Abstract
The housefly, Musca domestica is a major domestic, medical and veterinary pest. The management of these flies reliance on insecticide, causes environmental constraints, insecticide resistance and residues in the meat, skin. Therefore one of the eco-friendly alternate methods is by using biological agents such as entomopathogenic nematodes (EPN). In the present study evaluated the survival of EPN species Steinernema feltiae, Heterorhabditis indica, S. carpocapsae, S. glaseri and S. abbasi in poultry manure and also their efficacy against different developmental stages of house fly. After exposing to poultry manure, S. feltiae showed more survival as followed by H. indica, S. carpocapsae, S. glaseri and S. abbasi in all exposition period. When the exposition period extended to 96 h, all nematode species survivability was drastically reduced. After exposing these nematodes to poultry manure at 24 h their virulence capacity against wax moth, Galleria mellonella showed all the nematode species were able cause 100% mortality. However their progeny production was significantly reduced. Fly eggs and pupae were refractory to these nematode infection. Petri dish without artificial diet assay showed that, second and 3rd-instar larvae were highly susceptible to EPNs as compared to larvae provided with artificial diet. H. indica showed high virulence capacity compared to other nematodes tested. Poultry manure assay revealed that, H. indica and S. carpocapsae caused minimal mortality where as S. feltiae, S. glaseri and S. abbasi did not cause any mortality. This may be because of poor survival and limited movement of nematodes in poultry manure which may be due to ammonia, other toxic substances in poultry manure. The decrease in larval mortality in manure suggests that biocontrol of housefly by using EPNs is unlikely.
Keywords: EPN, Steinernematidae, Heterorhabditidae, Developmental stages, House fly, M. domestica, Poultry manure
Indian poultry industry is one of the fastest growing segments of the agricultural sector today in India. Nearly 20 million farmers are employed in poultry industry with around 1000 hatcheries operating across India. South India accounts for majority of total poultry production and consumption in the country. Andhra Pradesh, Karnataka, Kerala, Tamil Nadu and Maharashtra in the west and Haryana, Punjab in the north are key regions in this aspect (Abroader Consultancy India Pvt Ltd 2015). Arthropod pests, including flies, beetles and external parasites, cost poultry producers millions of rupees annually from reduced weight gains, meat quality, egg production losses and structural damage. Several species of manure breeding flies may be found associated with poultry production facilities among them the house fly Musca domestica L. (Diptera: Muscidae), is a major pest. It represents about 90% of the total fly species associated with poultry manure, especially in caged-layer operations. Accumulated poultry manure can be highly suitable for fly breeding, especially where general sanitations are poor. They cause flyspecking problems by regurgitated and faecal material on windows and walls of buildings, bird rearing cages, egg trays, and lights. Deposition of regurgitated and faecal material of the flies on the bird eggs that decreases aesthetic value of eggs (Muniyellappa 2010; Iqbal et al. 2014). High population density of the M. domestica in poultry farms spoils food, causes irritation to farm employees. It causes annoyance to neighbouring rural non-farm communities, this can often lead to poor community relations and potential litigation.
The housefly is also a major domestic, medical pest and is regarded as a public health hazard, predominantly in parts of the world where sanitary and hygienic conditions are poor. Furthermore, hygiene and sanitation are often relatively poor in rural areas especially in the developing countries (Khan et al. 2013). Houseflies’ can serve as vectors and reservoirs for food borne diseases (Barro et al. 2006; Khoobdel et al. 2008; Loftin et al. 2014). They are also widely recognized as potential vectors for transmitting bacterial diseases such as cholera, shigellosis and salmonellosis (Barin et al. 2010; De Jesus et al. 2004) and diphtheria, mycoses, yaws and leprosy (Keiding 1986). In addition to being mechanical vectors, houseflies feed on various kinds of food-stuffs, garbage and human excreta where they can pick up and transport pathogens. Accidentally when larvae of M. domestica swallowed in food material sometimes survive in the human gut and causes intestinal myiasis (Shekhawat et al. 1993; Sehgal et al. 2002; Achra et al. 2014).
No single method had proved to be successful in suppressing the pest population. Current management of these pests across the India relies upon the integration of mechanical, cultural, chemical methods, semiochemical approaches. The application of insecticides is the most popular farmer’s practice. However, indiscriminate use of insecticides leads to development of resistance, also increase the mortality of non-target organisms, risk of residue in the eggs, meat and environmental hazards. Use of biocontrol agents viz., virus, bacteria, fungi, nematodes, parasitic, parasitoid and predatory insects are being practiced as control measures for the M. domestica. Any attempt to scale down the use of chemical insecticides is welcome considering and the safety to flora and fauna of the environment. Due to environmental and regulatory pressures, research toward developing alternative pest control measures are warranted (Tomerlin 2000). Therefore, identifying biocontrol agents that control the soil-dwelling life stages is of paramount importance for the development of successful biological control against house fly.
Use of EPNs could offer an effective and safer alternative to chemical control (Poinar and O’Callaghan 1992). In this regard, Entomopathogenic nematodes (EPN) from the families Steinernematidae and Heterorhabditidae are excellent candidates. They are important biological control agents for a variety of economically important pests (Grewal et al. 2005). They have a mutualistic symbiosis with bacteria in the genera Xenorhabdus spp. and Photorhabdus spp., respectively. Some researchers suggested that nematodes were effective for controlling fly population in poultry houses (Belton et al. 1987). Where as many researchers indicated that EPNs are not effective for Musca domestica control in poultry manure (Renn et al. 1985; Geden et al. 1986; Georgis et al. 1987). The survivability and infectivity of EPN can vary as much among conspecific strains as between different species of nematodes (Molyneux et al. 1983) therefore evaluation of additional nematode strains may reveal differences in manure tolerance. Therefore, we have evaluated the survival of EPN in poultry manure and virulence of five EPN species S. feltiae, H. indica, S. carpocapsae, S. glaseri and S. abbasi against different developmental stages of house fly.
Materials and methods
Insect culture
House flies were initially collected from poultry house by using nylon net (Tamilam et al. 2010) located in Veterinary College, Bengaluru, Karnataka and were identified based on the morphological keys developed by Zumpt (1965), Van Emden (1965) and D’assis Fonseca (1968). The morphologically identified M. domestica was subjected to molecular method i.e. by DNA barcoding one of the emerging method for identification of insects up to species level and confirmed the species M. domestica (Hebert et al. 2003). Adult flies were provided with milk and granulated sucrose. Then flies were transferred to fly breeding chamber containing artificial diet to lay eggs [Wheat bran meal with wheat bran + milk powder + egg yolk (7:3:1)]. Eggs were collected and then transferred to fresh artificial diet. The eggs hatched into first instar larvae within 12 h. First instar larvae were reared on artificial diet and they developed to second instar within 24 h, the second instar larvae developed into third instar larvae within 24 h and the third instar larvae developed into pupae by 3–4 days. The pupae emerged after 5–6 days. The adults on emergence were fed with milk and granulated sucrose.
Fresh eggs were collected to test their susceptibility to EPNs while second, third instar and pupal stages were collected after 2, 3, and 6 days after introduction of eggs to the artificial diet, respectively (Geden et al. 1986).
Nematode culture
The entomopathogenic nematodes Heterorhabditis indica, Steinernema carpocapsae, S. glasseri, S. abbasi and S. feltiae used in this study were collected from the live nematode culture of the Division of Insect Systematics, National Bureau of Agricultural Insect Resources (NBAIR) Bengaluru, India and were maintained on late instar larvae of greater wax moth, Galleria mellonella (L.) (Lepidoptera: Pyralidae) at 25 ± 2 °C, using the method described by (Woodring and Kaya 1988). Nematode infective juveniles (IJ) emerging from the G. mellonella larvae within 3 day from the first day of emergence were collected using White traps. Nematode viability was 100%, unless otherwise stated. In all the experiments we have used the fresh IJs.
Nematode survival
Approximately 50,000 IJs of an each H. indica, S. carpocapsae, S. glasseri, S. abbasi and S. feltiae nematode species in 1 mL water were applied to each of 16 Petri dishes (5.0 cm), containing poultry manure. Manure had been field-collected and heat killed for any existing arthropods. After introduction of IJs, dishes were arranged in a completely randomised design with four replicates. At 24 h intervals, four Petri dishes were randomly selected to determine the survival of nematodes. Four manure samples were removed from each dish and placed in another dish containing 20 mL water (Georgis et al. 1987). The IJs were separated from manure by pouring the suspension through a sieve followed by an additional 500 mL of tap water. The IJs were washed from the sieve into clean flask and re-suspended in 10 mL of sterilized water. To assess the nematode mortality after 24, 48, 72, 96 h, 200 μL samples were taken from each treatment and placed in a 100-mm Petri dish lid to count the number of living and dead nematodes by using dissecting microscope. Immobile IJs were touched with a probe and considered dead if they did not react. The IJs were washed 4 times in sterilized, distilled water. The pathogenicity and reproducibility of nematodes was determined by adding 1 mL of suspension containing 400 IJs and two wax moth larvae to a 5 cm Petri dish lined with Whatman tissue paper (Patil et al. 2015). There were 4 Petri dishes per treatment. Wax moth larval mortality was observed after 2 days. Dead larvae removed and kept for incubation for 10 and 7 days for Heterorhabditis and Steinernema respectively, after incubation kept for emergence of IJs by keeping white traps for checking the reproduction.
Plate assay
Susceptibility of fly egg and pupa on filter paper
The efficacy of H. indica, S. carpocapsae, S. glasseri, S. abbasi and S. feltiae were tested against house fly eggs by placing 4 eggs in a Petri dish (5.0 cm) lined with a filter paper and adding 1 mL of a nematode suspension in distilled water at dosage levels of 0, 5000 and 10,000 nematodes per egg. Control treatments received only distilled water. Dishes were sealed with parafilm to avoid dehydration and kept 25 ± 2 °C. There were 4 replicates for each nematode dose and Petri dishes were placed in a completely random design. Egg mortality was checked based on larval emergence. The same protocol was followed for pupa except that mortality was based on adult fly emergence.
Susceptibility of fly larvae on filter paper
To determine the pathogenicity of EPNs against second and third instars of hose fly larvae. Experiment was conducted in Petri dishes (5.0 cm diam.) lined with a filter paper. Further, four second and third instar house fly larvae were placed in each Petri dishes separately. Petri dishes were inoculated with the treatments at 7 levels: 50,100, 200, 400, 800, 1600 and 3000 IJ/larva and control received only water. The containers were sealed with parafilm to avoid dehydration and kept 25 ± 2 °C. There were 4 replicates per treatment in a completely random design. Plastic dishes were examined at 2 and 3 days after treatment (DAT) and mortality was recorded. Cadavers were examined for signs of nematode infection (i.e., coloration) (Woodring and Kaya 1988). Dead grubs were kept on White traps to observe nematode emergence from nematode killed insects. The same protocol was followed for third-instar larvae except that mortality was based on adult fly emergence.
Susceptibility of fly larvae in rearing medium
The same protocol as above was followed here for second and third instar larvae except that here larvae were provided with artificial rearing medium and the mortality was based on adult fly emergence. In this experiment artificial diet provided to the larvae to see the differences in susceptibility, as the presence of food to larvae decrease the susceptibility of larvae to EPNs (Geden et al. 1986).
Susceptibility of fly larvae in poultry manure
To determine the pathogenicity of EPNs against second and third larval stages of house fly larvae. Experiment was conducted in plastic containers (5.8 cm diam., 200 cm3 soil capacity). Plastic containers were filled with 150 cm3 autoclaved manure. Manure moisture was maintained up to the field capacity level. Further, four second and third stages of house fly larvae were placed in each plastic container separately. Plastic containers were inoculated with the treatments: 50; 100; 200; 400; 800; 1600; 3000; 6000; 7000; 8000; 9000; 10,000; 12,000; 14,000; 16,000; 18,000; 20,000; 32,000; 64,000; 128,000; 256,000 IJs/larva and control received only water. There were 4 replicates per treatment in a completely random design. Plastic containers were examined at 2 and 5 days after treatment (DAT) and mortality was recorded. Cadavers were examined for signs of nematode infection (i.e., coloration) (Woodring and Kaya 1988).
Reproduction of EPN on M. domestica
Third instar larvae of house fly were exposed individually to IJs in petri dish (5 cm diam). Each petri dish was lined with filter papers and 3000 IJs were added in 1 mL of distilled water. The petri dishes were sealed with Parafilm and incubated in the dark at 25 ± 2 °C until larval death. The cadavers were rinsed in sterile distilled water to remove nematodes from their surface body. Then cadavers were incubated at 25 ± 2 °C for 7 and 10 days for Steinernema and Heterorabhdus respectively. The IJs that emerged from cadavers were collected over 2 days. There were 4 replicates and Petri plates were placed in a completely random design.
Statistical analysis
Probit analysis was used to calculate LC50 and LC90 values (numbers of IJs/individual causing 50 and 90% mortality) and to calculate the respective 95% confidence intervals using PROC Probit log10 (SAS version 9.3; SAS Institute, model r/n = con/lackfitinversecl;). The arcsine transformation was used to normalize the percentage data before an ANOVA was conducted. Analysis was undertaken on the transformed data and back transformed data only is presented. Nematode species and time, and their interactive effects on per cent survival and nematode species, dose and time, and their interactive effects on mortality percentage of different developmental stages of house fly using PROC GLM (SAS version 9.3; SAS institute).When ANOVA was significant, comparisons of relevant means were made using the Turkey’s significance test values at the 5% level of significance.
Results
Nematode survival
The survivability of EPNs in poultry manure at different time intervals, S. feltiae showed highest survival of about 85.25% at 24 h, whereas only 23.75% was recorded when exposition period was extended to 96 h. Whereas S. abbasi showed lowest survivability of 15.25% in 24 h and we could not notice any survivability at 48 h after exposition to the manure. The survivability assay revealed that, S. feltiae showed higher survivability in poultry manure followed by H. indica, S. carpocapsae, S. glaseri and S. abbasi Survival of EPNs was significantly (P < 0.05) reduced with increase in exposition period (Table 1).
Table 1.
Mean percent survival of five EPN species at different time intervals in poultry manure
| Hours after treatment | Survival in manure (%) | ||||
|---|---|---|---|---|---|
| S. feltiae | H. indica | S. carpocapsae | S. glasseri | S. abbasi | |
| 24 h | 85.25aa | 83.25a | 73a | 45a | 15.25a |
| 48 h | 62.75b | 58b | 52.25b | 9.75b | 0 |
| 72 h | 48.75c | 31.25c | 28c | 2.5c | 0 |
| 96 h | 23.75d | 12.25d | 14.25d | 0 | 0 |
| P value | |||||
| Exposure time (E) | P < 0.0001 | ||||
| Nematode (N) | P < 0.0001 | ||||
| E × N | P < 0.0001 | ||||
aMeans of four replications. Means followed by the same letter in a column are not significantly different at P < 0.05, as determined by Tukey’s test
Further, after exposing these nematodes to poultry manure we have tested their virulence capacity against wax moth, Galleria mellonella. The results of this revealed that all the nematode species were able cause 100% mortality in G. mellonella even after being exposed to the manure for 24 h. Progeny production assay revealed that approximately 2.5 lakh IJs were harvested from the cadaver that had been killed by fresh IJs whereas on an average of 19,000 IJs were harvested from the cadaver that had been killed by IJs after exposing to the poultry manure. These data indicated that poultry manure drastically reduced the survivability of nematodes and also affect the nematode reproduction.
Susceptibility of fly egg and pupa on filter paper
When fly eggs and pupae were exposed to H. indica, S. carpocapsae, S. glasseri, S. abbasi and S. feltiae at 5000 and 10,000 IJs/host, 24 h after nematode inoculation all eggs were hatched into larvae and whereas 5 days after nematode inoculation, pupae were emerged into adult flies. These results suggest that, the eggs and pupae of house fly were resistant to above five EPNs.
Susceptibility of fly larvae on filter paper
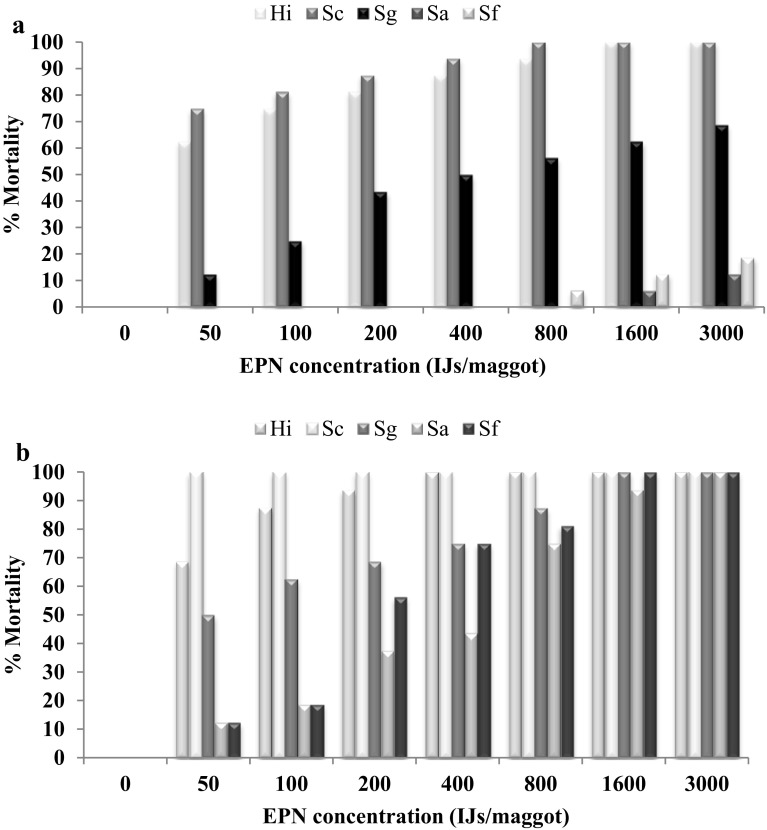
In the present study second and third instar larvae of house fly were susceptible to all the five EPNs. Comparing the H. indica, S. carpocapsae, S. glasseri, S. abbasi and S. feltiae susceptibility of second instar, there was no control mortality. S. carpocapsae had greater virulence to second instar of house fly at 2 and 3 DAT. S. carpocapsae caused highest mortality of 75% (100%), whereas lowest mortality of 0% (12.5%) was caused by S. abbasi at 50 (3000) IJs/larva 2DAT (Fig. 1a). S. carpocapsae caused highest mortality of 100%, whereas lowest mortality of 12.5% was observed in S. abbasi and S. feltiae at 50 IJs/larva. Three DAT 100% mortality was recorded in all the EPNs at 3000 IJs/larva (Fig. 1b).
Fig. 1.
a, b Mean percent mortality of second instar larvae of Musca domestica on filter paper to different dose of live EPNs at two and three days after treatment (DAT), in control there was no mortality
In this study the analysis of variance showed that second instar of M. domestica mortality was significantly (P < 0.05) influenced by IJ concentration (F = 125.09; df = 7, 240; P < 0.0001), EPN species (F = 201.49; df = 4, 240; P < 0.0001), exposure times (F = 280.99; df = 1, 240; P < 0.0001). The interaction between EPN species and IJ concentrations (F = 5.69; df = 28, 240; P = < 0.0001). The interaction between EPN species, IJ concentrations and exposure time (F = 4.64; df = 35, 240; P = < 0.0001) was significantly different. The calculated lowest LC50 and LC90 for S. carpocapsae, were 19 and 182 at 2DAT whereas LC values could not be calculated for 3DAT because 100% mortality was reached at all doses. The highest LC50 and LC90 were observed for S. abbasi where the LC values could not be calculated for 2DAT because there was no mortality, whereas LC50 and LC90 values of 309 and 1561 3DAT were observed (Table 2). The LC50 and LC90 values indicated that S. carpocapsae was more virulent against second instars of M. domestica compared to other EPNs.
Table 2.
LC50 and LC90 values of second instar larvae of Musca domestica on filter paper
| EPN species | DAT | LC50 | 95% FL | LC90 | 95% FL | Slope ± SE | χ 2 | P |
|---|---|---|---|---|---|---|---|---|
| H. indica | 2 | 30 | 2–68 | 363 | 195–1318 | 1.18 ± 0.33 | 12.27 | 0.0005 |
| 3 | 29 | 1–54 | 119 | 73–383 | 2.12 ± 0.78 | 7.33 | 0.0068 | |
| S. carpocapsae | 2 | 19 | 0–49 | 182 | 89–614 | 1.30 ± 0.44 | 8.45 | 0.0037 |
| 3 | * | * | * | * | * | * | * | |
| S. glasseri | 2 | 566 | 280–1387 | 19,261 | 4782–1,083,436 | 0.83 ± 0.21 | 14.92 | 0.0001 |
| 3 | 63 | 17–117 | 724 | 392–2662 | 1.21 ± 0.28 | 17.63 | <.0001 | |
| S. abbasi | 2 | ** | ||||||
| 3 | 309 | 211–447 | 1561 | 957–3571 | 1.82 ± 0.29 | 39.49 | <.0001 | |
| S. feltiae | 2 | 8775 | 3506–605,135,957 | 50,257 | 9524–2.61825 × 1014 | 1.69 ± 0.74 | 5.12 | 0.0237 |
| 3 | 203 | 140–283 | 821 | 540–1650 | 2.11 ± 0.34 | 37.71 | <.0001 |
DAT-days after treatment, LC50 and LC90 lethal concentration that kills 50 and 90% of exposed larvae respectively
FL fiducial confidence limits, SE standard error, χ 2 chi square value, P < 0.0001 highly significant, P < 0.05 significant
* Fiducial confidence limits could not be computed for 72 h because 100% mortality was observed in all the concentrations
** In S. abbasi at second day after treatment mortality was not observed
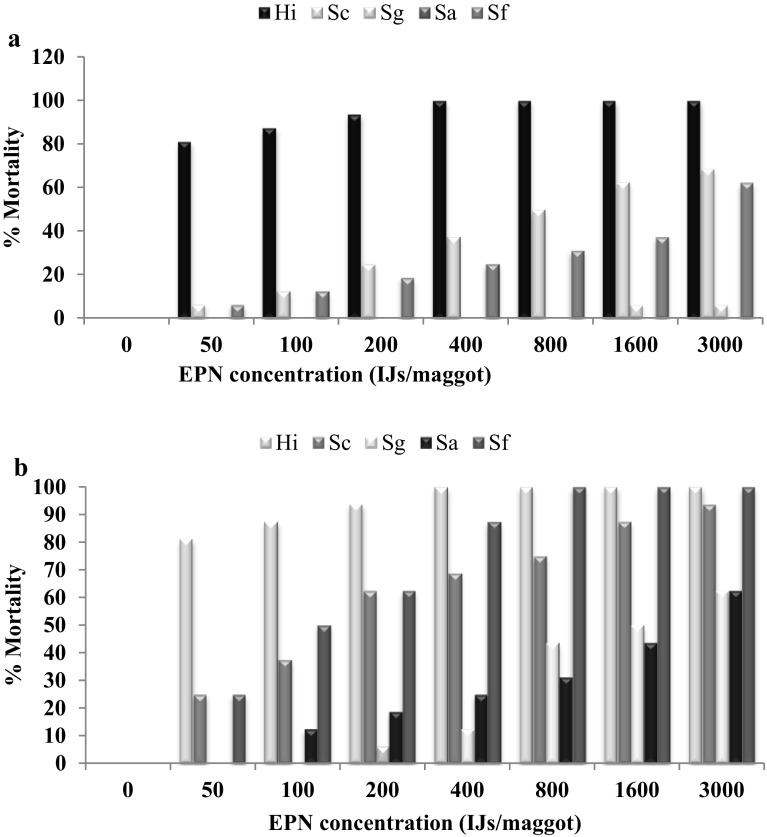
When third instar larvae exposed to all five EPNs, H. indica caused highest mortality of 81.25% (100%) at 50 (3000) IJs/larva 3(5) DAT. However S. glasseri and S. abbasi could not cause infection to M. domestica larva at 50 IJs/larva 3(5) DAT while S. abbasi at 3000IJs/larva 3DAT whereas lowest mortality of 62.5% observed 5DAT (Fig. 2a, b).
Fig. 2.
a, b Mean percent mortality of third instar on filter paper without food three and five day after treatment with different EPNs at different concentration
Analysis of variance showed that third instar of M. domestica mortality was significantly (P < 0.05) influenced by IJ concentration (F = 266.45; df = 7, 600; P < 0.0001), EPN species (F = 524.19; df = 4, 600; P < 0.0001), exposure times (F = 424.44; df = 4, 600; P < 0.0001). The interaction between EPN species and IJ concentrations (F = 13.76; df = 28, 600; P < 0.0001). The interaction between EPN species, IJ concentrations and exposure time (F = 5.11; df = 140, 600; P < 0.0001) was significantly different. The lowest LC50 and LC90 against third instar larvae was observed for H. indica were 814 (16) and 4363 (101) 3 (5) DAT, however the LC values could not be calculated for S. abbasi as there was no mortality 3DAT. The highest LC50 and LC90 were found for S. abbasi were 1798 and 26,477 5DAT (Table 3). The LC50 and LC90 values indicated that H. indica was more virulent against third instars of M. domestica.
Table 3.
LC50 and LC90 values of second instar larvae of Musca domesticaon filter paper with artificial food
| EPN species | DAT | LC50 | 95% FL | LC90 | 95% FL | Slope ± SE | χ 2 | P |
|---|---|---|---|---|---|---|---|---|
| H. indica | 3 | 88 | 25–165 | 1480 | 703–8242 | 1.04 ± 0.25 | 17.47 | <.0001 |
| 5 | 41 | 9–76 | 295 | 175–847 | 1.51 ± 0.40 | 14.15 | 0.0002 | |
| S. carpocapsae | 3 | 1109 | 602–3186 | 23,930 | 6322–799,429 | 0.96 ± 0.23 | 17.45 | <.0001 |
| 5 | 144 | 73–231 | 1321 | 720–4257 | 1.33 ± 0.26 | 25.86 | <.0001 | |
| S. glasseri | 3 | 996 | 669–1664 | 6068 | 3095–22,324 | 1.63 ± 0.29 | 30.89 | <.0001 |
| 5 | 171 | 112–245 | 804 | 511–1752 | 1.91 ± 0.32 | 34.18 | <.0001 | |
| S. abbasi | 3 | 2453 | 1502–6411 | 15,327 | 6015–160,161 | 1.61 ± 0.37 | 18.81 | <.0001 |
| 5 | 378 | 230–612 | 3655 | 1790–14,642 | 1.30 ± 0.24 | 29.28 | <.0001 | |
| S. feltiae | 3 | 8775 | 3506–605,135,957 | 50,257 | 9524–2.61825 × 1014 | 1.69 ± 0.74 | 5.12 | 0.0237 |
| 5 | 3482 | 1424–51,533 | 116,441 | 15,332–187,114,667 | 0.84 ± 0.24 | 11.43 | 0.0007 |
DAT-days after treatment, LC50 and LC90 lethal concentration that kills 50 and 90% of exposed larvae respectively
FL fiducial confidence limits, SE standard error, χ 2 chi square value, P < 0.0001 highly significant, P < 0.05 significant
Susceptibility of fly larvae on filter paper with artificial diet
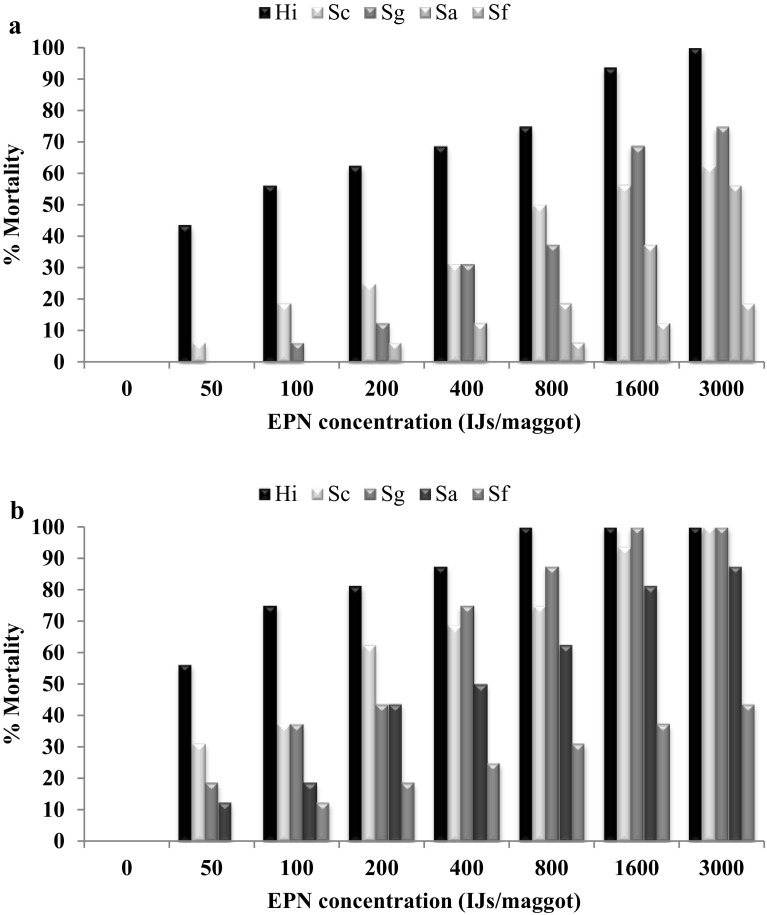
The results of susceptibility of second instar larvae to EPNs on filter paper with artificial diet was similar to that without providing diet to larvae on filter paper but when larvae provided with diet there was a decrease in percent mortality. Three DAT 100% mortality was observed in H. indica whereas lowest mortality of 18.75% observed in S. feltiae at 3000 IJs/larva (Fig. 3a). 100% mortality was observed in H. indica, S. carpocapsae, S. glasseri however only 43.75% was observed for S. feltiae at 3000 IJs/larva 5DAT (Fig. 3b).
Fig. 3.
a, b Mean percent mortality of second instar larvae of Musca domestica on filter paper provided with artificial food three and five day after treatment of different EPNs at different concentrations
The Analysis of variance observed for second instar of M. domestica mortality was significantly (P < 0.05) influenced by IJ concentration (F = 298.32; df = 7, 600; P < 0.0001), EPN species (F = 293.58; df = 4, 600; P < 0.0001), exposure times (F = 266.18; df = 4, 600; P < 0.0001). The interaction between EPN species and IJ concentrations (F = 8.57; df = 28, 600; P < 0.0001). The interaction between EPN species, IJ concentrations and exposure time (F = 2.77; df = 140, 600; P < 0.0001) was significantly different. The calculated lowest LC50 and LC90 were for H. indica, on second instar house fly larvae with artificial diet 3 (5) DAT were 88 (41) and 1480 (295), whereas lowest of 8775 (3482) and 50,257(116,441) for S. feltiae (Table 4). The LC50 and LC90 values indicated that H. indica, was more virulent against second instars of M. domestica with artificial diet.
Table 4.
LC50 and LC90 values of third instar of Musca domestica on filter paper with artificial food
| EPN species | DAT | LC50 | 95% FL | LC90 | 95% FL | Slope ± SE | χ 2 | P |
|---|---|---|---|---|---|---|---|---|
| H. indica | 3 | 814 | 560–1259 | 4363 | 2433–12,824 | 1.75 ± 0.30 | 33.71 | <.0001 |
| 5 | 16 | 0–42 | 101 | 32–377 | 1.62 ± 0.70 | 5.32 | 0.0211 | |
| S. carpocapsae | 3 | 886 | 526–1862 | 11,921 | 4309–118,606 | 1.13 ± 0.23 | 22.71 | <.0001 |
| 5 | 163 | 77–271 | 1983 | 987–8224 | 1.18 ± 0.24 | 23.81 | <.0001 | |
| S. glasseri | 3 | ** | ||||||
| 5 | 1560 | 1045–2853 | 8394 | 4096–38,998 | 1.75 ± 0.35 | 24.85 | <.0001 | |
| S. abbasi | 3 | ** | ||||||
| 5 | 1798 | 982–5837 | 26,477 | 7403–701,938 | 1.09 ± 0.25 | 18.30 | <.0001 | |
| S. feltiae | 3 | 2197 | 1051–12,364 | 55,536 | 10,611–8,104,407 | 0.91 ± 0.24 | 14.46 | 0.0001 |
| 5 | 110 | 70–154 | 416 | 277–881 | 2.21 ± 0.43 | 26.49 | <.0001 |
FL fiducial confidence limits, SE standard error, χ 2 chi square value, P < 0.0001 highly significant, P < 0.05 significant
** Mortality was not recorded at three day after treatment of EPNs in third instar larvae of Musca domestica. DAT-days after treatment, LC50 and LC90 lethal concentration that kills 50 and 90% of exposed larvae respectively
Mortality of third instar larvae when exposed to all the EPN in artificial diet, H. indica showed highest mortality of 56.25 (100%), whereas S. feltiae showed lowest mortality of 12.25(37.5%) at 3000IJs/larva 3(5) DAT (Fig. 4a, b).
Fig. 4.
a, b Mean percent mortality of third instar larvae of Musca domestica on filter paper with artificial food at three and five days after treatment with different dose of different EPNs
Analysis of variance observed that third instar of M. domestica with artificial diet mortality was significantly (P < 0.05) influenced by IJ concentration (F = 92.49; df = 7, 480; P < 0.0001), EPN species (F = 111.58; df = 4, 480; P < 0.0001), exposure times (F = 57.75; df = 3, 480; P < 0.0001). The interaction between EPN species and IJ concentrations (F = 3.03; df = 28; P < 0.0001). The interaction between EPN species, IJ concentrations and exposure time (F = 0.63; df = 105, 480; P = 0.9977) was not significantly different. The calculated lowest LC50 and LC90 for H. indica, on third instar house fly larvae 3 (5) DAT were 1456 (69) and 51,270 (1525), whereas the LC values could not be calculated for S. feltiae as there was no mortality 3DAT, the lowest LC50 and LC90 were for S. feltiae 5241 and 135,607 5DAT (Table 5). The LC50 and LC90 values indicated that H. indica was more virulent against third instars of M. domestica.
Table 5.
LC50 and LC90 values of third instar larvae of Musca domestica on filter paper with artificial food
| EPN species | DAT | LC50 | 95% FL | LC90 | 95% FL | Slope ± SE | χ 2 | P |
|---|---|---|---|---|---|---|---|---|
| H. indica | 3 | 1456 | 708–7033 | 51,270 | 9278–11,606,858 | 0.82 ± 0.22 | 13.41 | 0.0002 |
| 5 | 69 | 13–141 | 1525 | 685–11,362 | 0.95 ± 0.24 | 14.94 | 0.0001 | |
| S. carpocapsae | 3 | 2487 | 1103–21,299 | 84,453 | 12,823–47,568,034 | 0.83 ± 0.23 | 12.54 | 0.0004 |
| 5 | 429 | 171–1154 | 27,175 | 5122–10,387,651 | 0.71 ± 0.21 | 11.41 | 0.0007 | |
| S. glasseri | 3 | ** | ||||||
| 5 | 2772 | 1185–29,611 | 100,336 | 13,994–95,961,730 | 0.82 ± 0.23 | 12.01 | 0.0005 | |
| S. abbasi | 3 | 4568 | 3002–9.7 | 10,437 | 4808–2.4644 × 1036 | 3.57 ± 1.7 | 4.00 | 0.0456 |
| 5 | 1216 | 785–2286 | 8918 | 4011–46,833 | 1.48 ± 0.28 | 26.85 | <.0001 | |
| S. feltiae | 3 | |||||||
| 5 | 5241 | 1989–128,117 | 135,607 | 1716–336,567,111 | 0.90 ± 0.27 | 10.92 | 0.0010 |
FL fiducial confidence limits, SE standard error, χ 2 chi square value, P < 0.0001 highly significant, P < 0.05 significant
** The mortality was not recorded in third instar larvae of Musca domestica against S. glasseri at three day after treatment. DAT-days after treatment, LC50 and LC90 lethal concentration that kills 50 and 90% of exposed larvae respectively
All EPN species caused significantly (P < 0.05) higher mortality with increasing IJs concentrations. Fifth DAT irrespective of the IJs concentrations, the mortality of both house fly larval stages were significantly (P < 0.05) increased compared with the 3 DAT. Among the larval stages, irrespective of EPN species, second larval stage was significantly (P < 0.05) more susceptible compared with third instar larva of house fly.
Susceptibility of fly larvae in poultry manure
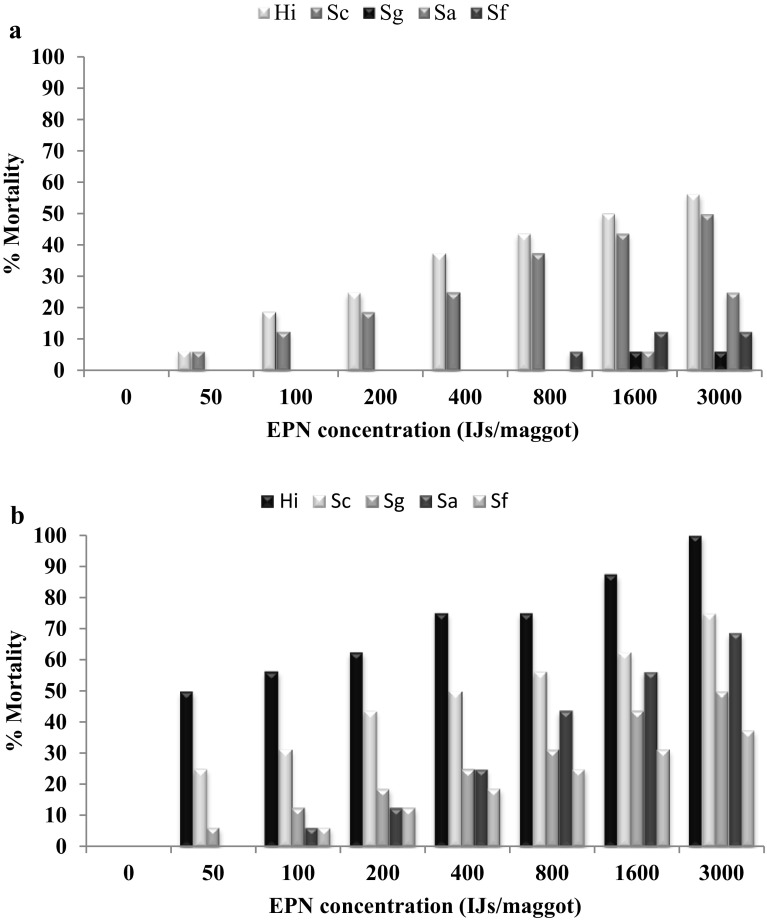
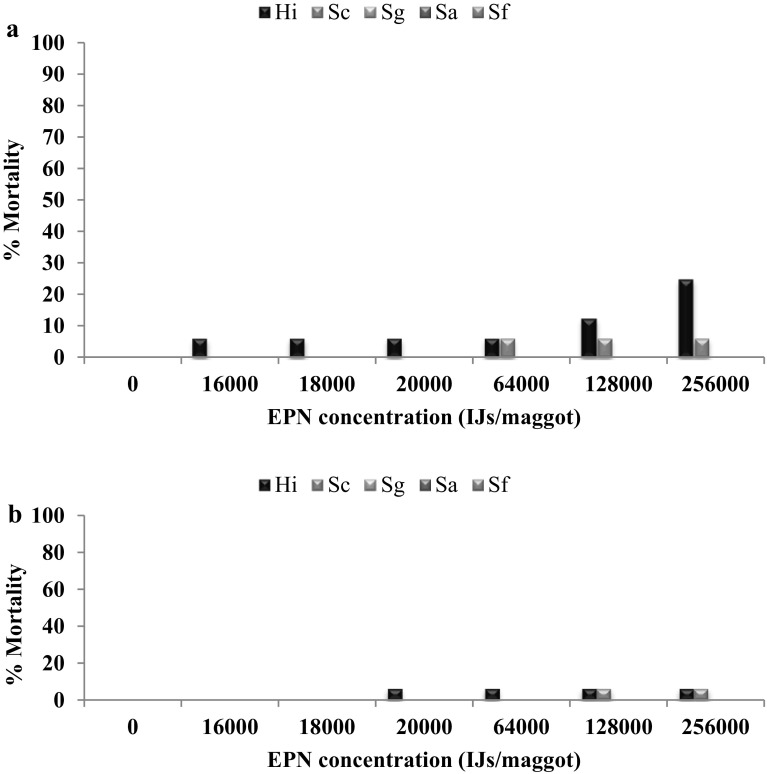
When comparing the efficacy of EPNs against larvae of M. domestica in plastic containers containing poultry manure, we could not notice larval mortality at even 50–3000 IJs/larva. Therefore EPNs dose has been increased 6000; 7000; 8000; 9000; 10,000; 12,000; 14,000; 16,000; 18,000; 20,000; 32,000; 64,000; 128,000; 256,000 IJs/larva. H. indica caused 6.25% mortality of second instar at 16,000 IJs/larva, whereas H. indica caused 6.25% mortality of third instar at 32,000 IJs/larva. S. carpocapsae caused 6.25% mortality of second instar at 64,000 IJs/larva, whereas S. carpocapsae caused 6.25% mortality third instar at 128,000/larva. Other three EPNs S. glasseri, S. abbasi and S. feltiae they do not cause any mortality in both second and third instar larvae even at the highest dose of 256,000 IJs/larva (Fig. 5a, b).
Fig. 5.
a, b Mean percent mortality of second and third instar larvae of Musca domestica against different EPN dose in poultry manure at two days after treatment
Progeny production
The progeny production assay revealed that all the five nematodes were able to reproduce in the insect larvae and produce IJs 1240, 1100, 595, 570 and 1080 IJs larva−1 respectively. H. indica produced significantly more number of infective juveniles per insect larva than the other nematode species. Among the five EPNs studied, the least progeny production was recorded for S. abbasi.
Discussion
In the present study revealed that, the survivability of EPNs in poultry manure was decreased as the exposure time increases. Among the EPN species tested in this study, infective juveniles of S. feltiae, H. indica, and S. carpocapsae showed higher survivability when compared to S. glasseri and S. abassi in poultry manure. After 24 h exposure of EPNs to the poultry manure, virulence capacity was remain 100% but their reproduction capacity decreased, this may be due to effect of toxic manure contents on the EPNs and poultry manure has negative effects on nematode survival. In poultry manure the presence of live IJs of S. feltiae, H. indica, and S. carpocapsae were recovered up to 96 h whereas, S. glasseri was recorded up to 72 h and S. abassi 24 h. Geden et al. (1986) reported that H. heliothidis juveniles were dead within 1 h of application to manure. Georgis et al. (1987) reported that 100% mortality was observed in S. feltiae, H. heliothidis and 93% in S. bibionis within 24 h of exposure of EPNs to the poultry manure. Renn et al. (1985) also reported that survivability of H. heliothidis and S. feltiae was reduced after exposing to the poultry manure. These data showed that, the survival of EPNs in poultry manure varies with nematode species and time of exposure. Many researchers reported that the decrease in the survival of EPNs in poultry manure may be due to presence of ammonia, salts or other materials associated with the poultry manure were probably toxic to the EPNs.
When we exposed fly eggs and pupae to EPNs at 5000 and 10,000 IJs/host, all the tested EPN species were unable to cause any mortality on eggs and pupae of M. domestica. Nevertheless, some researchers reported that the efficacy of EPNs against insect eggs e.g. ovicidal activity up to 84% was observed at 200 IJ/50 and 100 eggs of Helicoverpa armigera and Spodoptera litura, respectively (Kalia et al. 2014). Egg hatching period for H. armigera is 3–5 days (Patel et al. 2011). However, M. domestica egg hatching period is 8–12 h at 25–35 °C (Zumpt 1965) 6–24 h (Muniyellappa 2010) since nematodes need at least 24–72 h exposure time for killing of host, therefore in the present we could not notice any egg mortality. In the present study pupae were resistant to all EPNs tested. Similarly, Renn et al. (1985) pupae were refracted to the EPNs but Geden et al., reported that nematodes had entered and parasitized a small number of pupae upon dissection of pupae i.e. 1 and 2% in case of S. feltiae and H. heliothidis respectively but they suggested that these infections may be due to result of physical damage to the puparia during handling before treatment in Petri dishes. The house fly pupae were refractory the parasitism which may be due to the formidable barrier presented by the puparium. The pupae of some insects appear to be less susceptible to nematode infection than the larval stages (Kaya and Hara 1980). The only portal of entry to the insect puparia of M. domestica is via the spiracles, but the presence of spiracular slits within these openings may have prevented penetration (Bedding and Molyneux 1982).
In the present study the LC50 and LC90 values indicated that second instar larvae were more susceptible to all EPN species compared to third instar larvae. Similarly, Geden et al. (1986) reported that second instar larvae of M. domestica were more susceptible than third instar larvae. Whereas, Renn et al. (1985) and Mahmoud et al. (2007) reported that third instar larvae of M. domestica were more susceptible than second instar larvae. The lower susceptibility of third instar larvae compared with second instar larvae was probably may be due to the shorter effective exposure period of the larvae and also cuticular changes in the larvae before pupation may further reduce the successful infection by H. heliothidis (Geden et al. 1986). Susceptibility of M. domestica to EPNs depends on developmental stages of the insect and their effect varies with the nematode species. In the present study, among the EPN species tested, H. indica showed high virulence capacity compared to other EPN species. This is in contrast to the finding of an earlier report where S. feltiae was most virulent than H. heliothidis (Renn et al. 1985). S. feltiae was the most virulent species toward maggots followed by S. carpocapsae in the filter paper assay (Taylor et al. 1998). Mullens et al. (1987) and Taylor et al. (1998) reported that Heterorhabditis species had low virulence whereas Geden et al. (1986) reported that H. heliothidis was most virulent than S. feltiae. However in present study, we could record the pathogenicity of S. glasseri on larvae of M. demoestica whereas Geden et al. (1986) reported that larvae were not susceptible to S. glasseri. These results indicated that pathogenicity of EPN species may also varies with isolate to isolate. Therefore, for the effective management of target pests, choosing the appropriate nematode species and isolates are paramount.
We found that, larvae were more susceptible to EPNs on filter paper without artificial diet compared to when provided with artificial diet. Similarly, Geden et al. (1986) also reported that, in artificial rearing media the mortality has been reduced. This may be due to presence of food to the larvae, the larvae develop fast leading to shorter exposure period and also changes in the cuticle leads to reduced infection by EPNs. The moist inner region of the medium where the larvae concentrated has poor oxygen level leads to the poor survival Geden et al. (1986). We also speculate that, due to sticky nature of artificial diet makes reduced nematode movement leads to poor host searching ability of EPNs. When we studied the efficacy of EPNs against M. demosetica in poultry manure, H. indica caused 25% mortality in second instar maggots whereas, S. carpocapsae caused 6.25% mortality at 256,000 IJs/larva and rest of the EPN species not cause any mortality in this study. Whereas, Renn et al. (1985) reported that maggots of M. domestica were parasitized after inoculation of S. feltiae and H. heliothidis at dose of 5000/larva in poultry manure. However, in absence of manure EPN species caused 100% mortality to M. domestica. These results indicated that, the poor survival and limited movement of nematodes in poultry manure appear to make them unlikely candidates for biological control of M. domestica.
To be successful as an insect pathogen, reproduction and recycling of EPNs in the host play a vital role in their persistence in the environment after application, and thus in overall effectiveness of pest control (Harlan et al. 1971; Georgis and Hague 1981; Sharifi et al. 2014; Patil et al. 2016). In this present study, all EPN species reproduced in third instar of M. domestica and their IJs emerged from the host cadavers. However, progeny production assay revealed H. indica was significantly greater than that of S. feltiae, S. carpocapsae, S. glasseri, S. abbassi. Moreover, our results of progeny production agree with the data of Mannion and Jansson (1992); Yadav and Lalramliana 2012; Gowda et al. (2016) and Patil et al. (2016). One possible reason for this difference in EPNs progeny production in M. domestica, may be related to the type of reproduction of Heterorhabditids. H. indica is a hermaphrodite that produces more offspring than Steinernematids, which are amphimictic (Poinar 1990; Mannion and Jansson 1992).
In conclusion, our findings showed that S. feltiae, H. indica, and S. carpocapsae showed higher survivability in poultry manure compared to S. glasseri and S. abbassi which were susceptible to manure. The fly eggs and pupae were refractory to all the five different EPNs used. Second instar larvae were more susceptible to all EPNs compared to third instar larvae. The fly larvae were susceptible to all the five EPN species and all these EPNs successfully reproduced in third instar larvae. Larvae were more susceptible to EPNs on filter paper without artificial diet compared to when provided with artificial diet. H. indica showed high virulence capacity compared to other EPNs (Steinernematid) used. Poultry manure assay revealed that, H. indica and S. carpocapsae caused minimal mortality where as S. feltiae, S. glaseri and S. abbasi did not cause any mortality. The decrease in larval mortality in manure suggests that biocontrol of housefly by using EPNs is unlikely. This may be because of poor survival and limited movement of nematodes in poultry manure which may be due to ammonia, other toxic substances in poultry manure. It may, therefore, be concluded from this study that these EPN isolates are not potential biocontrol agents against house fly M. domestica in poultry manure.
Acknowledgements
The authors thankfully acknowledge Dr. Abraham Verghese, Director, NBAIR, for giving permission and providing facilities for conducting the work. The facilities provided by the ICAR Centre of Advanced Faculty Training in Veterinary Parasitology are gratefully acknowledged. We thank the Department of Science and Technology for awarding INSPIRE fellowship for PhD programme of the first author. The paper is based on a part of the PhD thesis by the first author to the KVAFSU, Bidar.
References
- Achra A, Prakash P, Verma B, Amar A. Unusual presentation of intestinal myiasis due to Musca domestica: a report of two cases. Asian J MedSci. 2014;6(1):124–126. [Google Scholar]
- Barin A, Arabkhazaeli F, Rahbari S, Madani S. The house fly, Musca domestica, as a possible mechanical vector of Newcastle disease virus in the laboratory and field. Med Vet Entomol. 2010;24:88–90. doi: 10.1111/j.1365-2915.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barro N, Aly S, Tidiane OCA, Sababenedjo TA. Carriage of bacteria by proboscises, legs, and feces of two species of flies in street food vending sites in Ouagadougou, Burkina Faso. J Food Protect. 2006;69(8):2007–2010. doi: 10.4315/0362-028X-69.8.2007. [DOI] [PubMed] [Google Scholar]
- Bedding RA, Molyneux AS. Penetration of insect cuticle by infective juveniles of Heterorhabditis spp. (Heterorhabditidae: Nematoda) Nematologica. 1982;28:354–359. doi: 10.1163/187529282X00402. [DOI] [Google Scholar]
- Belton P, Rutherford TA, Trotter DB, Webster JM. Heterorhabditis heliothidis: a potential biological control agent of house flies in caged-layer poultry barns. J Nematol. 1987;19:263–266. [PMC free article] [PubMed] [Google Scholar]
- D’assis Fonseca (1968) Handbooks for the identification of British insects. Diptera Cyclorrhapha Calyptrate section (b) Muscidae. Royal Entomological society of London
- De jesus AJ, Olsen AR, Bryce JR, Whiting RC. Quantitative contamination and transfer of Escherichia coli from foods by houseflies, Musca domestica L. (Diptera: Muscidae) Int J Food Microbiol. 2004;9:259–262. doi: 10.1016/j.ijfoodmicro.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Geden CJ, Axtell RC, Brooks WM. Susceptibility of the house fly, Musca domestica (Diptera: Muscidae), to the entomogenous nematodes Steinernema feltiae, S. glaseri (Steinernematidae), and Heterorhabditis heliothidis (Heterorhabditidae) J Med Entomol. 1986;23(3):326–332. doi: 10.1093/jmedent/23.3.326. [DOI] [PubMed] [Google Scholar]
- Georgis R, Hague NGM. A neoaplectanid nematode in the larch sawfly Cephalcia lariciphila (Hymenoptera: Pamphiliidae) Ann Appl Biol. 1981;99:171–177. doi: 10.1111/j.1744-7348.1981.tb05144.x. [DOI] [Google Scholar]
- Georgis R, Mullens BA, Meyer JA. Survival and movement of insect parasitic nematodes in poultry manure and their infectivity against Musca domestica. J Nematol. 1987;19(3):292–295. [PMC free article] [PubMed] [Google Scholar]
- Gowda M, Patil J, Mansheppa D, Rangasamy V, Verghese A. Entomopathogenic nematodes: a potential biocontrol agent against eggplant ash weevil Myllocerus subfaciatus Guerin (Coleoptera: Curculionidae) Nematology. 2016;18:743–750. doi: 10.1163/15685411-00002989. [DOI] [Google Scholar]
- Grewal PS, Koppenhofer AM, Choo HY. Lawn, turfgrass and pasture applications. In: Grewal PS, Ehlers RU, Shapiro-Ilan DI, editors. Nematodes as biocontrol agents. Wallngford: CABI Publishing; 2005. pp. 115–146. [Google Scholar]
- Harlan DP, Dutky SR, Padgett GR, Mitchell JA, Shaw ZA, Barlett FJ. Parasitism of Neoaplectana dutkyi in white-fringed beetle larvae. J Nematol. 1971;3:280–283. [PMC free article] [PubMed] [Google Scholar]
- Hebert PDN, Cywinska A, Ball SL, Dewaard JR. Biological identifications through DNA barcodes. Proc R Soc Lond B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal W, Malik MF, Sarwar MK, Azam I, Iram N, Rashda A. Role of housefly (Musca domestica, Diptera; Muscidae) as a disease vector; a review. J Entomol Zool Stud. 2014;2(2):159–163. [Google Scholar]
- Kalia V, Sharma G, Shapiro-Ilan DI, Ganguly S. Biocontrol potential of Steinernema thermophilum and its symbiont Xenorhabdus indica against Lepidopteran pests: virulence to egg and larval stages. J Nematol. 2014;46(1):18–26. [PMC free article] [PubMed] [Google Scholar]
- Kaya HK, Hara AH. Differential susceptibility of Lepidopterous pupae to infection by the nematode Neoaplectana carpocapsae. J Invertebr Pathol. 1980;36:389–393. doi: 10.1016/0022-2011(80)90043-9. [DOI] [Google Scholar]
- Keiding J. The housefly: biology and control. Geneva: World Health Organization; 1986. p. 63. [Google Scholar]
- Khan HAA, Shad SA, Akram W. Resistance to new chemical insecticides in the housefly (Musca domestica) from dairies in Punjab, Pakistan. Parasitol Resist. 2013;9:18. doi: 10.1007/s00436-013-3365-8. [DOI] [PubMed] [Google Scholar]
- Khoobdel M, Jonaidi N, Seiedi M. Blowfly and flesh (Diptera: Cyclorrhpha) fauna in Tehran, Iran. J Entomol. 2008;5(3):85–92. [Google Scholar]
- Loftin KM, Hopkins JD, Corder R (2014) Biology and control of flies in poultry facilities. Division of agriculture research & extension University of Arkansas System University of Arkansas, United States Department of Agriculture, and County Governments Cooperating
- Mahmoud MF, Mandour NS, Pomazkov YI. Efficacy of the entomopathogenic nematode Steinernema feltiae cross n 33 against larvae and pupae of four fly species in the laboratory. Nematol Medit. 2007;35:221–226. [Google Scholar]
- Mannion CM, Jansson RK. Comparison of ten entomopathogenic nematodes for control of sweet potato weevil (Coleoptera: Apionidae) J Econ Entomol. 1992;85:1642–1650. doi: 10.1093/jee/85.5.1642. [DOI] [Google Scholar]
- Molyneux AS, Bedding RA, Ackhurst RJ. Susceptibility of larvae of the sheep blow fly Lucilia cuprina to various Heterorhabditis spp., Neoaplectana spp. and an undescribed steinernematid (Nematoda) J Invertebr Pathol. 1983;42:1–7. doi: 10.1016/0022-2011(83)90196-9. [DOI] [PubMed] [Google Scholar]
- Mullens BA, Meyer JA, Cyr TL. Infectivity of insect-parasitic nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) for larvae of some manure-breeding flies (Diptera: Muscidae) Environ Entomol. 1987;16:769–773. doi: 10.1093/ee/16.3.769. [DOI] [PubMed] [Google Scholar]
- Muniyellappa HK (2010) Fly menace- A social, economic and health burden to poultry farmers. Varshavahini 4(3)
- Patel RS, Patel KAK, Patil S, Toke NR. Biology of Helicoverpa armigera Hub. on rose in laboratory condition. Pest Manag Hortic Ecosyst. 2011;17(2):144–148. [Google Scholar]
- Patil J, Rajkumar, Subhaharan K. Synergism of entomopathogenic nematode and imidacloprid: a curative tool to coconut white grub, Leucopholis coniophora (Coloeptera: Melolonthinae) Vegetos. 2015;28(1):184–190. [Google Scholar]
- Patil J, Rangasamy V, Verghesee A. Efficacy of indigenous Steinernema abbasi and Heterorhabditis indica isolates as potential biocontrol agent against Holotrichia consanguinea blanch. (Coleoptera: Scarabaeidae) Nematology. 2016;18:1045–1052. doi: 10.1163/15685411-00003013. [DOI] [Google Scholar]
- Poinar GO. Taxonomy and biology of steinernematidae and heterorhabditidae. In: Gaugler R, Kaya HK, editors. Entomopathogenic nematodes in biological control. Boca Raton: CRC Press; 1990. pp. 23–61. [Google Scholar]
- Poinar GO, O’Callaghan M. Nematodes associated with scarabaeidae. In: Glare TR, Jackson TA, editors. Use of pathogens in scarabs pest management. Andover: Intercept; 1992. pp. 93–106. [Google Scholar]
- Renn N, Barson G, Richardson PN. Preliminary laboratory tests with two species of entomophilic nematodes for control of Musca domestica in intensive animal units. Ann Appl Biol. 1985;106:229–233. doi: 10.1111/j.1744-7348.1985.tb03112.x. [DOI] [Google Scholar]
- Sehgal R, Bhatti HPS, Bhasin DK, Sood AK, Nada R, Malla N, et al. Intestinal myiasis due to Musca domestica: a report of two cases. Jpn J Infect Dis. 2002;55:191–193. [PubMed] [Google Scholar]
- Sharifi S, Karimi J, Hosseini M, Rezapanah M. Efficacy of two entomopathogenic nematode species as potential biocontrol agents against the rosaceae longhorned beetle, Osphranteria coerulescens, under laboratory conditions. Nematology. 2014;16:729–737. doi: 10.1163/15685411-00002802. [DOI] [Google Scholar]
- Shekhawat PS, Joshi KR, Shekhawat R. Contaminated milk powder and intestinal myiasis. Ind Pediatr. 1993;30:1138–1139. [PubMed] [Google Scholar]
- Tamilam TV, Ponnudurai G, Harikrishnan TJ, Balasubramanian GA. Efficacy of thermocole coated with spores of Metarhizium anisopliae and Baeuveria bassiana against adult Musca domestica. J Vet Parasitol. 2010;24(2):155–158. [Google Scholar]
- Taylor DB, Szalanski AL, Adams BJ, Peterson RD. Susceptibility of house fly (Diptera: Muscidae) larvae to entomopathogenic nematodes (Rhabditida: Heterorhabditidae, Steinernematidae) Environ Entomol. 1998;27(6):1514–1519. doi: 10.1093/ee/27.6.1514. [DOI] [Google Scholar]
- Tomerlin RJ. The US food quality protection act—policy implications of variability and consumer risk. Food Addit Contam. 2000;17:641–648. doi: 10.1080/026520300412573. [DOI] [PubMed] [Google Scholar]
- Van Emden FI (1965) The fauna of India and the adjacent countries. Diptera. Manager of publications, Government of India, Delhi 7(1)
- Woodring JL, Kaya HK (1988). Steinernematidand heterorhabditid nematodes. In: A handbook of techniques. Series Bulletin 331. Fayetteville, Arkansas Agricultural Experiment Station 30
- Yadav A, Lalramliana K. Evaluation of the efficacy of three indigenous strains of entomopathogenicnematodes from Meghalaya, India against mustard sawfly, Athalia lugens proxima Klug (Hymenoptera:Tenthredinidae) J Parasit Dis. 2012;36(2):175–180. doi: 10.1007/s12639-012-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumpt F. Myiasis in man and animals in the old world: a textbook for physicians, veterinarians and zoologists. London: Butterworths; 1965. [Google Scholar]