
- CLP
cecal ligation and puncture
- MHCII
major histocompatibility complex class II
- MFI
median fluorescence intensity
- NET
neutrophil extracellular trap
- NTA
nanoparticle tracking analysis
- PS
phosphatidylserine
- P. aeruginosa
Pseudomonas aeruginosa
- SEM
scanning electron microscopy
- veh
vehicle
Footnotes
Conflict of Interest: Patents for the in vivo use of CDNPs have been applied for by Varicula Biotec GmbH, and KU.K. is listed as an inventor on these applications. KU.K. is also a shareholder in Varicula Biotec GmbH. K.K. and KU.K. are part time scientific consultants for Varicula Biotec GmbH.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, R S. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193(3):259–72. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195–203. doi: 10.1016/j.molmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Opal SM, Dellinger RP, Vincent JL, Masur H, Angus DC. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C? Crit Care Med. 2014;42(7):1714–21. doi: 10.1097/CCM.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence. 2014;5(1):45–56. doi: 10.4161/viru.26516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–8. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106(15):6303–8. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasten KR, Muenzer JT, Caldwell CC. Neutrophils are significant producers of IL-10 during sepsis. Biochem Biophys Res Commun. 2010;393(1):28–31. doi: 10.1016/j.bbrc.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93(3):1247–88. doi: 10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solisch P. Hitherto unknown pattern of reaction in vertebrate cells--ultrastructural studies of virus-host cell relationship and cell cultures. 1. Zentralbl Allg Pathol. 1986;131(1):49–57. [PubMed] [Google Scholar]
- 12.Ronnau C, Liebermann HE, Helbig F, Staudt A, Felix SB, Ewert R, Landsberger M. The bio-complex “reaction pattern in vertebrate cells” reduces cytokine-induced cellular adhesion molecule mRNA expression in human endothelial cells by attenuation of NF-kappaB translocation. BMB Rep. 2009;42(2):106–12. doi: 10.5483/bmbrep.2009.42.2.106. [DOI] [PubMed] [Google Scholar]
- 13.Protter DS, Parker R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016;26(9):668–79. doi: 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osteikoetxea X, Sodar B, Nemeth A, Szabo-Taylor K, Paloczi K, Vukman KV, Tamasi V, Balogh A, Kittel A, Pallinger E. Differential detergent sensitivity of extracellular vesicle subpopulations. Org Biomol Chem. 2015;13(38):9775–82. doi: 10.1039/c5ob01451d. [DOI] [PubMed] [Google Scholar]
- 15.Solisch P, Nockler A, Schafer D, Riebe R, Holl U, Schirrmeier H, Kretzschmar C, Wazel W, Dietz G, Sulk J. A hitherto unknown reaction pattern in vertebrate cells (RiV). 2. The protective effect of RiV particle preparations against foot-and-mouth disease in guinea pigs. Zentralbl Allg Pathol. 1986;131(6):563–8. [PubMed] [Google Scholar]
- 16.Solisch P, Wazel W, Steinmann W, Riebe R, Meyer U. A hitherto unrecognized pattern of reactions in vertebrate cells (RiV). 3. Experimental therapy with the help of RiV particle preparations in mice with mammary carcinomas. Zentralbl Allg Pathol. 1987;133(3):293–8. [PubMed] [Google Scholar]
- 17.Gemoll T, Epping F, Heinrich L, Fritzsche B, Roblick UJ, Szymczak S, Hartwig S, Depping R, Bruch HP, Thorns C, et al. Increased cathepsin D protein expression is a biomarker for osteosarcomas, pulmonary metastases and other bone malignancies. Oncotarget. 2015;6(18):16517–26. doi: 10.18632/oncotarget.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao H, Liu W, Simmons BN, Harris HK, Cox TC, Massiah MA. Purifying natively folded proteins from inclusion bodies using sarkosyl, Triton X-100, and CHAPS. Biotechniques. 2010;48(1):61–4. doi: 10.2144/000113304. [DOI] [PubMed] [Google Scholar]
- 19.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184(7):3768–79. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice TC, Seitz AP, Edwards MJ, Gulbins E, Caldwell CC. Frontline Science: Sphingosine rescues burn-injured mice from pulmonary Pseudomonas aeruginosa infection. J Leukoc Biol. 2016;100(6):1233–1237. doi: 10.1189/jlb.3HI0416-197R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalies K, Blessenohl M, Nietsch J, Westermann J. T cell zones of lymphoid organs constitutively express Th1 cytokine mRNA: specific changes during the early phase of an immune response. J Immunol. 2006;176(2):741–9. doi: 10.4049/jimmunol.176.2.741. [DOI] [PubMed] [Google Scholar]
- 22.Patino T, Soriano J, Barrios L, Ibanez E, Nogues C. Surface modification of microparticles causes differential uptake responses in normal and tumoral human breast epithelial cells. Sci Rep. 2015;5:11371. doi: 10.1038/srep11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17(6):463–7. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric: Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simms HH, D'Amico R. Lipopolysaccharide induces intracytoplasmic migration of the polymorphonuclear leukocyte CD11b/CD18 receptor. Shock. 1995;3(3):196–203. doi: 10.1097/00024382-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Liu JR, Han X, Soriano SG, Yuki K. The role of macrophage 1 antigen in polymicrobial sepsis. Shock. 2014;42(6):532–9. doi: 10.1097/SHK.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 27.Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32(8):1175–83. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 28.Ocuin LM, Bamboat ZM, Balachandran VP, Cavnar MJ, Obaid H, Plitas G, DeMatteo RP. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J Leukoc Biol. 2011;89(3):423–32. doi: 10.1189/jlb.0810479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heper Y, Akalin EH, Mistik R, Akgoz S, Tore O, Goral G, Oral B, Budak F, Helvaci S. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur J Clin Microbiol Infect Dis. 2006;25(8):481–91. doi: 10.1007/s10096-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 30.Kalechman Y, Gafter U, Gal R, Rushkin G, Yan D, Albeck M, Sredni B. Anti-IL-10 therapeutic strategy using the immunomodulator AS101 in protecting mice from sepsis-induced death: dependence on timing of immunomodulating intervention. J Immunol. 2002;169(1):384–92. doi: 10.4049/jimmunol.169.1.384. [DOI] [PubMed] [Google Scholar]
- 31.Arnold P, Lu X, Amirahmadi F, Brandl K, Arnold JM, Feng Q. Recombinant human annexin A5 inhibits proinflammatory response and improves cardiac function and survival in mice with endotoxemia. Crit Care Med. 2014;42(1):e32–41. doi: 10.1097/CCM.0b013e3182a63e01. [DOI] [PubMed] [Google Scholar]
- 32.Dalrymple SA, Slattery R, Aud DM, Krishna M, Lucian LA, Murray R. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect Immun. 1996;64(8):3231–5. doi: 10.1128/iai.64.8.3231-3235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One. 2014;9(4):e94188. doi: 10.1371/journal.pone.0094188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng M, Scott MJ, Loughran P, Gibson G, Sodhi C, Watkins S, Hackam D, Billiar TR. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J Immunol. 2013;190(10):5152–60. doi: 10.4049/jimmunol.1300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101(2):311–20. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215(4):356–62. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barkhausen T, Tschernig T, Rosenstiel P, van Griensven M, Vonberg RP, Dorsch M, Mueller-Heine A, Chalaris A, Scheller J, Rose-John S, et al. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit Care Med. 2011;39(6):1407–13. doi: 10.1097/CCM.0b013e318211ff56. [DOI] [PubMed] [Google Scholar]
- 38.Rand JH, Wu XX, Lin EY, Griffel A, Gialanella P, McKitrick JC. Annexin A5 binds to lipopolysaccharide and reduces its endotoxin activity. MBio. 2012;3(2) doi: 10.1128/mBio.00292-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee DY, Huang CM, Nakatsuji T, Thiboutot D, Kang SA, Monestier M, Gallo RL. Histone H4 is a major component of the antimicrobial action of human sebocytes. J Invest Dermatol. 2009;129(10):2489–96. doi: 10.1038/jid.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unsinger J, Burnham CA, McDonough J, Morre M, Prakash PS, Caldwell CC, Dunne WM, Jr, Hotchkiss RS. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J Infect Dis. 2012;206(4):606–16. doi: 10.1093/infdis/jis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2011;16(3):235–49. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10(5):1463–75. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43(3):168–75. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Brien XM, Biron BM, Reichner JS. Consequences of extracellular trap formation in sepsis. Curr Opin Hematol. 2017;24(1):66–71. doi: 10.1097/MOH.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CDNPs isolated from cell cultures contain a highly reproducible mixture of proteins. A) CDNPs were isolated from 4 distinct cell cultures of mouse fibroblasts, run on 4-12% Gel and stained with Coomassie blue. MALDI-TOF and Western blot analysis revealed that the three major bands are annexins, mainly AnxA5 and AnxA1. B) To compare CDNP preparations among different species, CDNPs were isolated from porcine and bovine embryonic porcine kidney cells (EFN-R, KN-R1, both Friedrich-Loeffler-Institute, Insel Riems, Germany) and mouse embryonic fibroblast cells MC3T3-E1 (DSMZ, Braunschweig, Germany) and analyzed by SDS-PAGE.
Figure S2. CDNPs isolated from cell cultures contain small amounts of RNA and DNA. CDNPs were isolated from cell cultures, digested with DNase (left panel) or RNase (middle panel) and run on 1% Agarose gel. No nuclease was added to mock controls (right panel).