Introduction
The classic renin-angiotensin system (RAS) is a well-defined endocrine system consisting of liver production of angiotensinogen which is converted to angiotensin I by renin, followed by a second cleavage to angiotensin II (AngII) by angiotensin converting enzyme (ACE) located on the surface of lung endothelium1, 2. AngII is the major effector hormone of the RAS, playing a key role in in the maintenance of extracellular volume, blood pressure, renal hemodynamics, and tubular sodium transport, as well as in the pathogenesis of cardiovascular and renal diseases3–5. AngII acts on two types of membrane receptors, AngII type 1 receptor (AT1R) and type 2 receptor (AT2R). The RAS interventions targeting AT1R and ACE are widely used for management of hypertension, renal disease, and cardiovascular disease. During the past several decades the RAS has evolved into a highly complex system as highlighted by the discovery of ACE2/Ang 1-7/Mas pathway and the local RAS found in a number of tissues such as the kidney, the heart, and the brain1, 6, 7. The RAS activity is largely controlled by renin-mediated conversion of angiotensinogen to Ang I, a rate-limiting step in the RAS. Renin is a protease produced by juxtaglomerular (JG) cells of the afferent arteriole as well as collecting duct principal cells8, mast cells9, adipocytes10, etc. Prorenin, a precursor of renin, contains a prosegment covering the catalytic domain and undergoes proteolytic cleavage to generate the mature renin. Plasma prorenin levels are elevated in diabetic patients and predict microvascular complications, whereas plasma renin is normal or low11. Animal data suggests that excess prorenin over active renin in diabetes originates from the collecting duct (CD) rather than from the juxtaglomerular apparatus12. High prorenin state is also seen normal pregnancy13, 14.
In 2002, Nguyen et al. cloned a novel receptor for prorenin and renin, termed (pro)renin receptor (PRR), and assigned a renin-regulatory function to this receptor15. Renin bound to PRR exhibits a three- to five fold increased renin activity15. Furthermore, when PRR binds prorenin, the later undergoes conformational changes to unfold the inhibitory prosegment of prorenin, leading to non-proteolytic activation15. A soluble form of PRR (sPRR) is generated by intracellular cleavage by furin and secreted in plasma16. Based on the structure, PRR belongs to type I transmembrane receptor family, consisting of a large N-terminal extracellular domain, a single transmembrane protein, and a short cytoplasmic domain15,17. Amino acid sequence analysis reveals that the intracellular domain of PRR is highly conserved among all species whereas the extracellular domain shares high homology only in mammals. The conservation scores (identity/AA) are 6.8 for the intracellular domain (ICD, M8.9) and 3.8 for the extracellular domain (sPRR) among all species, and 8.7 for the ICD and 8.9 for sPRR in mammals (Fig. 1). This result is compatible with the concept that sPRR may serve an important function in mammals whereas M8.9 may participate in fundamental survival functions across all species.
Fig. 1.

Distinct conservations of different domains of PRR. The protein sequence alignment was generated by the Clustal × 2.0 multiple sequence alignment program using default parameters. The PRR protein sequences were from GenBank accessions numbers NP_005756.2 (human), NP_001126837.1(P. abelii), AAH79339.1(rat), NP_081715.1(mouse), NP_001080880.1(xenopus Laevis), NP_998188.2(Zebrafish), NP_510360.1(C. elegans), and NP_649876.1(drosophila melanogaster). (A) Alignment of amino acid sequence of PRR. The score depicts conservation between different species. The scores on the bottom two rows depict degrees of conservation across all species and the mammals only. TM, transmembrane domain; ICD, intracellular domain. The TM, the ICD, and furin-mediated cleavage site are highlighted. (B) The summary of the conservation scores.
Within the kidney, PRR is expressed in multiple cell types and is most abundantly detected in the intercalated cells of the CD18–20, a site for fine-tuning urinary excretion of water and electrolytes; the expression of PRR in the CD is stimulated by chronic AngII infusion21 or Na+ depletion22, 23. During the past decade, understanding the physiological function of PRR has been hindered by the lethal phenotype of complete or conditional deletion of PRR and also the lack of an effective PRR inhibitor with the controversial antagonistic effect of handle region peptide (HRP) on the PRR24–26. In recent years, the availability of various strains of viable renal PRR knockout (KO) models along with the newly developed PRR decoy inhibitor PRO20 has greatly facilitated the process in unraveling the physiological function of renal PRR. The existing in vivo data suggests that PRO20 acts via inhibition of the RAS during both AngII infusion and deoxycorticosterone acetate (Deoxycorticosterone acetate Deoxycorticosterone acetate DOCA)-salt hypertension27, 28 although in vitro data shows the RAS-independent effect of this inhibitor on epithelial Na+ channel (ENaC) activity29. The goal of this review is to highlight recent advances in identifying the roles of PRR in the nephron, particularly in the CD, for regulation of Na+ and water balance and blood pressure.
PRR regulation of Na+ homeostasis
A series of recent studies from our group and others have identified the prorenin/PRR pathway as a potential regulator of ENaC in the CD. Since prorenin/renin and PRR are co-expressed in the CD, we hypothesized that their interactions in an autocrine or paracrine fashion may affect ENaC activity. To address this possibility, we performed electrophysiological studies using mpkCCD cell line grown on Transwells. Using this system, we made an initial observation that prorenin treatment in the nanomolar range induced a rapid and transient increase in ENaC-mediated Na+ transport (Fig. 2) through Nox4-derived H2O229. Interestingly, renin treatment was largely ineffective. In agreement of this observation, multiple studies suggest that prorenin has higher affinity to PRR than renin30, 31. The dependence of The Na+ stimulatory effect of prorenin on PRR was validated by using a PRR decoy inhibitor PRO2029. This effect was independent of canonical RAS since AT1 receptor inhibition with losartan was ineffective (Fig. 2). These results suggest that prorenin via PRR induces Nox4-derived ROS resulting in a direct activation of ENaC. In agreement with this finding, Ramkumar et al. observed a similar stimulatory effect of prorenin on ENaC activity in isolated split-open CD32. Moreover, the prorenin effect was similarly unaffected by AT1 blockade32. Besides the ENaC regulation at activity level, PRR also upregulates α-ENaC mRNA expression in mIMCD3 cells exposed to low salt media or AngII through serine/threonine-protein kinase-1 and phosphorylated Nedd4-2 {also known as Nedd4L (neural precursor cell-expressed developmentally downregulated gene 4-like)}33. Together, the in vitro results suggest that PRR can modulate ENaC expression and activity through distinct mechanisms.
Fig. 2.
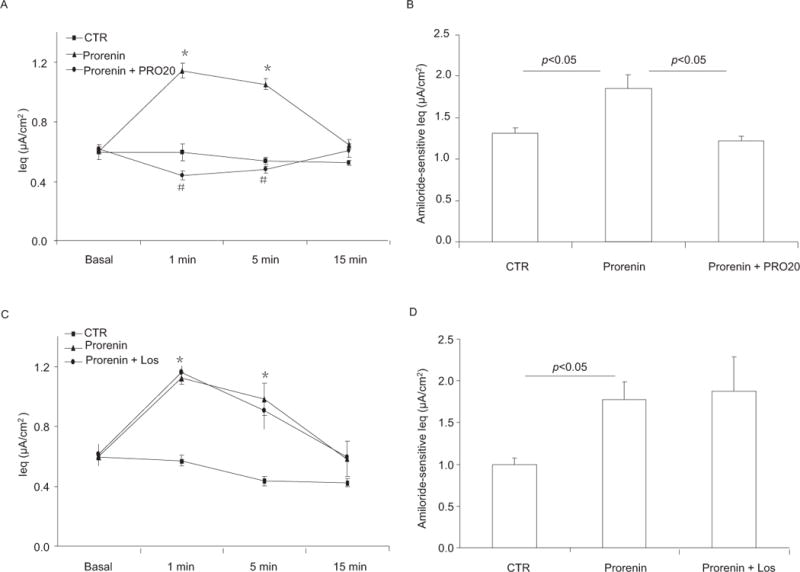
Effect of prorenin/PRR on ENaC activity in cultured CD cells. Confluent mpkCCD cells grown on Transwells were pretreated for 30 min with PRO20 (1.5 μM) or losartan (1.0 μM) and then treated with prorenin (10 nM). Ieq was monitored for a 15-min period by using an epithelial volt-ohmmeter (EVOM). In a separate experiment, the time point of peak stimulation of Na+ transport at 1 min was chosen for measuring amiloride-sensitive Ieq, an index of ENaC activity. (A) Timecourse of Ieq changes in Control, Prorenin, and Prorenin + PRO20 groups. (B) Corresponding amiloride-sensitive Ieq for experiments in (A). (C) Timecourse of Ieq changes in Control, Prorenin, and Prorenin + Losartan groups. (D) Corresponding amiloride-sensitive Ieq for experiments in (C). Los, losartan. * p < 0.05 vs. basal in the same group; # p < 0.05 vs. prorenin. N = 6–12 per group. Data are means ± SE. Adapted from Fig. 1 of a previous study29.
Besides the above-mentioned in vitro data, strong in vivo data from 3 independent studies all support an important role of PRR in ENaC regulation under basal and/or pathophysiological conditions29, 32, 33. To our surprise, PRR regulation of ENaC appears to occur only in the renal medulla but not the cortex. In this regard, we found that the renal inner medullary α-ENaC under basal condition or following AngII infusion was reduced in CD PRR KO mice34. In contrast, renal cortical α-ENaC expression was unaffected by AngII or CD PRR KO. Moreover, the other ENaC subunits (β & γ) remain unchanged. In agreement with our results, Quadri et al. showed that in vivo delivery of shRNA against PRR to the kidney of Sprague-Dawley rats selectively reduced α-ENaC expression in the renal medulla but not in the cortex despite the gene knockdown in the whole kidney33. These results suggest that PRR may primarily target renal medullary (or inner medullary) ENaC to regulate Na+ balance and BP.
As compared with the overwhelming information about Na+ transport process in the early part of the CD35–38, little attention has been paid to the terminal part of the CD, e.g. the inner medullary collecting duct (IMCD). Part of the reason is that the IMCD in rabbits or rats is highly branched or difficult to dissect due to tightly adherent tissues and therefore is not easily accessible to microperfusion as for the cortical CD (CCD). The literature concerning net Na+ transport in the IMCD is controversial. It has been difficult to demonstrate net Na+ reabsorption in isolated perfused IMCD by measurement of Na+ concentrations in the perfusate39. However, other studies reported robust Na+ reabsorption in this nephron segment40, 41. Furthermore, the apical membrane conductance in the IMCD due to passive movement of Na+ is detected and attributed to amiloride-sensitive conductive pathway42. In addition, Na+ reabsorption in the IMCD has been demonstrated by using micropuncture techniques. The IMCD is accessible to micropuncture through a retrograde placement of thin pipettes from the tip of the IMCD. Early micropuncture studies using this technique have demonstrated active net Na+ reabsorption in the IMCD43, 44. More importantly, Na+ reabsorption in the IMCD fluctuates in response to altered plasma volume so that it is increased during hypovolemia but suppressed during volume expansion44–47 despite a conflicting report48. Overall, these results suggest a potential role of Na+ transport in the IMCD in the control of Na+ excretion during alteration of plasma volume. Unfortunately, this area has almost remained uninvestigated during the past decade. It is anticipated that the demonstration of PRR as a regulator of renal medullary ENaC will provide an impetus for re-evaluation of importance of Na+ transport in the IMCD.
As discussed above, PRR regulation of renal medullary α-ENaC contributes to pathogenesis of AngII-induced hypertension34. In addition, dysregulation of renal medullary ENaC has been implicated in the development of salt-sensitive hypertension in Dahl salt-sensitive (S) rats49. Based on the conductive permeability to Na+ and the rate of Na+ transport, the IMCD cells from Dahl S rats transport twice as much Na+ as the cells from Dahl salt-resistant (R) rats50, 51. Consistent with this finding, the mRNA of β- and γ-ENaC and the protein abundance of all 3 ENaC subunits are increased in the renal medulla of Dah S rats under basal condition and following high salt intake as compared with Dahl R rats49. Additionally, aberrant activation of intrarenal RAS has been suggested to play a role in the Dahl S model which is traditionally considered as a low renin hypertension model. Despite the similar suppression of plasma angiotensinogen (AGT) in both strains, high salt enhances renal and urinary levels of AGT in Dahl S but not Dahl R rats52. Future studies need to address the precise location of the intrarenal RAS activation and possible involvement of PRR in salt-sensitive hypertension in the Dahl S model.
PRR regulation of water homeostasis
Most mammals can produce highly concentrated urine when water consumption is reduced so that a constant plasma osmolality can be maintained. Such urine concentrating capability resides in the renal medulla and is chiefly determined by arginine vasopressin (AVP)53, 54. The antidiuretic action of AVP is mediated by enhancement of water permeability of the CD as well as the generation of osmotic gradient due to increased NaCl reabsorption in the thick acceding limb (TAL)54, 55. In the CD, AVP via arginine vasopressin receptor 2 (V2R) activates cAMP/PKA pathway that induces aquaporin-2 (AQP2) trafficking to the apical membrane and expression leading to increased water permeability53. We discovered that AVP-stimulated AQP2 expression depends on prorenin activation of PRR56. In primary rat IMCD cells on Transwells, AVP induced a marked increase in AQP2 protein expression. To address the involvement of PRR, we employed three independent approaches to inhibit PRR: a pharmacological inhibitor, a siRNA, and a neutralizing PRR antibody. Inhibition of PRR with either one of these approaches was highly effective to suppress AQP2 upregulation by AVP. Furthermore, activation of PRR with prorenin but not renin induced a marked increase in AQP2 expression (Fig. 3). AVP also stimulated release of prorenin, and sPRR in primary rat IMCD cells56. In M-1 CD cell line, a similar enhancement of renin mRNA, prorenin and renin is observed following desmopressin (ddAVP) treatment57. Together, the in vitro data demonstrates a potential role of PRR in mediating AVP-induced AQP2 expression in the CD cells. However, whether PRR regulates AVP-induced AQP2 trafficking remains uninvestigated.
Fig. 3.
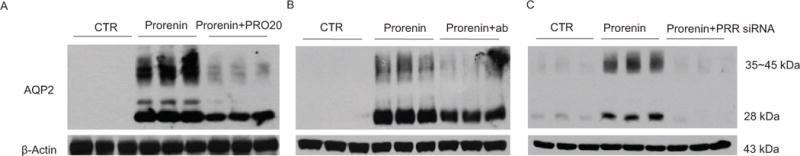
The direct role of prorenin/PRR in regulation of AQP2 expression in the CD cells. Primary rat IMCD cells were pretreated with PRO20 or anti-PRR-N antibody or pre-transfected with PRR siRNA, and then treated for 24 h with 10 nM prorenin. AQP2 protein expression was determined by immunoblotting and normalized by β-actin. (A) Effect of prorenin alone or in combination with PRO20 on AQP2 expression (n = 6 per group). (B) Effect of prorenin alone or in combination with anti-PRR-N antibody on AQP2 expression (n = 6 per group). (C) Effect of prorenin alone or in combination with PRR knockdown on AQP2 expression (n = 6 per group). Adapted from Fig. 4 of a previous study56.
In agreement with the in vitro data as discussed above, compelling in vivo data is also available to support antidiuretic action of renal PRR. First, renal fPRR protein abundance is consistently elevated in rats following 48-h or 72-h water deprivation (WD)56, 58. Interestingly, this maneuver increases urinary sPRR excretion56 but decreases plasma sPRR55. This result may suggest differences in the origin of urinary and plasma sPRR. Second, pharmacological inhibition of intrarenal PRR results in impaired urine concentrating capability56. In this regard, PRO20 is administered via an infusion catheter chronically implanted in the kidney of nephrectomized rats followed by 48-h WD. Intrarenal PRO20 infusion partially attenuates the reduction of urine volume and the increase of urine osmolality and the expression of AQP2 in the WD rats. Lastly, conditional deletion of PRR56 in the CD and the whole nephron59, 60 causes a common polyuria phenotype. CD PRR KO mice generated by using AQP2-Cre are not associated with renal developmental abnormality seen in the null model produced by using Hoxb7-Cre61. The whole nephron PRR deletion is generated by using an inducible way wherein PRR ablation occurs in adulthood so that the developmental stage is bypassed. All of these KO models have increased urine volume and decreased urine osmolality under basal condition and exhibit impaired ability to concentrate urine following WD. Evidence is available to suggest impairment of AVP signaling in these models. For example, renal AQP2 expression is consistently downregulated in these models and AVP sensitivity is also blunted in the nephron PRR KO mice. Besides AQP2, renal expression of Na/K/2Cl cotransporter (NKCC2), another target of AVP in the TAL55, is also suppressed in the null mice. These results suggest that PRR is indispensable for AVP signaling in both the CD and the TAL. However, it is important to note that interpretation of the phenotype of the nephron PRR KO model is complicated by renal structural abnormalities due to accumulation of autophagosome particularly in the TAL. This result raises a possibility that the downregulation of transporter proteins such as NKCC2 in this model may be secondary to an autophagic defect in the renal epithelial cells.
EP4 regulation of PRR
Further evidence suggests that prostaglandin EP4 receptors are required for enhancement of PRR and hence AQP2 following AVP treatment. PGE2 is one of the major cyclooxygenase-derived metabolites of arachidonic acid produced in the kidney, particularly the IMCD62, 63. PGE2 acts on four distinct G protein-coupled receptors, designated EP1–4, to induce a wide spectrum of biological functions64. PGE2 exerts a prominent, yet complex, role in the regulation of water homeostasis through distinct EP receptors. In particular, PGE2 and sulprostone, an EP1/3 agonist, blunt AVP-induced AQP2 trafficking and urine concentration65–68 whereas EP2 and EP4 agonists induces AQP2 trafficking to the apical membrane and alleviate the symptoms of nephrogenic diabetes insipidus (NDI) in rodents69.A nonbiased gene expression analysis of G protein coupled receptor (GPCR) in mouse IMCD cells identified the prostaglandin EP4 subtype as the highly expressed GPCR in addition to the V2R69. In microdissected nephron segments, EP4 mRNA was abundantly detected in the cortical collecting duct70 where PGE2 stimulated cAMP production71. Following 24-hour water deprivation, EP4 protein expression is remarkably upregulated contrasting to unchanged expression of the other 3 EP subtypes72, indicating a unique role of EP4 receptors in regulation of water homeostasis. This notion has been confirmed by generation of nephron- (Ksp-EP4-/-) and CD (AQP2-EP4-/-) -specific deletion of EP4, both of which impair urine concentrating capability72. An earlier study from our group showed that EP4 acting downstream of COX-2 mediates upregulation of renal medullary PRR and the intrarenal RAS during AngII-induced hypertension73, 74. Therefore, we proposed a hypothesis that PGE2/EP4 pathway may mediate the antidiuretic action of AVP via targeting PRR and the intrarenal RAS. We found that in primary rat IMCD cells, activation of EP4 receptors was able to stimulate AQP2 expression and conversely inhibition of this receptor subtype effectively attenuates AVP-induced AQP2 expression56. These results suggest the EP4 receptor as a mediator of AVP-induced AQP2 expression. Since EP4 agonist stimulated PRR expression74 and EP4-induced AQP2 expression was blunted by inhibition of PRR, the EP4 receptor should act downstream of AVP and upstream of PRR in regulation of AQP2 expression.
The biological function of sPRR
PRR contains a protease cleavage site at the N-terminal domain near the transmembrane domain15. sPRR was originally shown to be generated by intracellular cleavage by furin and detected in the conditioned medium of cultured cells16. This conclusion was based on the observations that LoVo colon carcinoma cells deficient in furin didn’t produce sPRR despite the expression of fPRR and that overexpression of α1-anttrypsin Portland variant, an inhibitor of furin completely suppressed sPRR production in Chinese hamster ovary cells16. However, the role of furin as the cleaving enzyme was not confirmed by another study75. In the later study, ADAM19 but not furin is shown to be responsible for the cleavage process75. Moreover, in cultured vascular smooth muscle cells (VSMC), PRR is mainly localized to the endoplasmic reticulum (ER) and the Golgi, and shed by ADAM19 in the Golgi, leading to the secretion of sPRR to the extracellular space where it enhances renin activity of prorenin75. Thus the relative importance of furin versus ADAM19 in this cleavage event remains elusive.
A large number of clinical studies suggest importance of circulating sPRR. sPRR can be measured by ELISA76 which offers a simple tool for assessment of sPRR in the body fluid. By ELISA, serum levels of sPRR are increased during pregnancy77, 78 and in patients with heart failure79, gestational diabetes mellitus80, obstructive sleep apnea syndrome81, primary epithelial ovarian cancer82. In 374 patients with chronic kidney disease (CKD) due to hypertension and type 2 diabetes, serum sPRR is positively associated with serum creatinine, blood urea nitrogen, and proteinuria, and inversely with estimated glomerular filtration rate (eGFR)83. It is puzzling however that in this study serum sPRR levels were lower in patients with primary hypertension or diabetes than in those without these conditions83. In 25 patients who underwent uninephrectomy, plasma sPRR further correlated with renal tubulointerstitial fibrosis84. Despite the lack of correlation of plasma sPRR with circulating renin, prorenin, and aldosterone in healthy subjects85, sPRR correlates positively with urinary AGT in patients with essential hypertension86. Urinary AGT is quite widely considered as an index of intrarenal RAS87 despite some evidence for its hepatic origin88. Overall, most of the previous studies favor plasma sPRR as a biomarker of certain diseases likely due to its association with the intrarenal RAS.
A clue suggesting a biological function of renal PRR came from our immunostaining studies comparing antibodies against the different PRR domains. A commercial antibody against the C-terminus of PRR (anti-PRR-C antibody)18, 20 reproducibly labeled intercalated cells. However, to our surprise, an antibody against the N-terminus of PRR (sPRR) only stained the apical membrane of principal cells. The anatomical evidence suggested an intriguing possibility that sPRR might be secreted from intercalated cells and secreted to the luminal side and then bind an as yet unknown protein on the apical membrane of principal cells, revealing a paracrine loop linking the two cell types in the CD; this paracrine mechanism may play a role in regulation of Na+ and water transport in the principal cells of the CD. To test this hypothesis, we generated a histidine-tagged recombinant sPRR, termed as sPRR-His, and examined its effect on AQP2 expression in primary IMCD cells. sPRR-His at the nanomolar range remarkably induced AQP2 mRNA and protein expression (Fig. 4)89. The parallel increase in AQP2 promoter activity (Fig. 4) suggested that the transcriptional mechanism may account for the upregulation of AQP2 expression by sPRR-His89. We employed SPRING to predict proteins that interact with sPRR. This led to the discovery of frizzled-8 (FZD8) that interacted with sPRR to activate Wnt/β-catenin pathway and thus enhancement of AQP2 gene transcription89. However, this mechanism doesn’t appear to contribute to AQP2 redistribution following an acute AVP treatment89. Since AVP stimulated sPRR release, we speculate that the sPRR/β-catenin/AQP2 pathway may primarily mediate the upregulation of AQP2 transcription during chronic AVP treatment. While previous studies extensively focus on the detailed signaling mechanism of AQP2 trafficking between intracellular vesicles and apical plasma membrane which occurs within minutes of AVP treatment53, 54, 90, 91, little is known about the mechanism of transcriptional regulation of AQP2 gene under chronic condition. Our results uncovered a novel role of sPRR in transcriptional regulation of AQP2 expression.
Fig. 4.
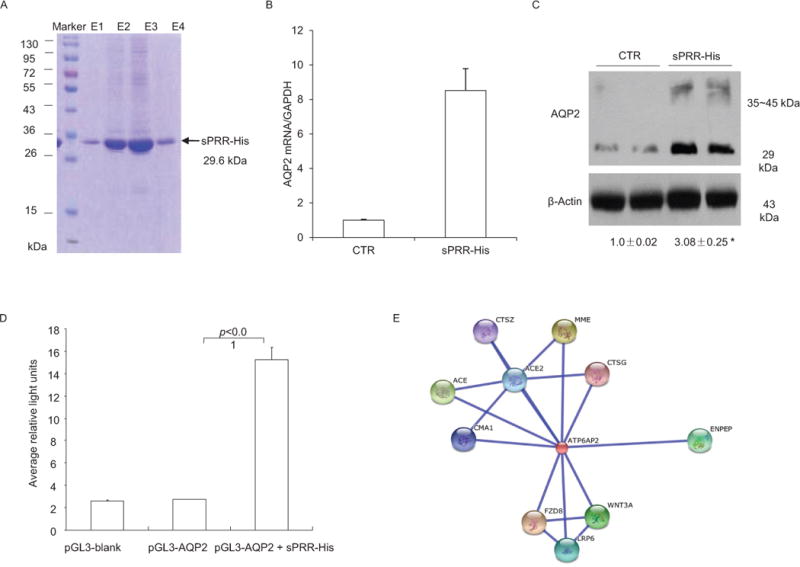
Characterization of function of sPRR. A recombinant PRR, termed sPRR-His, was expressed in a mammalian expressing system as a fusion protein that contained sPRR, a histidine tag in the C terminus, and a secretion signal peptide in the N terminus. (A) sPRR-His was purified from the medium as a single 29.6 kDa band in 12% SDS polyacrylamide gel electrophoresis (PAGE). E1-4 denotes sequential fractions from the elution. (B&C) Primary rat IMCD cells grown in Transwells were exposed to 10 nM sPRR-His for 24 h, and AQP2 expression was analyzed by qRT-PCR and immunoblotting (n=5 per group). (D) In a separate experiment, the cultured CD cells were transfected with an AQP2-luciferase construct and allowed to grow to confluence, and then treated with vehicle or 10 nM sPRR-His for 24 h and the luciferase activity was assayed (n=5 per group). (E) SPRING is a template-based algorithm for protein-protein structure prediction. We employed SPRING to identify proteins that interact with sPRR. This analysis revealed 10 hits: CTSG (cathepsin G), ACE2 (angiotensin 1 converting enzyme 2), CMA1 (chymase 1), MME (membrane metallo-endopeptidase), ACE (angiotensin 1 converting enzyme 1), CTSZ (cathepsin Z), ENPEP (glutamyl aminopeptidase), FZD8 (frizzled family receptor 8), WNT3A (wingless-type MMTV intergration 3A), and LRP6 (low density lipoprotein receptor-related protein 6). Adapted from Fig. 2 of a previous study89.
The Wnt/β-catenin pathway is a multifunctional network critically involved in embryogenesis92. Dephosphorylated β-catenin translocates to the nucleus where it recruits several transcription factors, such as lymphoid enhancer–binding factor 1/T-cell-specific transcription factor93. In low vertebrates including zebrafish and Xenopus, PRR functions as a key component of the membrane receptor complex in Wnt signaling to control embryogenesis94. In addition, the Wnt/β-catenin pathway is also involved in physiological and pathophysiological processes. Aberrant activation of this pathway contributes to pathogenesis of CKD95–97. Our recent work demonstrates that the coupling of PRR with β-catenin signaling is operative in the setting of physiological regulation of water transport in the distal nephron89. In support of this observation, Jung et al. reported that tankyrase-mediated β-catenin pathway mediated AVP-induced AQP2 expression in mpkCCDc14 cells98.
The mechanism of how AVP regulates sPRR production, particularly the involvement of V1R versus V2R and intercalated cells versus principal cells largely remains elusive. It has been shown that within the CD, V1R is mainly expressed in the intercalated cells regulating urine acidification99. Given colocalization of V1R and PRR to the intercalated cells, it seems possible that V1R may mediate AVP-induced sPRR production. Another possibility also exists thatV2R in principal cells may affect intercalated cell sPRR production through an intermediate mediator such as prostaglandins. Indeed, we found involvement of the prostaglandin EP4 receptor in mediating AVP-induced activation of PRR56.
The in vitro capability of sPRR-His to regulate AQP2 expression as discussed above predicts an antidiuretic action of sPRR. This prediction has been confirmed by in vivo studies using two types of DI modes induced by inhibition of V2R and liver X receptor (LXR)89. Administration of sPRR-His via an intravenous route effectively attenuated polydipsia and polyuria, and partially restored urine osmolality in both models89. These results demonstrate therapeutic potential of sPRR-His for treatment of diabetes insipidus, particularly NDI. Central diabetes insipidus (CDI) is caused by deficiency of AVP and thus can be treated with supplementing the missing hormone100, 101. NDI results from the insensitivity of the kidney to AVP usually due to genetic abnormalities with 90% of families having a mutation in V2R102, 103 or with AQP2 mutations accounting for rest of the other families104. Patients with NDI usually develop very severe polyuria and polydipsia. It is particularly astonishing that children with congenital NDI can produce 20 liters of urine daily and must drink a comparable amount of water to avoid dehydration101. Infants with this disease may fail to thrive. So far, a specific therapy for NDI is lacking. The existing therapy for NDI consisting of a thiazide diuretic, a low salt diet, and indomethacin is only minimally effective101. Our results suggest that patients with NDI may benefit from administration of sPRR-His. This calls for future clinical evaluation of the antidiuretic potential of sPRR-His in NDI patients. It is also needed to screen NDI patients for mutations of PRR that resides on X chromosome. Indeed, the congenial NDI is mostly an X-linked disease.
PRR regulation of proton transport
Prior to the cloning of the full-length PRR, a truncated fragment, termed M8.9, was identified from adrenal secretory vesicle membrane as part of the vacuolar H+-ATPase (V-ATPase) and thus named ATPap2 (ATPase 6 accessory protein 2)105. V-ATPase is a multimeric protein complex consisting of a Vo domain responsible for proton translocation, and a V1 domain for ATP hydrolysis to provide energy for proton transport. Therefore, V-ATPase acts as an ATP-dependent proton pump involved in many cellular functions. V-ATPase is expressed in all cell types and are mainly expressed in the intracellular compartments where it pumps proton into the vesicle to maintain the acidic range needed for cellular degradation, autophagy, endocytosis, protein tracking, Wnt/β-catenin signaling, etc.106. Defective lysosomal acidification due to impairment of V-ATPase contributes to multiple neurodegenerative diseases such as Alzheimer disease, Parkinson disease, and frontotemporal lobar degeneration106. In some cell types such as the intercalated cells of the CD, V-ATPase is expressed on the apical membrane responsible for pumping proton to the urine. Mutations of renal specific V-ATPase subunits cause distal renal tubular acidosis107.
In vitro evidence demonstrates that PRR is requited for integrity of V-ATPase in cultured Madin-Darby canine kidney (MDCK) cells108. In this study, prorenin at low nanomolar concentrations increased V-ATPase activity, which was blunted by the V-ATPase inhibitor bafilomycin or small siRNA against PRR108. However, the acute stimulation with prorenin in freshly isolated CD cells fails to affect H+-ATPase activity109. Given these conflicting results, it remains unclear as to whether PRR regulation of H+-ATPase is ligand-dependent. The association of PRR with V-ATPase appears to be essential for cell survival. Impairment of V-ATPase has been proposed as an important mechanism for lethal heart failure due to suppressed autophagic function in mice with cardiomyocyte-specific deletion of PRR110. This mechanism may also explain severe podocyte injury and chronic renal failure induced by podocyte-specific deletion of PRR111.
The abundant expression of PRR on the apical membrane of the intercalated cells of the CD18 implies its functional association of V-ATPase for regulation of proton secretion to the urine. This issue was examined recently by using a mouse model of inducible deletion of PRR in the nephron60. Under basal condition, blood pH, PCO2, and [HCO3−] were not different between KO and control animals. However, when challenged with acid load, the KO mice developed severe acidosis due to impaired ability to decrease urine pH, contrasting to well-maintained acid-base balance in the acid-loaded control animals60. Moreover, the transport activity of V-ATPase in the individual intercalated cells of isolated perfused cortical CDs was also measured by determining the recovery in sodium-free solutions of intracellular pH after an acute acid load. The majority of the intercalated cells of the KO mice exhibited blunted V-ATPase activity as compared wild-type controls60. Overall, these results represent quite convincing evidence supporting a role of PRR in regulation of V-ATPase-dependent proton secretion from the intercalated cells of the CD.
Perspectives
Our data suggests an intriguing possibility that sPRR may be derived from the intercalated cells and secreted to the lumen and acts on the principal cells, revealing a paracrine pathway mediating the communication between the two CD cell types (Fig. 5). However, to establish this concept, many questions need to be answered with solid experimental data. For example, the concept is heavaly relied on immunobting with antibodies reocngzining the extrcaelular and intracellular domains. The generation of intercalated cell-specific deletion of PRR will be necessary in answering the source of sPRR. However, this attempt was unsuccessful with the use of V-ATPase B1-Cre112 since the null mice died around perinatal period (data not shown). The AQP2-Cre non-selectively targets both intercalated and principal cells56, 113. Therefore, novel approaches are needed to define the contribution of each of these cell types to sPRR production along the CD. We have shown dependence of PRR regulation by the EP4 receptor during antidiuresis. How and where does AVP stimulate PGE2 synthesis and activate the EP4 receptor? Specifically, will this occur in intercalated cells or principal cells via V1R or V2R? Additionally, autophagosome accumulation has been postulated to contribute to downregulate NKCC2 as well as AQP2 expression in the nephron PRR KO mice60. Future studies are needed to clarify the causality between the autophagic defect and downregulation of the transporter proteins.
Fig. 5.
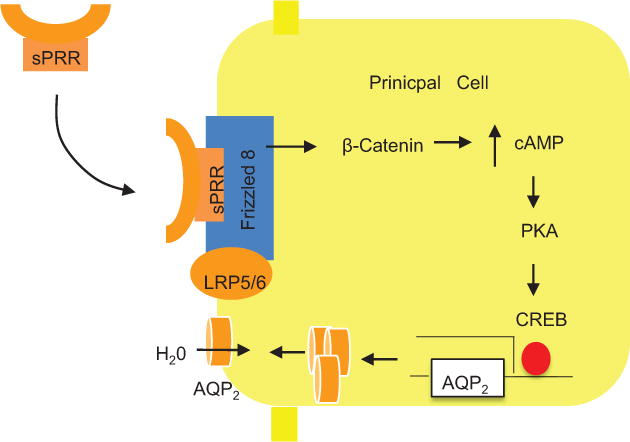
Schematic illustration of the mechanism of action of sPRR. sPRR is generated from the intercalated cells and secreted to the lumen. Then, sPRR binds FZD8 in a receptor complex on apical membrane of principal cells, resulting in activation of β-catenin which promotes cAMP accumulation, ultimately leading to increased AQP2 transcription and enhanced urine concentration. Adapted from Fig. 8 with modifications of a previous study89.
Recent studies have demonstrated that PRR selectively targets α-ENaC in the renal medulla. This is unexpected in light of the traditional view of CCD as a major site for active sodium transport along the CD. It is however unclear as to what signaling mechanism mediates PRR regulation of α-ENaC expression and whether this phenomenon has a broader implication in other types of hypertension in addition to the AngII infusion model.
Na+ and water reabsorption in the CD is traditionally thought to be independently controlled by Aldo and AVP. The capability of PRR to simultaneously target both ENaC and AQP2 suggests a common regulatory pathway mediated by PRR that coordinates renal handling of Na+ and water. While PRR is shown to mediate the antidiuretic action of AVP, it remains elusive whether PRR expression in the CD is also regulated by Aldo. If so, what mechanisms coordinate the actions of AVP and aldosterone in modulating the expression/activity of PRR?
The discovery of prorenin as a possible physiological ligand of PRR in regulation of Na+ and water transport sheds new lights on the long-debated relationship between PRR and the RAS. Strong in vitro data demonstrates that prorenin in the nanomolar range stimulates ENaC activity and AQP2 expression in the CD cells whereas renin is largely effective. Furthermore, these effects of prorenin are independent of the canonical activity of the RAS. However, the in vivo data to support prorenin as a true physiological ligand of PRR is insufficient. In particular, the conditional deletion of renin and PRR in the CD or the whole nephron doesn’t produce the same phenotype. This observation may indicate an additional as yet unknown physiological ligand of PRR may exist in regulation of water homeostasis. Another possibility is that prorenin from extrarenal source may act in an endocrine fashion to activate renal PRR.
PRR is associated with V-ATPase with a truncated fragment of PRR, M8.9, as an important component of the H+-ATPase complex. PRR has been shown to be an important determinant of V-ATPase activity in the intercalated cells, promoting urine acidification following an acid load. In addition, nephron PRR deletion causes renal structural abnormalities characterized by prominent autophagosome accumulation in the TAL. In the latter case, it is likely that V-ATPase activity of PRR in the TAL may contribute to cell survival through regulation of organelle acidification and autophagy.
The diversity of PRR’s biological functions may be ascribed to the protease-mediated cleavage that generates the two distinct subunits, sPRR and M8.9. It is likely that following the cleavage, sPRR serves a paracrine function to regulate Na+ and water transport in the principal cells whereas M8.9 retains in the intercalated cells to control proton secretion (Fig. 6). I anticipate that a further investigation of the functions of PRR’s different domains will provide novel insights into the complex role of PRR in the kidney.
Fig. 6.
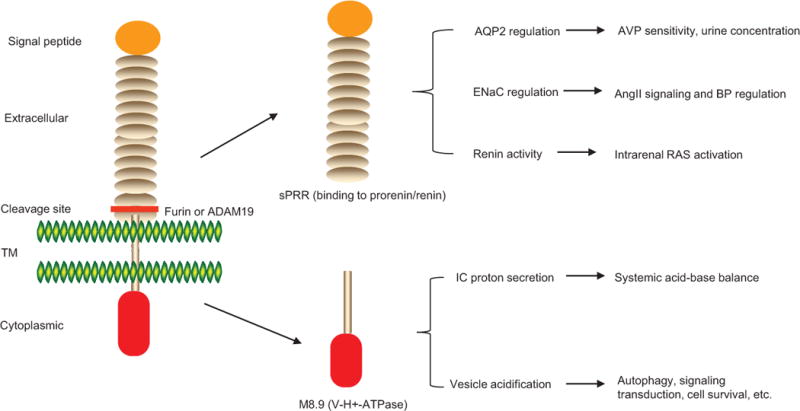
The proposed functions of PRR’s different domains generated by protease-mediated cleavage. After the cleavage, sPRR serves a paracrine function to control water and Na+ reabsorption in the principal cells via regulation of AQP2 and ENaC. M8.9 likely retains in the intercalated cells to control proton secretion to the urine. In addition, V-ATPase activity of M8.9 may also regulate vesicle acidification needed for some basic cellular functions such as autophagy, signal transduction, etc.
Acknowledgments
I thank Drs. Chang-Jiang Zou and Chuanming Xu for technical assistance.
Sources of funding
This work was supported by National Institutes of Health Grants DK104072 and DK094956, and VA Merit Review from the Department of Veterans Affairs, and National Natural Science Foundation of China Grants No. 91439205 and No. 31330037. T. Yang is Research Career Scientist in the Department of Veterans Affairs.
Footnotes
Disclosures
None.
References
- 1.Zhuo JL, Ferrao FM, Zheng Y, Li XC. New frontiers in the intrarenal renin-angiotensin system: A critical review of classical and new paradigms. Frontiers Endocrinology. 2013;4:166. doi: 10.3389/fendo.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzau VJ. Evolving concepts of the renin-angiotensin system. Focus on renal and vascular mechanisms. Am J Hypertens. 1988;1:334S–337S. doi: 10.1093/ajh/1.4.334s. [DOI] [PubMed] [Google Scholar]
- 3.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol. 2007;27:144–152. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Giani JF, Janjulia T, Taylor B, Bernstein EA, Shah K, Shen XZ, McDonough AA, Bernstein KE, Gonzalez-Villalobos RA. Renal generation of angiotensin ii and the pathogenesis of hypertension. Curr Hypertens Rep. 2014;16:477. doi: 10.1007/s11906-014-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey RM, Wang ZQ, Siragy HM. Update: Role of the angiotensin type-2 (at(2)) receptor in blood pressure regulation. Curr Hypertens Rep. 2000;2:198–201. doi: 10.1007/s11906-000-0082-3. [DOI] [PubMed] [Google Scholar]
- 6.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacological Reviews. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 7.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin ii and its contribution to the genesis of chronic hypertension. Current Opinion Pharmacology. 2011;11:180–186. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: A major player in angiotensin ii-dependent hypertension. Journal American Society of Hypertension: JASH. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid AC, Silver RB, Levi R. Renin: At the heart of the mast cell. Immunological Reviews. 2007;217:123–140. doi: 10.1111/j.1600-065X.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- 10.Iwai M, Horiuchi M. Role of renin-angiotensin system in adipose tissue dysfunction. Hypertens Res. 2009;32:425–427. doi: 10.1038/hr.2009.55. [DOI] [PubMed] [Google Scholar]
- 11.Yokota H, Nagaoka T, Tani T, Takahashi A, Sato E, Kato Y, Yoshida A. Higher levels of prorenin predict development of diabetic retinopathy in patients with type 2 diabetes. Journal Renin-angiotensin-aldosterone System: JRAAS. 2011;12:290–294. doi: 10.1177/1470320310391327. [DOI] [PubMed] [Google Scholar]
- 12.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sealey JE, McCord D, Taufield PA, Ales KA, Druzin ML, Atlas SA, Laragh JH. Plasma prorenin in first-trimester pregnancy: Relationship to changes in human chorionic gonadotropin. American Journal Obstetrics Gynecology. 1985;153:514–519. doi: 10.1016/0002-9378(85)90464-8. [DOI] [PubMed] [Google Scholar]
- 14.Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, Boomsma F, Damm P, Mathiesen ER. A high concentration of prorenin in early pregnancy is associated with development of pre-eclampsia in women with type 1 diabetes. Diabetologia. 2011;54:1615–1619. doi: 10.1007/s00125-011-2087-7. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin ii production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009;53:1077–1082. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 17.Burckle C, Bader M. Prorenin and its ancient receptor. Hypertension. 2006;48:549–551. doi: 10.1161/01.HYP.0000241132.48495.df. [DOI] [PubMed] [Google Scholar]
- 18.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (pro)renin receptor: Site-specific and functional linkage to the vacuolar h+-atpase in the kidney. Hypertension. 2009;54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez AA, Green T, Luffman C, Bourgeois CR, Gabriel Navar L, Prieto MC. Renal medullary cyclooxygenase-2 and (pro)renin receptor expression during angiotensin ii-dependent hypertension. Am J Physiol Renal Physiol. 2014;307:F962–970. doi: 10.1152/ajprenal.00267.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin ii-independent upregulation of cyclooxygenase-2 by activation of the (pro)renin receptor in rat renal inner medullary cells. Hypertension. 2013;61:443–449. doi: 10.1161/HYPERTENSIONAHA.112.196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin ii-dependent hypertensive rats. Hypertension. 2011;57:859–864. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Siragy HM. Sodium depletion enhances renal expression of (pro)renin receptor via cyclic gmp-protein kinase g signaling pathway. Hypertension. 2012;59:317–323. doi: 10.1161/HYPERTENSIONAHA.111.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matavelli LC, Huang J, Siragy HM. In vivo regulation of renal expression of (pro)renin receptor by a low-sodium diet. Am J Physiol Renal Physiol. 2012;303:F1652–1657. doi: 10.1152/ajprenal.00204.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen G. Renin and prorenin receptor in hypertension: What’s new? Curr Hypertens Rep. 2011;13:79–85. doi: 10.1007/s11906-010-0172-9. [DOI] [PubMed] [Google Scholar]
- 25.Feldt S, Maschke U, Dechend R, Luft FC, Muller DN. The putative (pro)renin receptor blocker hrp fails to prevent (pro)renin signaling. J Am Soc Nephrol. 2008;19:743–748. doi: 10.1681/ASN.2007091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T. Renal medullary (pro)renin receptor contributes to angiotensin ii-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Medicine. 2015;13:278. doi: 10.1186/s12916-015-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (pro)renin receptor antagonist pro20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension. 2015;65:352–361. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X, Wang F, Liu M, Yang KT, Nau A, Kohan DE, Reese VR, Richardson RS, Yang T. Activation of enac in collecting duct cells by prorenin and its receptor prr: Involvement of nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol. 2016;310:F1243–1250. doi: 10.1152/ajprenal.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabi AH, Suzuki F. Biochemical properties of renin and prorenin binding to the (pro)renin receptor. Hypertens Res. 2010;33:91–97. doi: 10.1038/hr.2009.201. [DOI] [PubMed] [Google Scholar]
- 31.Nabi AH, Biswas KB, Nakagawa T, Ichihara A, Inagami T, Suzuki F. Prorenin has high affinity multiple binding sites for (pro)renin receptor. Biochimica Biophysica Acta. 2009;1794:1838–1847. doi: 10.1016/j.bbapap.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Ramkumar N, Stuart D, Mironova E, Bugay V, Wang S, Abraham N, Ichihara A, Stockand JD, Kohan DE. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol. 2016;311:F186–194. doi: 10.1152/ajprenal.00088.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quadri S, Siragy HM. (pro)renin receptor contributes to regulation of renal epithelial sodium channel. Journal Hypertension. 2016;34:486–494. doi: 10.1097/HJH.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng K, Lu X, Wang F, Nau A, Chen R, Zhou SF, Yang T. Collecting duct (pro)renin receptor targets enac to mediate angiotensin ii-induced hypertension. Am J Physiol Renal Physiol. doi: 10.1152/ajprenal.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: The story of our internal environment revisited. Physiol Rev. 2015;95:297–340. doi: 10.1152/physrev.00011.2014. [DOI] [PubMed] [Google Scholar]
- 36.Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mcap1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11:828–834. doi: 10.1681/ASN.V115828. [DOI] [PubMed] [Google Scholar]
- 37.Liu W, Pastor-Soler NM, Schreck C, Zavilowitz B, Kleyman TR, Satlin LM. Luminal flow modulates h+-atpase activity in the cortical collecting duct (ccd) Am J Physiol Renal Physiol. 2012;302:F205–215. doi: 10.1152/ajprenal.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leviel F, Hubner CA, Houillier P, et al. The na+-dependent chloride-bicarbonate exchanger slc4a8 mediates an electroneutral na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sands JM, Nonoguchi H, Knepper MA. Hormone effects on nacl permeability of rat inner medullary collecting duct. Am J Physiol. 1988;255:F421–428. doi: 10.1152/ajprenal.1988.255.3.F421. [DOI] [PubMed] [Google Scholar]
- 40.Kudo LH, van Baak AA, Rocha AS. Effect of vasopressin on sodium transport across inner medullary collecting duct. Am J Physiol. 1990;258:F1438–1447. doi: 10.1152/ajprenal.1990.258.5.F1438. [DOI] [PubMed] [Google Scholar]
- 41.Rocha AS, Kudo LH. Atrial peptide and cgmp effects on nacl transport in inner medullary collecting duct. Am J Physiol. 1990;259:F258–268. doi: 10.1152/ajprenal.1990.259.2.F258. [DOI] [PubMed] [Google Scholar]
- 42.Stanton BA. Characterization of apical and basolateral membrane conductances of rat inner medullary collecting duct. Am J Physiol. 1989;256:F862–868. doi: 10.1152/ajprenal.1989.256.5.F862. [DOI] [PubMed] [Google Scholar]
- 43.Zeidel ML. Hormonal regulation of inner medullary collecting duct sodium transport. Am J Physiol. 1993;265:F159–173. doi: 10.1152/ajprenal.1993.265.2.F159. [DOI] [PubMed] [Google Scholar]
- 44.Bengele HH, Lechene C, Alexander EA. Sodium and chloride transport along the inner medullary collecting duct: Effect of saline expansion. Am J Physiol. 1980;238:F504–508. doi: 10.1152/ajprenal.1980.238.6.F504. [DOI] [PubMed] [Google Scholar]
- 45.Sonnenberg H. Medullary collecting-duct function in antidiuretic and in salt- or water-diuretic rats. Am J Physiol. 1974;226:501–506. doi: 10.1152/ajplegacy.1974.226.3.501. [DOI] [PubMed] [Google Scholar]
- 46.Sonnenberg H. Collecting duct function in deoxycorticosterone acetate-escaped, normal, and salt-deprived rats. Response to hypervolemia. Circ Res. 1976;39:282–288. doi: 10.1161/01.res.39.2.282. [DOI] [PubMed] [Google Scholar]
- 47.Sonnenberg H. Secretion of salt and water into the medullary collecting duct of ringer-infused rats. Am J Physiol. 1975;228:565–568. doi: 10.1152/ajplegacy.1975.228.2.565. [DOI] [PubMed] [Google Scholar]
- 48.Osgood RW, Reineck HJ, Stein JH. Further studies on segmental sodium transport in the rat kidney during expansion of the extracellular fluid volume. J Clin Invest. 1978;62:311–320. doi: 10.1172/JCI109131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amin MS, Reza E, El-Shahat E, Wang HW, Tesson F, Leenen FH. Enhanced expression of epithelial sodium channels in the renal medulla of dahl s rats. Canadian Journal Physiology Pharmacology. 2011;89:159–168. doi: 10.1139/Y11-005. [DOI] [PubMed] [Google Scholar]
- 50.Husted RF, Takahashi T, Stokes JB. Imcd cells cultured from dahl s rats absorb more na+ than dahl r rats. Am J Physiol. 1996;271:F1029–1036. doi: 10.1152/ajprenal.1996.271.5.F1029. [DOI] [PubMed] [Google Scholar]
- 51.Husted RF, Takahashi T, Stokes JB. The basis of higher na+ transport by inner medullary collecting duct cells from dahl salt-sensitive rats: Implicating the apical membrane na+ channel. The Journal Membrane Biology. 1997;156:9–18. doi: 10.1007/s002329900182. [DOI] [PubMed] [Google Scholar]
- 52.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: From molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 54.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev. 2007;87:1083–1112. doi: 10.1152/physrev.00053.2006. [DOI] [PubMed] [Google Scholar]
- 55.Mutig K, Borowski T, Boldt C, Borschewski A, Paliege A, Popova E, Bader M, Bachmann S. Demonstration of the functional impact of vasopressin signaling in the thick ascending limb by a targeted transgenic rat approach. Am J Physiol Renal Physiol. 2016;311:F411–423. doi: 10.1152/ajprenal.00126.2016. [DOI] [PubMed] [Google Scholar]
- 56.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T. Antidiuretic action of collecting duct (pro)renin receptor downstream of vasopressin and pge2 receptor ep4. J Am Soc Nephrol. 2016;27:3022–3034. doi: 10.1681/ASN.2015050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez AA, Cifuentes-Araneda F, Ibaceta-Gonzalez C, Gonzalez-Vergara A, Zamora L, Henriquez R, Rosales CB, Navar LG, Prieto MC. Vasopressin/v2 receptor stimulates renin synthesis in the collecting duct. Am J Physiol Renal Physiol. 2016;310:F284–293. doi: 10.1152/ajprenal.00360.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamura Y, Mori N, Xu B, Nakamura T, Yamakoshi S, Hirose T, Ito O, Totsune K, Takahashi K, Kohzuki M. Water deprivation increases (pro)renin receptor levels in the kidney and decreases plasma concentrations of soluble (pro)renin receptor. The Tohoku Journal Experimental Medicine. 2016;239:185–192. doi: 10.1620/tjem.239.185. [DOI] [PubMed] [Google Scholar]
- 59.Ramkumar N, Stuart D, Calquin M, Quadri S, Wang S, Van Hoek AN, Siragy HM, Ichihara A, Kohan DE. Nephron-specific deletion of the prorenin receptor causes a urine concentration defect. Am J Physiol Renal Physiol. 2015;309:F48–56. doi: 10.1152/ajprenal.00126.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trepiccione F, Gerber SD, Grahammer F, Lopez-Cayuqueo KI, Baudrie V, Paunescu TG, Capen DE, Picard N, Alexander RT, Huber TB, Chambrey R, Brown D, Houillier P, Eladari D, Simons M. Renal atp6ap2/(pro)renin receptor is required for normal vacuolar h+-atpase function but not for the renin-angiotensin system. J Am Soc Nephrol. 2016;27:3320–3330. doi: 10.1681/ASN.2015080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song R, Preston G, Ichihara A, Yosypiv IV. Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PLoS One. 2013;8:e63835. doi: 10.1371/journal.pone.0063835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farman N, Pradelles P, Bonvalet JP. Determination of prostaglandin e2 synthesis along rabbit nephron by enzyme immunoassay. Am J Physiol. 1986;251:F238–244. doi: 10.1152/ajprenal.1986.251.2.F238. [DOI] [PubMed] [Google Scholar]
- 63.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annual Review Physiology. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 64.Yang T. Crosstalk between (pro)renin receptor and cox-2 in the renal medulla during angiotensin ii-induced hypertension. Current Opinion Pharmacology. 2015;21:89–94. doi: 10.1016/j.coph.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jackson BA. Prostaglandin e2 synthesis in the inner medullary collecting duct of the rat: Implications for vasopressin-dependent cyclic amp formation. Journal of Cellular Physiology. 1986;129:60–64. doi: 10.1002/jcp.1041290109. [DOI] [PubMed] [Google Scholar]
- 66.Maeda Y, Terada Y, Nonoguchi H, Knepper MA. Hormone and autacoid regulation of camp production in rat imcd subsegments. Am J Physiol. 1992;263:F319–327. doi: 10.1152/ajprenal.1992.263.2.F319. [DOI] [PubMed] [Google Scholar]
- 67.Noland TD, Carter CE, Jacobson HR, Breyer MD. Pge2 regulates camp production in cultured rabbit ccd cells: Evidence for dual inhibitory mechanisms. Am J Physiol. 1992;263:C1208–1215. doi: 10.1152/ajpcell.1992.263.6.C1208. [DOI] [PubMed] [Google Scholar]
- 68.Hebert RL, Jacobson HR, Fredin D, Breyer MD. Evidence that separate pge2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am J Physiol. 1993;265:F643–650. doi: 10.1152/ajprenal.1993.265.5.F643. [DOI] [PubMed] [Google Scholar]
- 69.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, Anderson SA, Deng CX, Knepper MA, Wess J. A selective ep4 pge2 receptor agonist alleviates disease in a new mouse model of x-linked nephrogenic diabetes insipidus. J Clin Invest. 2009;119:3115–3126. doi: 10.1172/JCI39680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen BL, Stubbe J, Hansen PB, Andreasen D, Skott O. Localization of prostaglandin e(2) ep2 and ep4 receptors in the rat kidney. Am J Physiol Renal Physiol. 2001;280:F1001–1009. doi: 10.1152/ajprenal.2001.280.6.F1001. [DOI] [PubMed] [Google Scholar]
- 71.Torikai S, Kurokawa K. Distribution of prostaglandin e2-sensitive adenylate cyclase along the rat nephron. Prostaglandins. 1981;21:427–438. doi: 10.1016/0090-6980(81)90088-5. [DOI] [PubMed] [Google Scholar]
- 72.Gao M, Cao R, Du S, Jia X, Zheng S, Huang S, Han Q, Liu J, Zhang X, Miao Y, Kang J, Gustafsson JA, Guan Y. Disruption of prostaglandin e2 receptor ep4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc Natl Acad Sci U S A. 2015;112:8397–8402. doi: 10.1073/pnas.1509565112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F, Lu X, Peng K, Zhou L, Li C, Wang W, Yu X, Kohan DE, Zhou SF, Yang T. Cox-2 mediates angiotensin ii-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol Renal Physiol. 2014;307:F25–32. doi: 10.1152/ajprenal.00548.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin e-prostanoid4 receptor mediates angiotensin ii-induced (pro)renin receptor expression in the rat renal medulla. Hypertension. 2014;64:369–377. doi: 10.1161/HYPERTENSIONAHA.114.03654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T. The (pro)renin receptor is cleaved by adam19 in the golgi leading to its secretion into extracellular space. Hypertens Res. 2011;34:599–605. doi: 10.1038/hr.2010.284. [DOI] [PubMed] [Google Scholar]
- 76.Maruyama N, Segawa T, Kinoshita N, Ichihara A. Novel sandwich elisa for detecting the human soluble (pro)renin receptor. Frontiers Bioscience. 2013;5:583–590. doi: 10.2741/e640. [DOI] [PubMed] [Google Scholar]
- 77.Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, Morimoto S, Jwa SC, Egawa M, Arai Y, Suzuki F, Sago H, Ichihara A. Soluble (pro)renin receptor and blood pressure during pregnancy: A prospective cohort study. Hypertension. 2012;60:1250–1256. doi: 10.1161/HYPERTENSIONAHA.112.197418. [DOI] [PubMed] [Google Scholar]
- 78.Watanabe N, Morimoto S, Fujiwara T, Suzuki T, Taniguchi K, Mori F, Ando T, Watanabe D, Kimura T, Sago H, Ichihara A. Prediction of gestational diabetes mellitus by soluble (pro)renin receptor during the first trimester. The Journal Clinical Endocrinology Metabolism. 2013;98:2528–2535. doi: 10.1210/jc.2012-4139. [DOI] [PubMed] [Google Scholar]
- 79.Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Abe T, Suga T, Takada S, Kadoguchi T, Okita K, Matsushima S, Tsutsui H. Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. International Journal Cardiology. 2013;168:4313–4314. doi: 10.1016/j.ijcard.2013.04.176. [DOI] [PubMed] [Google Scholar]
- 80.Bonakdaran S, Azami G, Tara F, Poorali L. Soluble (pro) renin receptor is a predictor of gestational diabetes mellitus. Current Diabetes Reviews. 2016 Sep 19; doi: 10.2174/1573399812666160919100253. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 81.Nishijima T, Tajima K, Yamashiro Y, Hosokawa K, Suwabe A, Takahashi K, Sakurai S. Elevated plasma levels of soluble (pro)renin receptor in patients with obstructive sleep apnea syndrome in parallel with the disease severity. The Tohoku Journal Experimental Medicine. 2016;238:325–338. doi: 10.1620/tjem.238.325. [DOI] [PubMed] [Google Scholar]
- 82.Kreienbring K, Franz A, Richter R, Dragun D, Heidecke H, Dechend R, Muller DN, Sehouli J, Braicu EI. Predictive and prognostic value of sprr in patients with primary epithelial ovarian cancer. Analytical Cellular Pathology. 2016;2016:6845213. doi: 10.1155/2016/6845213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamada K, Taniguchi Y, Shimamura Y, et al. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clinical Experimental Nephrology. 2013;17:848–856. doi: 10.1007/s10157-013-0803-y. [DOI] [PubMed] [Google Scholar]
- 84.Ohashi N, Isobe S, Ishigaki S, Suzuki T, Iwakura T, Ono M, Fujikura T, Tsuji T, Otsuka A, Ishii Y, Furuse H, Kato A, Ozono S, Yasuda H. Plasma soluble (pro)renin receptor reflects renal damage. PLoS One. 2016;11:e0156165. doi: 10.1371/journal.pone.0156165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen G, Blanchard A, Curis E, Bergerot D, Chambon Y, Hirose T, Caumont-Prim A, Tabard SB, Baron S, Frank M, Totsune K, Azizi M. Plasma soluble (pro)renin receptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertension. 2014;63:297–302. doi: 10.1161/HYPERTENSIONAHA.113.02217. [DOI] [PubMed] [Google Scholar]
- 86.Morimoto S, Ando T, Niiyama M, Seki Y, Yoshida N, Watanabe D, Kawakami-Mori F, Kobori H, Nishiyama A, Ichihara A. Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens Res. 2014;37:642–648. doi: 10.1038/hr.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin ii. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou SF, Gustafsson JA, Yang T. Soluble (pro)renin receptor via beta-catenin enhances urine concentration capability as a target of liver x receptor. Proc Natl Acad Sci U S A. 2016;113:E1898–1906. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-cd water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knepper MA, Verbalis JG, Nielsen S. Role of aquaporins in water balance disorders. Curr Opin Nephrol Hypertens. 1997;6:367–371. doi: 10.1097/00041552-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annual Review Cell Developmental Biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 93.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 94.Rousselle A, Sihn G, Rotteveel M, Bader M. (pro)renin receptor and v-atpase: From drosophila to humans. Clinical Science. 2014;126:529–536. doi: 10.1042/CS20130307. [DOI] [PubMed] [Google Scholar]
- 95.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y. Multiple genes of the renin-angiotensin system are novel targets of wnt/beta-catenin signaling. J Am Soc Nephrol. 2015;26:107–120. doi: 10.1681/ASN.2014010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He W, Kang YS, Dai C, Liu Y. Blockade of wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou L, Liu Y. Wnt/beta-catenin signaling and renin-angiotensin system in chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25:100–106. doi: 10.1097/MNH.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jung HJ, Kim SY, Choi HJ, Park EJ, Lim JS, Frokiaer J, Nielsen S, Kwon TH. Tankyrase-mediated beta-catenin activity regulates vasopressin-induced aqp2 expression in kidney collecting duct mpkccdc14 cells. Am J Physiol Renal Physiol. 2015;308:F473–486. doi: 10.1152/ajprenal.00052.2014. [DOI] [PubMed] [Google Scholar]
- 99.Yasuoka Y, Kobayashi M, Sato Y, Zhou M, Abe H, Okamoto H, Nonoguchi H, Tanoue A, Kawahara K. The intercalated cells of the mouse kidney omcd(is) are the target of the vasopressin v1a receptor axis for urinary acidification. Clinical and Experimental Nephrology. 2013;17:783–792. doi: 10.1007/s10157-013-0783-y. [DOI] [PubMed] [Google Scholar]
- 100.Gabbi C, Kong X, Suzuki H, Kim HJ, Gao M, Jia X, Ohnishi H, Ueta Y, Warner M, Guan Y, Gustafsson JA. Central diabetes insipidus associated with impaired renal aquaporin-1 expression in mice lacking liver x receptor beta. Proc Natl Acad Sci U S A. 2012;109:3030–3034. doi: 10.1073/pnas.1200588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sands JM, Klein JD. Physiological insights into novel therapies for nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2016;311:F1149–F1152. doi: 10.1152/ajprenal.00418.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yun J, Schoneberg T, Liu J, Schulz A, Ecelbarger CA, Promeneur D, Nielsen S, Sheng H, Grinberg A, Deng C, Wess J. Generation and phenotype of mice harboring a nonsense mutation in the v2 vasopressin receptor gene. J Clin Invest. 2000;106:1361–1371. doi: 10.1172/JCI9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knoers NV, Monnens LL. Nephrogenic diabetes insipidus. Semin Nephrol. 1999;19:344–352. [PubMed] [Google Scholar]
- 104.Dollerup P, Thomsen TM, Nejsum LN, Faerch M, Osterbrand M, Gregersen N, Rittig S, Christensen JH, Corydon TJ. Partial nephrogenic diabetes insipidus caused by a novel aqp2 variation impairing trafficking of the aquaporin-2 water channel. BMC Nephrology. 2015;16:217. doi: 10.1186/s12882-015-0213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schagger H. Identification and characterization of a novel 9.2-kda membrane sector-associated protein of vacuolar proton-atpase from chromaffin granules. J Biol Chem. 1998;273:10939–10947. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- 106.Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification-the emerging role of v-atpase in aging and neurodegenerative disease. Ageing Research Reviews. 2016;32:75–88. doi: 10.1016/j.arr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar h+-atpase. Physiol Rev. 2004;84:1263–1314. doi: 10.1152/physrev.00045.2003. [DOI] [PubMed] [Google Scholar]
- 108.Lu X, Garrelds IM, Wagner CA, Danser AH, Meima ME. (pro)renin receptor is required for prorenin-dependent and -independent regulation of vacuolar h(+)-atpase activity in mdck.C11 collecting duct cells. Am J Physiol Renal Physiol. 2013;305:F417–425. doi: 10.1152/ajprenal.00037.2013. [DOI] [PubMed] [Google Scholar]
- 109.Daryadel A, Bourgeois S, Figueiredo MF, Gomes Moreira A, Kampik NB, Oberli L, Mohebbi N, Lu X, Meima ME, Danser AH, Wagner CA. Colocalization of the (pro)renin receptor/atp6ap2 with h+-atpases in mouse kidney but prorenin does not acutely regulate intercalated cell h+-atpase activity. PLoS One. 2016;11:e0147831. doi: 10.1371/journal.pone.0147831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H. The (pro)renin receptor/atp6ap2 is essential for vacuolar h+-atpase assembly in murine cardiomyocytes. Circ Res. 2010;107:30–34. doi: 10.1161/CIRCRESAHA.110.224667. [DOI] [PubMed] [Google Scholar]
- 111.Ichihara A. (pro)renin receptor and autophagy in podocytes. Autophagy. 2012;8:271–272. doi: 10.4161/auto.8.2.18846. [DOI] [PubMed] [Google Scholar]
- 112.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. V-atpase b1-subunit promoter drives expression of egfp in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. American journal of physiology Cell Physiology. 2005;288:C1134–1144. doi: 10.1152/ajpcell.00084.2004. [DOI] [PubMed] [Google Scholar]
- 113.Wu H, Chen L, Zhou Q, Zhang X, Berger S, Bi J, Lewis DE, Xia Y, Zhang W. Aqp2-expressing cells give rise to renal intercalated cells. J Am Soc Nephrol. 2013;24:243–252. doi: 10.1681/ASN.2012080866. [DOI] [PMC free article] [PubMed] [Google Scholar]


