Abstract
Orexin neurons are exclusively localized in the lateral hypothalamic area and project their fibers to the entire central nervous system, including the histaminergic tuberomammillary nucleus (TMN). Dysfunction of the orexin system results in the sleep disorder narcolepsy, but the role of orexin in physiological sleep–wake regulation and the mechanisms involved remain to be elucidated. Here we provide several lines of evidence that orexin A induces wakefulness by means of the TMN and histamine H1 receptor (H1R). Perfusion of orexin A (5 and 25 pmol/min) for 1 hr into the TMN of rats through a microdialysis probe promptly increased wakefulness for 2 hr after starting the perfusion by 2.5- and 4-fold, respectively, concomitant with a reduction in rapid eye movement (REM) and non-REM sleep. Microdialysis studies showed that application of orexin A to the TMN increased histamine release from both the medial preoptic area and the frontal cortex by ≈2-fold over the baseline for 80 to 160 min in a dose-dependent manner. Furthermore, infusion of orexin A (1.5 pmol/min) for 6 hr into the lateral ventricle of mice produced a significant increase in wakefulness during the 8 hr after starting infusion to the same level as the wakefulness observed during the active period in wild-type mice, but not at all in H1R gene knockout mice. These findings strongly indicate that the arousal effect of orexin A depends on the activation of histaminergic neurotransmission mediated by H1R.
The neuropeptides orexin A and B (also called hypocretin 1 and 2) were recently isolated and identified from rat hypothalamic extracts (1, 2), and implicated in feeding, energy homeostasis, and reproduction (3–6). An extensive extrahypothalamic network of orexin-like immunoreactive fibers and distribution of orexin receptor mRNA in the rat brain suggest that orexins may have broader effects within the central nervous system (7–15). Pre-pro-orexin (the precursor for both orexins A and B) gene knockout (KO) mice exhibit a phenotype with many similarities to human narcolepsy (14), a disabling neurological disorder characterized by excessive daytime sleepiness, sleep attacks, sleep fragmentation, cataplexy, sleep onset rapid eye movement (REM) sleep periods, and hypnagogic hallucinations (16–18). Thus, mice lacking orexin peptide display increases in REM and non-REM (NREM) sleep and a decrease in awake time during the active period of normal rodents (14). When rats were treated with saporin–orexin conjugates to selectively produce lesions in orexinergic neurons in the lateral hypothalamus, they had markedly decreased locomotor activity and clear hypersomnolence in addition to some features of narcolepsy (48). It had been shown earlier that canine narcolepsy is caused by a mutation in the orexin-2 receptor gene (19). Furthermore, human narcolepsy is not associated with orexin mutations, but most patients have deficient orexin systems such as a significant decrease in the orexin A concentration in the cerebrospinal fluid (20), and absence of orexin mRNA and peptide in the central nervous system (21, 22). These findings indicate that orexin–orexin-2 receptor interaction is involved in pathological sleep regulation in humans and animals. However, the role of orexin in physiological sleep and the mechanisms involved in vigilance control are still unknown.
In the rodent central nervous system, orexin-containing cells are a small group of neurons restricted to the lateral and posterior hypothalamus and perifornical areas (1, 7, 10, 14, 23). Despite their highly restricted origin, immunohistochemical studies have shown that orexin neurons project widely throughout the entire neuroaxis (6). Particularly abundant projections are found in monoaminergic cell groups, including histaminergic cells of the tuberomammillary nucleus (TMN) (7, 14), in which orexin-2 receptors are enriched (24, 25). It is well known that activation of the histaminergic system promotes wakefulness through activation of histamine H1 receptor (H1R) (26–28). Furthermore, administration of modafinil, an increasingly popular wake-promoting drug used for the treatment of narcolepsy, produces wakefulness in rats in association with activation of the TMN (29). These observations suggest that the histaminergic and orexinergic systems may play a role in the regulation of wakefulness in the TMN.
In the present study, we applied an in vivo microdialysis probe to deliver orexin A into the TMN in rats to study the behavioral changes and histamine release from the medial preoptic area (MPO) and frontal cortex (FrCx), both of which have been implicated in the arousal effect of histamine (30–32). We used H1R KO mice to clarify the roles of H1R in the sleep–wake effect of orexin A. Here, we found that orexin A perfusion into the TMN of rats promptly induced wakefulness, with a reduction in NREM and REM sleep. The arousal effect induced by orexin A paralleled an increase in histamine release from the MPO and FrCx. Furthermore, orexin A significantly increased arousal in wild-type (WT) mice, but not at all in H1R KO mice, strongly indicating that the histaminergic system plays a crucial role in the arousal effect of orexin A.
Materials and Methods
Animals.
Male Sprague–Dawley rats, weighing 260–320 g (7–11 weeks old), were purchased from Shizuoka Laboratory Animal Center, Shizuoka, Japan. Male H1R KO and WT mice of the inbred C57BL/6 strain (33), weighing 20–26 g (11–13 weeks old), were maintained at Oriental Bioservice (Kyoto, Japan) and used in these experiments. They were housed at a constant temperature (24 ± 0.5°C for rats and 22 ± 0.5°C for mice) with a relative humidity (60 ± 2%) on an automatically controlled 12:12 light/dark cycle (light on at 8 a.m.), and had free access to food and water. The experimental protocols were approved by the Animal Care Committee of Osaka Bioscience Institute.
Chemicals.
Orexin A (Peptide Institute, Osaka, Japan) was dissolved in sterile saline, and the pH was adjusted to ≈7 with 0.5 μM NaOH (34). Aliquots were prepared and frozen at −20°C for each experiment and thawed immediately before use. All other chemicals were of analytical grade.
Electroencephalogram (EEG) and Electromyogram (EMG) Recordings with Orexin A Perfusion into the TMN in Rats.
Under pentobarbital anesthesia (50 mg/kg, i.p.), rats underwent surgery for implantation of electrodes for EEG and EMG recordings and placement of a guide cannula for the microdialysis probe as described (35, 36). Briefly, a guide cannula (outer diameter, 0.6 mm) with an in-dwelling stylet was directed stereotaxically into the TMN. The coordinates of the guide tip were as follows: anteroposterior (AP), −4.5 mm; left–right (LR), −0.8 mm; dorsoventral, −7.2 mm from bregma according to the atlas of Paxinos and Watson (37). When perfusion was started, the stylet was replaced by the dialysis probe, which protruded 2 mm beyond the guide tube. The cannula and electrodes were fixed on the skull with dental cement and four stainless steel screws were anchored to the skull. Two stainless steel wire electrodes for EMG recordings were placed into the neck muscles. Postoperatively, each rat was allowed 10 days of recovery, then transferred to a sound-proof recording chamber and connected with an EEG/EMG recording cable for 3 days of habituation to experimental conditions.
At least 20 hr before the recording session, the stylet of the microdialysis guide cannula was replaced by a microdialysis probe (CMA/Microdialysis, Stockholm) consisting of a semipermeable membrane having a tip length of 2 mm, an outer diameter of 0.5 mm, and a molecular cut-off size of 20 kDa. The probe was continuously perfused with artificial cerebrospinal fluid (140 mM NaCl/3 mM KCl/1.0 mM MgCl2/1.3 mM CaCl2/2 mM Na2HPO4/2 mM NaH2PO4, pH 7.4) at a flow rate of 2 μl/min. The EEG/EMG signals were amplified and filtered (EEG, 0.5–30 Hz; EMG, 16–128 Hz), then digitized at a sampling rate of 128 Hz and recorded by using a data acquisition program SLEEPSIGN (Kissei Comtec, Nagano, Japan) as described (38). Baseline and experimental recordings were taken in each rat for 2 consecutive 24-hr periods, starting at 8 a.m. On the experimental day, orexin A, at a dose of 1, 5, or 25 pmol/2 μl per min, was perfused from 9 to 10 a.m. in the experimental group.
EEG and EMG Recordings with Microinfusion of Orexin A in H1R KO Mice.
Under pentobarbital anesthesia (50 mg/kg, i.p.), mice were implanted with EEG and EMG electrodes for polysomnographic recordings. For monitoring EEG signals, two stainless steel screws were placed over the cortex (AP, 1.0 mm and LR, −1.5 mm from bregma or lambda). EMG activity was monitored by stainless steel, Teflon-coated wires bilaterally placed into both trapezius muscles. One stainless-steel cannula (outer diameter, 0.2 mm) for continuous infusion of orexin A was stereotaxically placed 2 mm lateral from bregma, and inserted to a depth of 2.2 mm from the surface of the cortex at an angle of 25° from the midsagittal plane, aimed at a site in the lateral ventricle according to the atlas of Franklin and Paxinos (39). The cannula and electrodes were fixed to the skull with dental cement. The infusion and recordings of EEG and EMG were carried out by means of a swivel and a slip ring, designed so that behavioral movement of the mouse was not restricted.
After a 10-day recovery period, the mice were placed in experimental cages for adaptation and the continuous infusion of artificial cerebrospinal fluid into the lateral ventricle was commenced at a speed of 1 μl/hr. After an acclimation period of 4 days, sleep–wakefulness states were monitored for a period of 48 hr, which comprised baseline and experimental days. The EEG/EMG signals were amplified and filtered (EEG, 0.5–30 Hz; EMG, 20–200 Hz), then digitized at a sampling rate of 128 Hz and recorded by using SLEEPSIGN as described (40). Baseline recordings were taken in each animal for 24 hr, beginning at 8 a.m., which served as the control for the same animal. On the next day orexin A was infused into the lateral ventricle at 1.5 pmol/min for 6 hr between 8 a.m. and 2 p.m.
Vigilance State Analysis.
The vigilance states were automatically classified off-line by 4-s epochs for mice and 10-s epochs for rats, into three stages of wake, NREM, and REM sleep by SLEEPSIGN, according to the standard criteria (38, 40, 41). As a final step, defined sleep–wake stages were examined visually, and corrected, if necessary.
Microdialysis Procedure.
Rats were anesthetized with urethane (1.2 g/kg, i.p.). Two microdialysis probes were inserted stereotaxically, one into the TMN (AP, −4.5 mm; LR, −0.8 mm; dorsoventral, −9.2 mm; membrane length, 2 mm) for administration of orexin A, and the other into the MPO (AP, −1.0 mm; LR, −0.5 mm; dorsoventral, −8.6 mm; membrane length, 2 mm) or the FrCx (AP, +3.2 mm; LR, −1.0 mm; dorsoventral, −4.8 mm; membrane length, 3 mm) for collecting the extracellular histamine. Both probes were perfused with artificial cerebrospinal fluid at a flow rate of 2 μl/min. Orexin A was diluted in artificial cerebrospinal fluid to the concentrations needed. Two hours after insertion of the microdialysis probes, dialysates were continuously collected from the MPO or FrCx at 20-min intervals (40 μl each) for 1 hr before the perfusion, during perfusion of orexin A for 1 hr, and until 2 hr after perfusion. The dialysates were kept at −20°C until they were assayed for histamine by HPLC and fluorometry (42). Histamine output was observed to be stable 2 hr after implantation of the probe. Thus, the mean value of histamine output found during the next 1 hr was defined as the basal output, and the subsequent fractions were expressed as percentages of this value.
Histological Verification.
When an experiment was over, both mice and rats were killed with an overdose of pentobarbital sodium and injected through the implanted cannulae, or perfused through the microdialysis probes with a microquantity of pontamine sky-blue dye solution (0.5% wt/vol) to verify the site of orexin A administration.
Statistical Analysis.
For vigilance studies, amounts of the different sleep–wake states were expressed in minutes. Statistical analyses were performed by use of the paired t test, with each animal serving as its own control. For the microdialysis data, two-way analysis of variance, followed by the Fisher's probable least-squares difference test, was used to determine whether the difference between groups was statistically significant. In all cases, P < 0.05 was taken as the level of significance.
Results
Orexin A Perfusion into the TMN Increased Wakefulness in Rats.
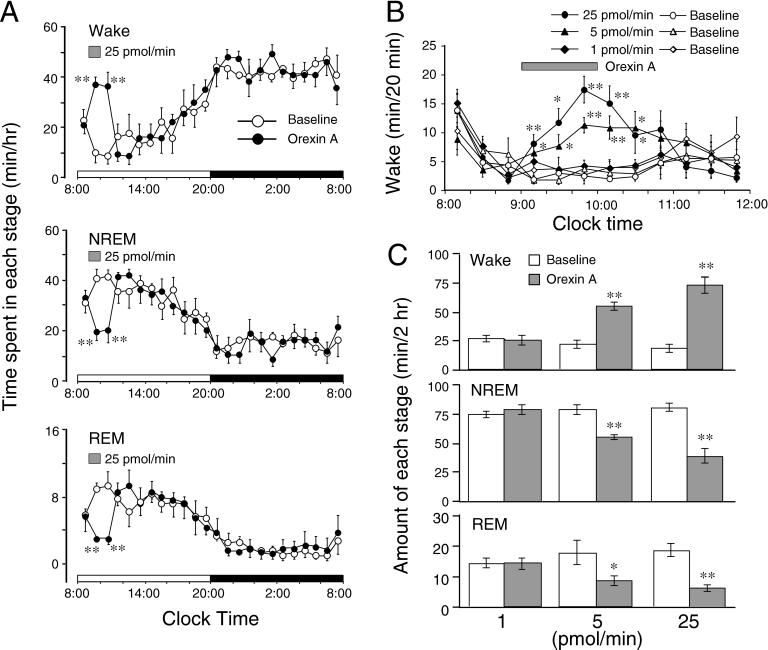
We perfused orexin A into the TMN through a microdialysis probe for 1 hr, from 9 to 10 a.m. to investigate the changes in sleep- stage distribution. As shown in Fig. 1A, orexin A perfusion at 25 pmol/min significantly and rapidly increased the wake time by 3.7-fold during the 1-hr orexin A perfusion; and the arousal effect was maintained even 1 hr after the perfusion, when the wakefulness was compared with that of the baseline day. This enhancement of wakefulness was concomitant with decreases in NREM and REM sleep. Orexin A (25 pmol/min) decreased NREM sleep by 50% and 52%, and reduced REM sleep by 66% and 70% during the 1-hr perfusion and 1 hr after dosing, respectively. The amounts of wakefulness, NREM, and REM sleep during these 2 hr were almost comparable to those observed during the night, an active period of rats. There was no further disruption of sleep architecture during the subsequent period. Similar time course profiles were observed with a lower concentration of orexin A. Orexin A (5 pmol/min) increased time spent awake by 2.1- and 3.1-fold, decreased amounts of NREM sleep by 20% and 43%, and reduced amounts of REM sleep by 45% and 56% during the 1-hr perfusion and 1 hr after dosing, respectively. When the time course changes in wakefulness were analyzed at 20-min intervals (Fig. 1B), orexin A dose-dependently increased wakefulness from the first 20 min after starting the perfusion at doses of 5 and 25 pmol/min, and the arousal effects of orexin A reached maximal at the third 20-min period, then gradually returned to the basal levels at 1 hr after finishing the perfusion. However, orexin A perfusion at 1 pmol/min had little effect on the sleep-stage distribution.
Figure 1.
Sleep-stage distribution produced by orexin A perfusion of the TMN of rats. (A) Time course changes in a 25 pmol/min treated group, with each circle representing the hourly mean ± SEM amounts of wake, NREM, or REM sleep. (B) Time course changes in wakefulness at 20-min intervals after orexin A perfusion, with each symbol representing mean ± SEM amount of wake in 20 min. In both A and B, open and closed symbols stand for the baseline and experimental day profiles, respectively. The horizontal shaded bars indicate orexin A perfusion between 9 and 10 a.m. on the experimental day. (C) Total time spent in wake, NREM, and REM sleep for 2 hr after starting the 1-hr perfusion. Open and shaded columns show the baseline and experimental day profiles, respectively. Values are means ± SEM (n = 6 in 1 and 25 pmol/min groups; n = 5 in the 5 pmol/min group). **, P < 0.01; *, P < 0.05 by paired t test.
We calculated the total time spent in wake, NREM, and REM sleep for 2 hr, that is, during orexin A perfusion for 1 hr and 1 hr postperfusion (Fig. 1C). Orexin A given at 5 and 25 pmol/min increased the total amounts of wake during those 2 hr by 2.5- and 4-fold, and reduced NREM sleep by 30% and 54% and REM sleep by 50% and 70%, respectively. There was essentially no difference between orexin A (1 pmol/min) perfusion and the baseline day. These results clearly indicate that orexin A dose-dependently increased wakefulness and concomitantly reduced NREM and REM sleep.
Orexin A Perfusion into the TMN Increased Histamine Release in Anesthetized Rats.
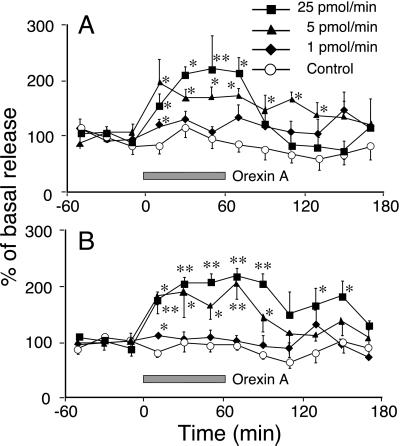
To examine the activation of the histaminergic system by orexin A, we monitored the histamine release from the MPO and FrCx after perfusion of the TMN with orexin A in urethane-anesthetized rats. As shown in Fig. 2A, orexin A perfusion of the TMN for 1 hr induced a significant increase in histamine release from the MPO in a dose-dependent manner. Orexin A at 5 pmol/min produced rapid and significant elevation of histamine release, with the maximal level of release being 2-fold over the baseline level at the first 20-min sampling. The elevated level was maintained during orexin A perfusion and for even 80 min after the perfusion. At its highest concentration (25 pmol/min) orexin A significantly induced histamine release at the first sampling and the release reached an even higher maximal level of 2.2-fold over the baseline. This maximal level was sustained for 60 min, and then gradually returned to the basal level. Orexin A at 1 pmol/min increased histamine release only by 1.3-fold at the first sampling.
Figure 2.
Orexin A-induced histamine release from the MPO (A) and the FrCx (B) in anesthetized rats. The horizontal shaded bar indicates the duration of orexin A perfusion. Each value represents the mean ± SEM of 5 or 6 rats. **, P < 0.01; *, P < 0.05, significantly different from control, as assessed by two-way ANOVA followed by Fisher's probable least-squares difference test.
The histamine release from the FrCx was also increased in a dose-dependent manner, when orexin A was perfused into the TMN. As shown in Fig. 2B, orexin A at doses of 5 and 25 pmol/min induced significant histamine release from the first sampling after orexin A perfusion, which was the same as that from the MPO. The increased histamine release was maintained for 160 min in the case of perfusion at 25 pmol/min and for 100 min at 5 pmol/min, with the maximal elevation of 2.2- and 2.1-fold over the baseline, respectively. Similar to its effect on histamine release from the MPO, orexin A perfusion at 1 pmol/min increased histamine release by 1.4-fold during the first 20 min.
Sleep–Wake Profiles in H1R KO Mice.
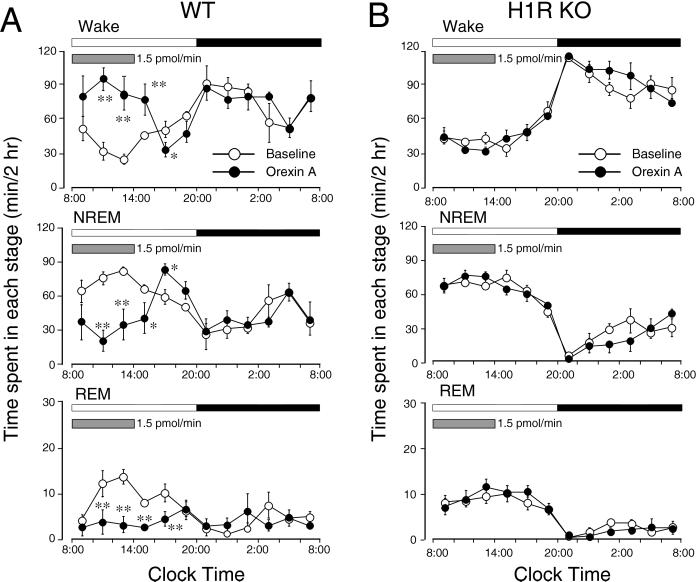
H1R has been reported to mediate histamine-induced wakefulness (26–28). To investigate the role of H1R in the arousal effect of orexin A, we used H1R KO mice. Under baseline conditions, the amounts and circadian profiles of sleep and wakefulness were statistically unchanged between H1R KO and WT mice (Fig. 3). When orexin A (1.5 pmol/min) was infused into the lateral ventricle of mice for 6 hr, the WT mice displayed significant changes in sleep–wake stages from the second 2 hr after infusion, although the first 2 hr showed a tendency toward increased wakefulness (Fig. 3A). The maximal changes in each stage were displayed in the second 2 hr, with an increase in wakefulness by 3.3-fold and a reduction in NREM and REM sleep by 58% and 80%, respectively, when the experimental day was compared with the baseline day. The amounts of wakefulness and of NREM and REM sleep during the orexin A infusion reached the same levels as the maximum wake and the minimum sleep observed, respectively, during the active period of mice. These effects were sustained during further orexin A administration and gradually declined but were still significant even 2 hr after the infusion had ended. The rebound responses, a decrease in wake and an increase in NREM sleep, were observed during the second 2 hr after the end of the perfusion. There was no further disruption of sleep architecture during the subsequent period. However, H1R KO mice did not exhibit any significant changes in time spent in wake, NREM, and REM sleep during or after orexin A administration (Fig. 3B).
Figure 3.
Sleep-stage distribution produced by orexin A infusion into the lateral ventricle in WT mice (A) and H1R KO mice (B) between 8 a.m. and 2 p.m. is indicated by horizontal shaded bars on the experimental day. Each circle represents 2-hr amounts of wake, NREM, or REM sleep. Open and closed circles stand for the baseline and experimental day profiles, respectively. Values are means ± SEM (n = 6 in H1R KO mice and n = 5 in WT mice). **, P < 0.01; *, P < 0.05 by paired t test.
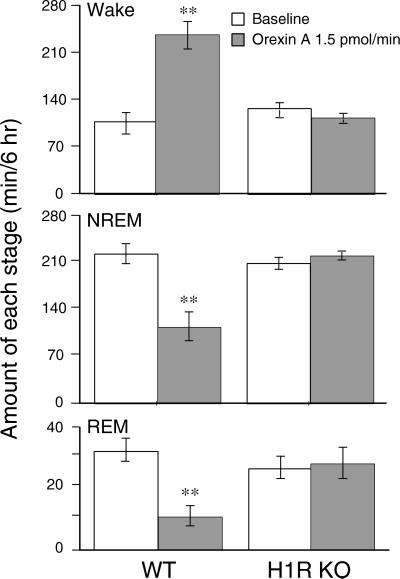
We calculated the total amounts of wake, NREM, and REM sleep during the 6-hr infusion of both groups of mice. As shown in Fig. 4, orexin A increased total amounts of wakefulness by 2.3-fold in the WT mice, and reduced NREM and REM sleep by 50% and 67%, respectively, in comparison with the baseline. In contrast, no difference was observed in H1R KO mice between the amounts during orexin A infusion and the baseline ones. These results clearly indicate that H1R plays a crucial role in orexin A-induced wakefulness.
Figure 4.
Total time spent in wake, NREM, and REM sleep during 6-hr orexin A infusion into the lateral ventricle of mice. Open and shaded columns stand for the baseline and experimental day profiles, respectively. Values are means ± SEM (n = 6 in H1R KO mice and n = 5 in WT mice). **, P < 0.01 by paired t test.
Discussion
Dysfunction of the orexin system in narcoleptic dogs (19), mice (14), and humans (20–22) suggests that this system has an important role in narcolepsy. However, the exact role of orexins in physiological sleep–wake regulation remains poorly characterized. Infusion of orexin A into the lateral ventricle of WT mice increased arousal from the second 2 hr, but did not significantly change the sleep-stage distribution during the first 2 hr of infusion (Fig. 3A), reflecting a delay in the peptide's reaching effective concentrations in some potential action sites. A similar 1-hr delay was also observed in rats after the intracerebroventricular injection of orexin A (10 and 30 μg per rat) (11, 34). However, when orexin A (5 and 25 pmol/min) was delivered directly to the TMN of rats through a microdialysis probe, wakefulness was increased promptly after the start of perfusion (Fig. 1 A and B). Moreover, the arousal effect was sustained even 1 hr after the perfusion had ended (Fig. 1 A and B). These results clearly indicate that the TMN or its vicinity is a direct action site for the arousal effect of orexin A. It is also consistent with recent studies showing that the TMN is a putative wake center (36, 43) with a dense distribution of orexin A immunoreactive processes (7, 9, 12) and the mRNA for orexin-2 receptors (24, 25).
The histaminergic neurons are located in the TMN (27, 28) and histamine promotes cortical wakefulness probably either through direct cortical projections or by tonic control over the sleep-generating mechanisms in the preoptic/anterior hypothalamus (30–32). To examine whether orexin activates the histaminergic system, we used an in vivo microdialysis method and found that perfusion of the TMN of rats with orexin A increased histamine release from both the MPO (Fig. 2A) and FrCx (Fig. 2B), suggesting that application of orexin A into the TMN activates the histaminergic system in the brain. The duration of the increase in histamine release from the MPO and FrCx almost paralleled that of the arousal effect (Figs. 1B and 2). These results are consistent with the observation that orexin A markedly increased the basal firing rate of histaminergic neurons in vitro (44) and suggest that activation of the histaminergic system is involved in the orexin A-induced wakefulness.
Histamine has been reported to promote wakefulness through activation of H1R (26, 30–32, 45, 46). H1R KO mice provided a valuable tool to study the role of the histaminergic system in orexin A-induced arousal. H1R KO mice were initially generated by Inoue et al. (33), and their behavior and neuropharmacological characteristics have been investigated in detail (33, 47). Although these authors reported that these mice lacking H1R showed a significant decrease in ambulation in an open field and on an activity wheel, electrophysiological studies have not been carried out thus far. Our results show that orexin A significantly increased wakefulness in WT mice but not at all in the H1R KO mice, although mice of each genotype displayed essentially the same amounts of sleep and wakefulness under basal conditions (Figs. 3 and 4), clearly indicating that H1R is essential for mediating orexin A-induced arousal. Nevertheless, contrary to the narcoleptic symptoms in orexin KO mice (14), these symptoms were not detected in any of the H1R KO mice examined, as judged by either electrophysiological examination or infrared video recordings (data not shown).
In summary, orexin A activated the histaminergic system and induced wakefulness in rats and WT mice, but did not induce arousal at all in H1R KO mice, strongly indicating that the arousal effect of orexin A depends on activation of the histaminergic system.
Acknowledgments
We thank Takehiko Watanabe of Tohoku University for his helpful discussion and Yuko Kuwahata for her excellent technical assistance. This study was supported in part by grants from the Core Research for Evolutional Science and Technology of the Japan Science and Technology Corporation (to Y.U.), the Japan Space Forum (to Y.U.), the Special Coordination Funds of the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y.U. and N.E.), the Grants-in-Aid for Japan Society for the Promotion of Science Fellows (to Z.-L.H.), and for Scientific Research (B) (13557016, to N.E.) of Japan Society for the Promotion of Science, the Ministry of Health and Welfare of Japan (100107, to O.H.) and Osaka City.
Abbreviations
- KO
knockout
- REM
rapid eye movement
- NREM
non-REM
- TMN
tuberomammillary nucleus
- H1R
histamine H1 receptor
- MPO
medial preoptic area
- FrCx
frontal cortex
- WT
wild type
- EEG
electroencephalogram
- EMG
electromyogram
- AP
anteroposterior
- LR
left–right
References
- 1.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli R M, Tanaka H, Williams S C, Richardson J A, Kozlowski G P, Wilson S, et al. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 2.Gautvik K M, de Lecea L, Gautvik V T, Danielson P E, Tranque P, Dopazo A, Bloom F E, Sutcliffe J G. Proc Natl Acad Sci USA. 1996;93:8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pu S, Jain M R, Kalra P S, Kalra S P. Regul Pept. 1998;78:133–136. doi: 10.1016/s0167-0115(98)00128-1. [DOI] [PubMed] [Google Scholar]
- 4.van den Pol A N, Gao X B, Obrietan K, Kilduff T S, Belousov A B. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilduff T S, Peyron C. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 6.Willie J T, Chemelli R M, Sinton C M, Yanagisawa M. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 7.Peyron C, Tighe D K, van den Pol A N, de Lecea L, Heller H C, Sutcliffe J G, Kilduff T S. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivedi P, Yu H, MacNeil D J, Van der Ploeg L H, Guan X M. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen C T, Dun S L, Kwok E H, Dun N J, Chang J K. Neurosci Lett. 1999;260:161–164. doi: 10.1016/s0304-3940(98)00977-x. [DOI] [PubMed] [Google Scholar]
- 10.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagan J J, Leslie R A, Patel S, Evans M L, Wattam T A, Holmes S, Benham C D, Taylor S G, Routledge C, Hemmati P, et al. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 13.Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei M A, Bloom S R. FEBS Lett. 1999;457:157–161. doi: 10.1016/s0014-5793(99)01030-3. [DOI] [PubMed] [Google Scholar]
- 14.Chemelli R M, Willie J T, Sinton C M, Elmquist J K, Scammell T, Lee C, Richardson J A, Williams S C, Xiong Y, Kisanuki Y, et al. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 15.Sutcliffe J G, de Lecea L. J Neurosci Res. 2000;62:161–168. doi: 10.1002/1097-4547(20001015)62:2<161::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Aldrich M S. N Engl J Med. 1990;323:389–394. doi: 10.1056/NEJM199008093230606. [DOI] [PubMed] [Google Scholar]
- 17.Nishino S, Mignot E. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 18.Siegel J M. Cell. 1999;98:409–412. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong P J, Nishino S, Mignot E. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 20.Nishino S, Ripley B, Overeem S, Lammers G J, Mignot E. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 21.Thannickal T C, Moore R Y, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel J M. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, et al. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 23.de Lecea L, Kilduff T S, Peyron C, Gao X, Foye P E, Danielson P E, Fukuhara C, Battenberg E L, Gautvik V T, Bartlett F S, II, et al. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X Y, Bagnol D, Burke S, Akil H, Watson S J. Horm Behav. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- 25.Marcus J N, Aschkenasi C J, Lee C E, Chemelli R M, Saper C B, Yanagisawa M, Elmquist J K. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 26.Monti J M, Orellana C, Boussard M, Jantos H, Olivera S. Brain Res Bull. 1990;25:229–231. doi: 10.1016/0361-9230(90)90065-8. [DOI] [PubMed] [Google Scholar]
- 27.Lin J S. Sleep Med Rev. 2000;4:471–503. doi: 10.1053/smrv.2000.0116. [DOI] [PubMed] [Google Scholar]
- 28.Brown R E, Stevens D R, Haas H L. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 29.Scammell T E, Estabrooke I V, McCarthy M T, Chemelli R M, Yanagisawa M, Miller M S, Saper C B. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J S, Sakai K, Vanni-Mercier G, Arrang J M, Garbarg M, Schwartz J C, Jouvet M. Brain Res. 1990;523:325–330. doi: 10.1016/0006-8993(90)91508-e. [DOI] [PubMed] [Google Scholar]
- 31.Lin J S, Sakai K, Jouvet M. Eur J Neurosci. 1994;6:618–625. doi: 10.1111/j.1460-9568.1994.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 32.Lin J S, Hou Y, Sakai K, Jouvet M. J Neurosci. 1996;16:1523–1537. doi: 10.1523/JNEUROSCI.16-04-01523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue I, Yanai K, Kitamura D, Taniuchi I, Kobayashi T, Niimura K, Watanabe T. Proc Natl Acad Sci USA. 1996;93:13316–13320. doi: 10.1073/pnas.93.23.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piper D C, Upton N, Smith M I, Hunter A J. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 35.Satoh S, Matsumura H, Suzuki F, Hayaishi O. Proc Natl Acad Sci USA. 1996;93:5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Proc Natl Acad Sci USA. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 38.Gerashchenko D, Okano Y, Urade Y, Inoue S, Hayaishi O. J Sleep Res. 2000;9:81–87. doi: 10.1046/j.1365-2869.2000.00175.x. [DOI] [PubMed] [Google Scholar]
- 39.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 40.Pinzar E, Kanaoka Y, Inui T, Eguchi N, Urade Y, Hayaishi O. Proc Natl Acad Sci USA. 2000;97:4903–4907. doi: 10.1073/pnas.090093997. . (First Published April 18, 2000; 10.1073/pnas.090093997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobler I, Deboer T, Fischer M. J Neurosci. 1997;17:1869–1879. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Z L, Mochizuki T, Watanabe H, Maeyama K. Neurosci Lett. 1999;270:181–184. doi: 10.1016/s0304-3940(99)00500-5. [DOI] [PubMed] [Google Scholar]
- 43.Hayaishi O. Philos Trans R Soc London B. 2000;355:275–280. doi: 10.1098/rstb.2000.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eriksson K S, Sergeeva O A, Brown R E, Haas H L. Eur J Physiol. 2001;441:R220. [Google Scholar]
- 45.Lin J S, Sakai K, Jouvet M. Neuropharmacology. 1988;27:111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- 46.Monti J M. Life Sci. 1993;53:1331–1338. doi: 10.1016/0024-3205(93)90592-q. [DOI] [PubMed] [Google Scholar]
- 47.Yanai K, Son L Z, Endou M, Sakurai E, Nakagawasai O, Tadano T, Kisara K, Inoue I, Watanabe T. Neuroscience. 1998;87:479–487. doi: 10.1016/s0306-4522(98)00167-5. [DOI] [PubMed] [Google Scholar]
- 48.Geraschenko, D., Kohls, M. D., Greco, M. A., Walen, N. S., Salin-Pascual, R., Kilduff, T. S., Lappi, D. A. & Shiromani, P. J. (2001) J. Neurosci. 21, in press. [DOI] [PMC free article] [PubMed]